Keywords
High-dose intravenous vitamin C (HDIVC), oncology, safety and efficacy, cancer-related morbidity and mortality
This article is included in the Oncology gateway.
High-dose intravenous vitamin C (HDIVC) has gained interest as an adjunctive therapy in cancer care due to its pro-oxidative antioxidative and cytotoxic effects. Despite growing research, a comprehensive synthesis of the evidence on the efficacy and safety of HDIVC in advanced solid tumors remains limited.
This review will follow the six steps of the Arksey and O’Malley framework, as adapted by Levac et al, namely: identifying the research question, identifying relevant studies, study selection eligibility, charting the data, collating, summarizing and reporting the results, and consultation. PubMed, Scopus, ScienceDirect, and Google Scholar databases will be used for the search of articles from 2000 – 2025. Grey literature will also be conducted. The Preferred Reporting Items for Systematic Reviews and the Meta-Analysis for Scoping Reviews (PRISMA-ScR) will be used as a guide for this scoping review protocol. A two-stage screening process will be used to determine the eligibility of articles with two independent reviewers. Discrepancies will be handled by consensus or by consulting an expert in cancer care. The selection of studies for the review is anticipated to be completed within 12 weeks, from 01 October to 31 December 2025. The extracted data will be analyzed and presented in a report.
This scoping review will consolidate evidence on HDIVC for advanced solid tumors, identifying efficacy outcomes, safety profiles, and patient experiences limited to African adult populations and other global studies applicable to African settings in African populations that will inform clinical practice and guide policy making.
Ethical approval is not required for this scoping review. However, the results of this search will be disseminated through academic presentations and publications in peer-reviewed journals.
High-dose intravenous vitamin C (HDIVC), oncology, safety and efficacy, cancer-related morbidity and mortality
This revised version of the scoping review protocol incorporates substantive revisions in response to peer reviewer feedback, aimed at enhancing methodological clarity, scope relevance, and scientific rigor. Key changes include the correction of a critical contradiction in the exclusion criteria: the original version mistakenly excluded "studies on advanced-stage disease," which conflicted with the review’s stated focus on advanced solid tumors. This has now been corrected to exclude studies on early-stage disease only, aligning the methodology with the intended research objectives.
Additionally, the search strategy has been revised to ensure inclusivity and balance. Previous inconsistencies in search terms such as the omission of "African females" and lack of clarity regarding the exclusion of pediatric populations have been addressed. The review now explicitly focuses on African adult populations, with revised Boolean search terms across all databases for consistency and to minimize selection bias.
The methodology section has been expanded to include greater detail on data extraction, stakeholder consultation, and grey literature retrieval. The updated version also clarifies that global studies applicable to African contexts will be included to compensate for the anticipated paucity of region-specific data, ensuring a meaningful synthesis of evidence.
Terminological precision has been improved. In the abstract and introduction, the description of HDIVC’s anticancer effects now correctly emphasizes its pro-oxidative mechanisms rather than antioxidative ones, referencing foundational studies by the Levine/Chen group (e.g., Chen et al., 2005).
Overall, these revisions strengthen the protocol’s coherence, scientific validity, and practical applicability to African oncology settings.
See the authors' detailed response to the review by Qi Chen
See the authors' detailed response to the review by Taufeeque Ali
Solid tumors represent approximately 90% of human malignancies and continuously contribute strongly to global cancer-related morbidity and mortality (Sung et al., 2021; Xiaoyu et al., 2024; Santucci et al., 2020). Significant progress has been made with targeted therapies, immune checkpoint inhibitors, and combination treatment strategies, but the clinical management of advanced solid tumors continues to pose a formidable challenge (Li et al., 2023). These cancers exhibit biological heterogeneity and varied tumor microenvironments (TME), and recurring resistance to conventional therapy including chemotherapy, radiation, and surgery (Garcia et al., 2024; Ge et al., 2022; Taeb et al., 2022). The tumor microenvironment strongly impacts tumor progression, immune evasion, metastasis, and therapeutic resistance, with factors such as hypoxia, acidity, and immune suppression presenting significant barriers to the success of therapy (Wang et al., 2023; Ghosh et al., 2024; Zhou et al., 2024).
There has been renewed interest in adjunctive therapies that may enhance the therapeutic index of standard treatments. Traditionally known for its antioxidant activity, vitamin C has garnered interest for its potential pro-oxidant effects when administered at pharmacologic concentrations achievable via high-dose intravenous infusion (Giansanti et al., 2021; Böttger et al., 2021; Pawlowska et al., 2019). High-dose intravenous vitamin C (HDIVC) has demonstrated the ability to modulate the tumor microenvironment by potentially reversing epithelial-to-mesenchymal transition, suppressing hypoxia-driven and oncogenic signaling pathways, and promoting anti-tumor immune responses (Böttger et al., 2021; Suraweera et al., 2018; Hoffer et al., 2015). HDIVC vitamin has gained interest as an adjunctive therapy in cancer care due to its potential pro-oxidative and cytotoxic effects (Chen et al., 2005). The pharmacologic intravenous ascorbate acts via pro-oxidative mechanisms, rather than classic antioxidative ones. For instance, Chen et al. (2008) demonstrated that high-dose ascorbate functions as a pro-drug for hydrogen peroxide generation in tissues, selectively killing cancer Subsequent work by the Levine & Chen group confirmed in vivo production of H2O2 in tumor interstitial fluid at pharmacologic doses (Roy et al., 2025).
HDIVC is proposed to selectively generate oxidative stress in tumor cells, inducing cytotoxicity while minimizing harm to normal tissues (Gibson et al., 2020; Paller et al., 2024). Preliminary clinical studies indicate that HDIVC is safe and effectively attains the pharmacologic plasma levels necessary to elicit anti-tumor effects (Böttger et al., 2021; Zhao et al., 2025). Furthermore, preclinical studies have highlighted the potent antitumor effects of HDIVC, including the induction of apoptosis, suppression of tumor cell proliferation, and disruption of metastatic mechanisms (Fan et al., 2023; Carr & Cook, 2018; Böttger et al., 2021). HDIVC has also demonstrated potential synergistic effects when combined with conventional chemotherapy and radiotherapy, enhancing tumoricidal efficacy while concurrently reducing the toxicities associated with standard treatments (Böttger et al., 2021; Carr & Cook, 2018; Didier et al., 2023). Nonetheless, although phase I and II trials increasingly support the safety and potential efficacy of high-dose intravenous vitamin C (HDIVC), conclusive evidence from phase III trials is still lacking, and reported outcomes related to tumor response, survival, and patient-reported measures remain inconsistent (Shah et al., 2022; Wang et al., 2022).
Existing studies are largely concentrated in North America, Europe, and parts of Asia, with limited relevance to the sociocultural, genetic, and healthcare infrastructure realities of African patients. Africa’s public health sector will benefit from adjunctive treatments like HDIVC, which offer potential benefits in mitigating treatment-related side effects, enhancing quality of life, and providing consistent, comprehensive oncology care. Synthesizing the current global and emerging local evidence will identify gaps in knowledge, underscore the need for context-specific evidence to inform tailored therapeutic interventions, and support the development of African-centered clinical guidelines. This review will be specifically limited to African adult populations and other global studies with applicability to African setting, as pediatric cancers often differ in biology, treatment response, and supportive care requirements.
This scoping review will adopt the Joanna Briggs Institute (JBI) methodological framework for scoping reviews and adhere to the PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) checklist for reporting (Hadie, 2024). These frameworks provide transparency, rigor, and reproducibility in scoping reviews by clearly articulating the processes for identifying, selecting, and synthesizing literature. This review also aligns with the enhanced methodology proposed by Arksey and O’Malley (2005) and later refined by Levac et al (Levac et al., 2010; Ouzzani et al., 2016), ensuring systematic rigor through six key steps ( Figure 1):
1. Identifying the research question
2. Identifying relevant studies
3. Study selection
4. Charting the data
5. Collating, summarizing, and reporting results
6. Consultation
The research question was developed using the technique suggested by Arksey and O’Malley (2005) and further refined by Levac et al. (2010), which builds on Arksey and O’Malley’s framework, with guidance from subsequent enhancements. The proposed scoping review methodology for scoping reviews and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis extension for Scoping Reviews (PRISMA-ScR, Figure 2) guidelines and checklist to allow us to make use of literature across study designs and in both peer-reviewed and grey literature. The process will involve five key stages and an optional sixth stage that we have included in our process.
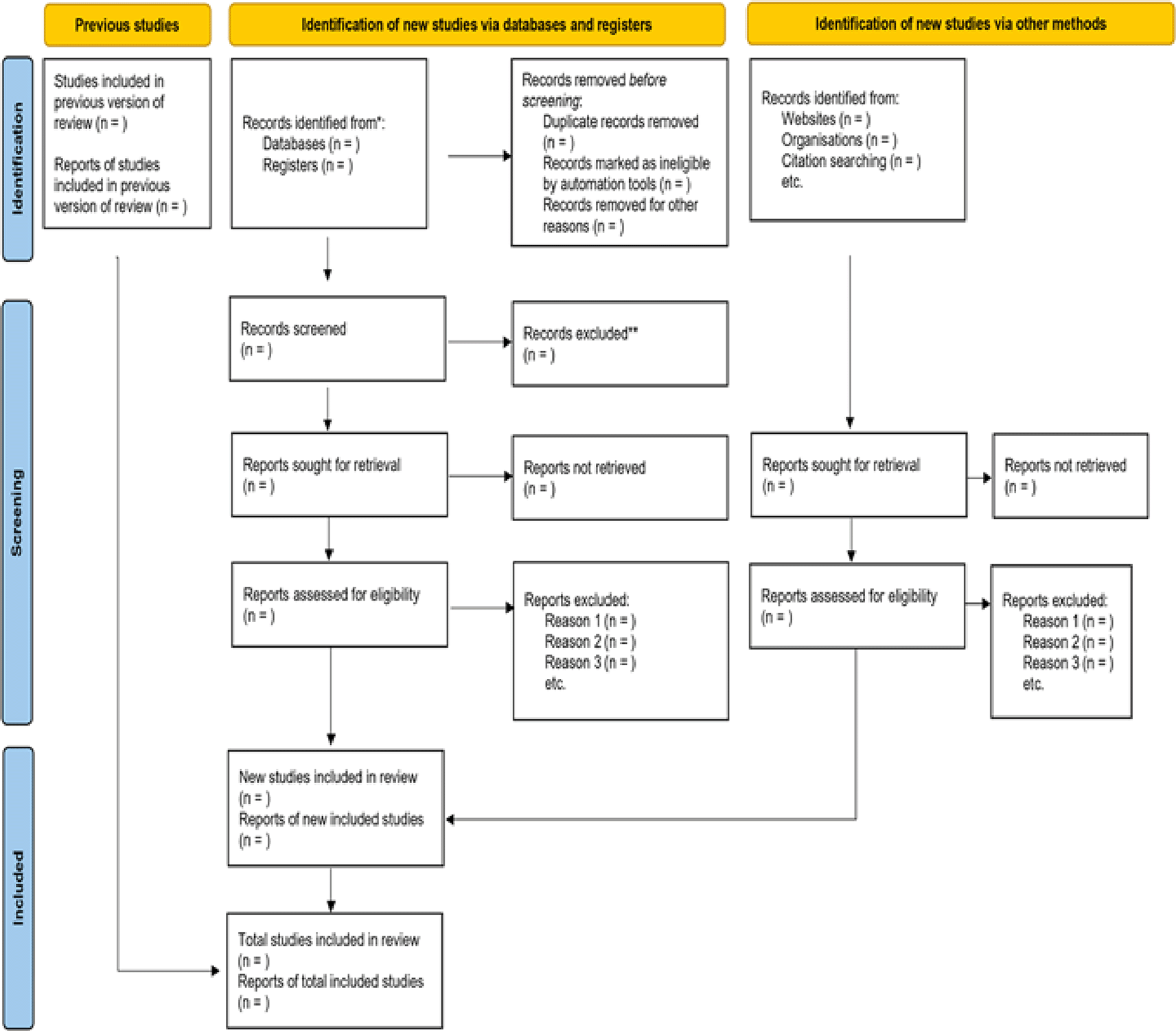
Source: Tricco et al. (2018).
The process will include: (1) Identifying the research question –clearly defining the purpose, concepts, and target population to maintain a balance between breadth and focus; (2) Identifying relevant studies – developing an iterative, comprehensive search strategy in collaboration with an information specialist, covering both peer-reviewed and grey literature; using predefined keywords and Boolean operators (3) Study selection – applying well-defined inclusion and exclusion criteria with independent screening of titles and abstracts and full articles by two reviewers, including pilot testing to ensure consistency; (4) Charting the data – using a standardized and iteratively refined extraction form to capture key study characteristics and contextual information; (5) Collating, summarizing, and reporting results – analyzing and presenting findings through descriptive numerical summaries, thematic synthesis, and interpretation of implications for research, policy, and practice; and (6) Consultation with stakeholders – engaging relevant experts and knowledge users to validate findings, identify gaps, and guide dissemination and inform implications for research and practice.
Research Sub-questions:
• What types of solid tumors have been studied concerning HDIVC?
• What adverse effects and safety concerns are documented with HDVIC?
• What are the reported clinical efficacy outcomes or quality-of-life measures with the use of HDIVC?
This study will use the Population, Intervention, Comparison, Outcomes, and Timeline (PICOT) format to align the study selection with the research question. This study’s applied PICOT framework is shown in Table 1. The timeline for this study will span 12 weeks from 01 October 2025 to 31 December 2025, and eligible studies will be from 2000 to 2025. The use of high-dose intravenous vitamin C as a potential adjunct in oncology started to receive renewed scientific attention in the early 2000s following the pioneering pharmacokinetic studies conducted by NIH researchers that showed intravenous but not oral vitamin C could achieve pharmacologic plasma concentrations with potential cytotoxic effects on tumor cells. Prior to 2000, most studies were anecdotal or lacked a thorough clinical approach. Over the past 20 years, there has been an increasing push to improve the infrastructure for clinical research in low- and middle-income countries (LMICs) and Africa (Böttger et al., 2021).
We will comprehensively search electronic databases (PubMed, ScienceDirect, Scopus, and Google Scholar) and grey literature sources for studies published between 2000 and 2025. An initial exploratory search strategy based on the PICOT framework will be developed on PubMed to determine some relevant terms to the research question. However, there is no comparator for this study hence PIOT framework will be used. A second search strategy will be developed using the most relevant Medical Subject Headings (MeSH) terms, while some keywords will be searched in the title, abstract, and subject headings of the other electronic databases. Additional grey literature will be searched through cancer research institutes’ organizational reports, and manual searching of reference lists from included studies, including consulting websites such as the World Health Organization (WHO) and the United Nations (UN). The strategy will be refined with the input of an academic librarian and subjected to internal peer review for completeness and replicability. The initial search strategy in the electronic databases is stated in Table 2.
To ensure a rigorous selection process, we will employ the PIOT framework (Population Intervention Outcome Time) as a guide for title and abstract screening, with the two reviewers working independently. It is anticipated that the selection of studies for the review will be completed within 12 weeks, and this process will adhere to the guidelines provided by the PRISMA-ScR checklist. Once all identified records have been extracted from all databases, duplicates will be eliminated using the EndNote X9 (Clarivate) software. Rayyan, a reviewing platform, will be used to execute the entire selection process to facilitate collaboration between the reviewers to maintain transparency and streamline the reviewing process. Any discrepancies between the two reviewers during the screening and selection stages will be resolved through discussion with an expert in cancer care to reach an agreement.
Titles and abstracts will be screened for eligibility by two independent reviewers based on the inclusion criteria:
Inclusion criteria:
• Published peer-reviewed studies and review articles that evaluated high-dose intravenous vitamin C (HDIVC) in patients with advanced solid tumors in African countries
• Conference proceedings and grey literature.
• Study designs: Randomized Clinical Trials, observational studies, cohort studies, case series, qualitative studies, and mixed-methods studies.
• Outcomes addressing efficacy (e.g., tumor response, survival), safety (e.g., adverse events), and patient-reported experiences (e.g., quality of life).
• Published studies in English from 2000-2025
• African adult population
• Global studies (with applicability for African setting)
Exclusion criteria:
• Studies on oral vitamin C.
• Studies from non-African countries
• Preclinical (animal or in vitro) studies.
• Studies exclusively involving hematologic malignancies.
• Studies not involving solid tumors or where the cancer type is hematologic in nature or preclinical studies simulating advanced-stage disease in non-human models.
A standardized data extraction form will be developed and piloted to ensure clarity and consistency. The form will capture key details shown in Table 3.
The key findings across studies will be synthesized. The NVivo software will be used for qualitative synthesis of data. Qualitative data will be summarized narratively in themes. Descriptive statistics will be used for quantitative outcomes, where applicable, and presented in tables and figures. A PRISMA-ScR flow diagram would be used to illustrate the selection process.
Quality assessment is not a primary focus in the scoping review. The study designs of included studies, such as qualitative, quantitative, and mixed methods, will be described to improve the quality of scoping reviews by enhancing transparency and uniformity in reporting. This assessment will help contextualize the strength of the evidence and identify methodological gaps. There will be no risk of bias assessment for this scoping review.
Stakeholders, including researchers, health care policymakers, oncologists and cancer advocates will be consulted to validate and interpret the findings of this scoping review. Though these stakeholders have not directly contributed to the design of this review, they will be consulted and involved in interpreting our findings for practical and evidence-based decision-making.
This scoping review will provide an evidence map of the role of HDIVC in treating advanced solid tumors in adult African populations with results for practice, policy, and future integrative oncology guidelines and research. Given a rising incidence of cancer in Africa, a synthesis of current global and emerging local evidence will identify gaps in knowledge and inform tailored therapeutic interventions, particularly in resource-constrained settings in Africa.
The review will follow a well-established scoping review methodology using the Arksey and O’Malley framework, refined by the Levac et al framework. The selection of articles will cover studies published in the last two decades (2000 –2025), synthesizing old and current evidence from the literature. The inclusion of grey literature strengthens our review by reducing publication bias and enhancing the comprehensiveness of our findings.
However, studies un-indexed in languages other than English, in the consulted databases, and in grey literature will be omitted from the review.
The current study did not produce or analyze any datasets, as no primary data will be collected, and all sources will be from publicly available literature. Upon research completion, all pertinent data will be made public. There will be a scoping review, and the outcomes will be presented. All data generated or analyzed during this scoping review will be included in the published article and its supplementary materials. No primary data will be collected, and all sources will be from publicly available literature through the systematic search process. Detailed search strategies, data charting forms, and extracted datasets will be provided as supplementary files to ensure transparency and reproducibility.
Figshare: A Scoping Review Protocol of the Efficacy and Safety of High-Dose Intravenous Vitamin C in the Treatment of Advanced Solid Tumors in African Populations, https://doi.org/10.25406/wsu.29930129.v1 (Ogbodu & Akapo, 2025).
This project contains the following data:
Data are available under the terms of the Creative Commons 1.0 Universal License (CC0 1.0).
The authors acknowledge and thank the library personnel at Walter Sisulu University for helping to optimize the search plan.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Vitamin C and Cancer, translational research, EMT, cancer stem cells
Is the rationale for, and objectives of, the study clearly described?
Partly
Is the study design appropriate for the research question?
Partly
Are sufficient details of the methods provided to allow replication by others?
Partly
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Drug Discovery, Epigenetics, Oncological Therapeutics
Is the rationale for, and objectives of, the study clearly described?
Partly
Is the study design appropriate for the research question?
Partly
Are sufficient details of the methods provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Yes
References
1. Chen Q, Espey M, Krishna M, Mitchell J, et al.: Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proceedings of the National Academy of Sciences. 2005; 102 (38): 13604-13609 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Vitamin C and Cancer, translational research, EMT, cancer stem cells
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 22 Dec 25 |
read | |
|
Version 1 22 Sep 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)