Keywords
decreased sound tolerance, auditory hypersensitivity, hyperacusis, autism spectrum disorder, attention, load theory
Decreased sound tolerance (DST) is common in individuals with autism spectrum disorder (ASD). This study examined how attentional control resources influence DST. Previous studies have shown that increasing cognitive load enhances the brain activity component (N100) to task-irrelevant noise. We hypothesized that individuals with DST would be more sensitive to cognitive load, showing a greater increase in their neural responses to noise as cognitive load increases. Because DST comprises multiple subjective symptoms, we analyzed the associations with specific symptoms.
Thirteen adults with ASD and twenty-two adults without ASD participated in the study. Participants who self-reported auditory hypersensitivity were classified as the DST group. All participants completed a DST questionnaire with six subscales: excessive loudness, ear pain, fear, annoyance, attention control, and attention to detail. Event-related potentials (ERPs) elicited by task-irrelevant noise were analyzed during an auditory n-back task. The n-back task included a low (1-back) and a high (2-back) cognitive load condition.
The prevalence of self-reported DST was significantly higher in the ASD group than in controls. Individuals who reported DST also tended to experience other types of sensory hypersensitivity. Attentional functions were associated with fear, annoyance, and loudness perception of sounds, and ear pain appeared to function independently of other DST symptoms. Scores for attentional control difficulties related to DST did not correlate with performance on the n-back task, which reflects susceptibility to noise interference. Contrary to previous findings, N100 amplitude did not differ between task-load conditions and did not vary according to the presence of DST. P200 amplitude was larger in the 1-back than in the 2-back condition but did not differ by ASD diagnosis or DST status.
The presence of DST or ASD was not associated with cognitive capacity or with the auditory processing of task-irrelevant noise.
decreased sound tolerance, auditory hypersensitivity, hyperacusis, autism spectrum disorder, attention, load theory
Decreased sound tolerance (DST) is a chronic condition commonly defined as a lowered tolerance to sounds that are perceived as normal by most people (Potgieter et al., 2022). DST is associated with distress and reduced quality of life (Fackrell et al., 2022) and has also been reported to impose limitations on learning and social participation (Potgieter et al., 2022). This sensitivity can be divided into four subcategories—excessive loudness, pain, annoyance, and fear—which may coexist or influence one another (Tyler et al., 2014). Daily behavioral responses to sounds can sometimes serve as indicators of DST.
Although terminology regarding auditory sensitivity is inconsistent, the term “DST” is used in this study to collectively refer to any decrease in sound tolerance. As the subjective experience of DST can be divided into several subcategories, each of which is expected to have different underlying mechanisms and coping strategies, it is necessary to distinguish among them and to improve our understanding of each domain.
Since the publication of the Diagnostic and Statistical Manual – Fifth Edition (DSM-5), the diagnostic criteria for autism spectrum disorder (ASD) have incorporated hyperreactivity or hyporeactivity to sensory stimuli (American Psychiatric Association, 2013a). Sensory hypersensitivity and hyposensitivity are common in ASD, with DST being particularly prevalent among children with ASD. Reported prevalence rates of DST vary widely depending on assessment instruments and severity criteria. A review synthesizing previous studies reported that the percentage of children with ASD who have DST ranges from 15 to 100% (Gomes et al., 2008). Nonetheless, when consistent assessment criteria are applied, individuals with ASD consistently show a higher percentage of DST than the general population (Danesh et al., 2021).
It has been suggested that characteristics of selective attention may be responsible for DST in ASD (Inafuku et al., 2013). The tendency to focus on details and the reduced cognitive flexibility observed in individuals with ASD (Gambra et al., 2024; Lage et al., 2024) may also contribute to heightened attention to unpleasant auditory stimuli. Descriptions provided by individuals with DST often include narratives that suggest an influence of attention. For example, individuals with DST report feels that various sounds are pushed in and out of the perceptual mix (Yokomichi, 2021). For these individuals, noises cannot be filtered out of consciousness, and all sounds are perceived as an undifferentiated mixture (NHK Special Reporting Team, 2018). They may also detect minute mechanical sounds that typically go unnoticed (NHK Special Reporting Team, 2018). It is possible that individuals with difficulties in attentional control have trouble disengaging their attention from unwanted auditory stimuli (Khalfa et al., 2002), and this cognitive trait may trigger DST. Although multiple individual factors may contribute to different subtypes of DST, difficulties in attentional control may play a central role by preventing disengagement from unpleasant stimuli.
Event-related potentials (ERPs) are among the most effective tools for objectively evaluating neural responses immediately after stimulus presentation. ERPs can detect changes in neural activity that may not be captured by behavioral indicators and can help clarify the neural mechanisms underlying behavior. Some ERP components reflect attentional processes (Karhson & Golob, 2016). Schwartz et al. (2020) studied adolescents with ASD and language delays and found a correlation between the percentage of time atypical auditory behaviors such as “covering the ears” and “humming” were observed during the Autism Diagnostic Observation Schedule (ADOS) and the P1-N1 peak-to-peak amplitude elicited by deviant stimuli in a passive oddball task. Chien et al. (2019) conducted an ERP study of sensory hypersensitivity in individuals with ASD using a paired-click paradigm to measure sensory gating. They analyzed the P50, N100, and P200 components and found reduced N100 suppression in the ASD group, reflected by larger N100 amplitudes to subsequent stimuli compared with typically developing individuals. Moreover, N100 amplitude was correlated with sensory hypersensitivity as assessed by a sensory processing questionnaire, and P200 amplitude in response to the preceding stimulus was correlated with Autism Spectrum Quotient (AQ) “attention-switching” scores in the ASD group.
In this study, we focus on attentional load theory. This theory posits that increasing the cognitive load of a task reduces the availability of attentional control resources, thereby increasing susceptibility to task-irrelevant stimuli (Lavie, 2005). From this perspective, higher cognitive load leaves fewer resources for attentional control functions, making it harder to inhibit task-irrelevant stimuli. Sabri et al. (2014) tested this hypothesis by presenting an n-back task (high load/low load) to one ear while delivering task-irrelevant syllables to the other ear. In the n-back task, participants responded whether the current sound matched the sound presented one or two trials earlier, with the number of trials manipulated to control cognitive load. The results revealed increased brain activity to task-irrelevant syllables under high-load conditions, indicating reduced selective attention or inhibition of attention to irrelevant stimuli, consistent with load theory.
There are numerous reports of altered executive functioning in individuals with ASD, suggesting difficulties with working memory tasks under dual-task conditions (Kercood et al., 2014). These difficulties may affect selective attention to auditory stimuli. ERP studies of DST have examined attentional functions such as the detection of deviant stimuli, habituation, and sensory gating; however, studies that impose attentional tasks remain limited (Bigras et al., 2023).
In light of the above, we analyzed the ERP components associated with task-irrelevant stimulus processing during an auditory n-back task. We also examined the relationship between these ERP measures and scores on a questionnaire assessing DST. To the best of our knowledge, no ERP studies have distinguished DST symptoms based on subjective symptom types. Commonly used tools such as the Sensory Profile (Dunn, 1997; Japanese version: Hagiwara et al., 2015) include indices of auditory processing and sensitivity; however, they have limitations in assessing auditory hypersensitivity itself or its subjective symptoms and do not adequately evaluate attention-related characteristics. Therefore, we developed a new questionnaire based on existing questionnaires and used it in this study. We hypothesized that poorer attentional control would be associated with a stronger tendency toward DST, and that a greater increase in ERP amplitude with increasing task load would be associated with greater DST tendency.
Participants were recruited through snowball sampling, the university’s disability support office, and postings on social media. The study targeted individuals aged 15 years and older. Participants were asked to self-report whether they had received a diagnosis of ASD; no diagnostic certificates or related documentation were required. Thirteen participants comprised the ASD group (mean age: 25.2 ± 8.3 years; 8 women, 5 men) and twenty-two participants comprised the control group (mean age: 25.4 ± 7.9 years; 15 women, 7 men). Both groups included individuals with comorbid developmental or psychiatric disorders, such as attention-deficit hyperactivity disorder (ADHD), depression, and anxiety disorders. Eight participants in the ASD group (61.5%) and five in the control group (22.7%) reported at least one of these conditions. The experiment was initiated on November 13, 2025. Written informed consent to participate in the study and consent to publish were obtained from all participants. For participants who were minors, written informed consent was obtained from their parents or legal guardians.
To identify participants with DST, we asked the following question: “If you have any sensory hypersensitivities, please select all that apply. If none apply, please choose ‘None’.” Response options were visual, auditory, tactile, olfactory, and gustatory hypersensitivity, as well as none. Participants were then divided into a self-identified DST (auditory hypersensitivity) group and a non-DST group. Individuals with hearing impairment were excluded from the study.
We developed a new questionnaire to assess DST on the basis of several existing questionnaires to cover a wide range of symptoms. Rather than creating new items, we selected items from existing questionnaires, as these were considered to have established content validity. For this purpose, we first identified questionnaire studies that assessed self-reported symptoms of DST. Studies were included if they met the following eligibility criteria: (1) the questionnaire was designed for adults, (2) it assessed the subjective aspects of DST, (3) the questionnaire items were available in the main text or appendix, and (4) it was published in English or Japanese. As a result of the literature search, we identified seven questionnaires, described below.
Sound-Level Tolerance Questionnaire (SLTQ)
The SLTQ (Gu et al., 2010) is a three-item questionnaire that assesses perceived loudness of everyday and low-intensity sounds. In the original version, respondents rate the extent to which they agree with each statement on a scale from 0 to 100.
Sound Sensitivity Symptoms Questionnaire (SSSQ)
The SSSQ is a five-item questionnaire that assesses the number of days on which loudness, pain, annoyance, and fear occurred as symptoms of hyperacusis (Aazh et al., 2022). As part of its psychometric validation, the authors reported a moderate correlation with uncomfortable loudness levels.
Japanese version of the Sensory Gating Inventory (SGI)
The SGI is a 36-item questionnaire assessing sensory gating functions (Nobuyoshi et al., 2018). It consists of four factors—Perceptual Modulation, Distractibility, Over-Inclusion, and Fatigue and Stress Vulnerability—and has been standardized.
Multidimensional Inventory of Sound Tolerance in Adults (MIST-A)
The MIST-A has four subscales—Misophonia, Hyperacusis, Fear/Overwhelm, and Anxiety/Avoidance (phonophobia) (Williams et al., 2023). Its reliability and validity have been examined in both autistic adults and the general adult population.
Khalfa Hyperacusis Questionnaire (KHQ)
The KHQ was developed to assess marked intolerance to ordinary environmental sounds (Khalfa et al., 2002). It is a 14-item questionnaire comprising three dimensions: attentional, social, and emotional. Its reliability and validity have been established in adult samples from the general population.
Severity Measure for Specific Phobia
This questionnaire comprises 10 items and was developed to assess specific phobia in adults (American Psychiatric Association, 2013b). It is a self-report measure of symptoms experienced over the past seven days, and targets various types of phobias rather than being limited to auditory symptoms. It is based on an operational definition.
Duke-Vanderbilt Misophonia Screening Questionnaire (DVMSQ)
The DVMSQ (Williams et al., 2022) is a questionnaire designed to measure misophonia based on the Revised Amsterdam Criteria (Jager et al., 2020). It consists of 21 multiple-choice items and 2 open-ended items. Its reliability and validity have been evaluated in adults with and without ASD.
Japanese versions of the Hyperacusis Questionnaire (Yamada et al., 2013) and the SGI (Nobuyoshi et al., 2018) were available and were therefore adopted in the current study with a Japanese population. The remaining instruments were translated into Japanese. Participants rated all items on a 4-point scale: “Strongly disagree,” “Somewhat disagree,” “Somewhat agree,” and “Strongly agree.” No examination of the reliability or validity of the subscale structure, nor any pilot testing, was conducted.
The new DST questionnaire contained subscales for excessive loudness, pain, fear, and annoyance, on the basis of a previously proposed theoretical framework (Tyler et al., 2014), as well as attentional control and attention to detail to examine the subjective attentional responses to sounds. The questionnaire contents and citation sources are shown in Table 1.
We adapted the tasks used during electroencephalography (EEG) measurements from a previous study (Sabri et al., 2014), which was similar to the present study. Sound stimuli were presented at approximately 45 dB via speakers. Each trial lasted 2000 ms, and one of three different frequencies of pure tones (1000, 1790, or 3375 Hz) appeared for 100 ms at the beginning of each trial. In some trials, 100 ms of task-irrelevant white noise appeared 700 ms after the start of the trial. The flow of each trial is shown in Figure 1.
Each block consisted of 17 trials, including 10 trials with white noise. Participants took a 12-s break after each block, and each run contained four blocks. Participants completed two runs each of the 1- and 2-back tasks, for a total of four runs. During the n-back task (1-/2-back), participants responded by pressing a button to indicate whether the pitch of the newly presented pure tone matched that of the immediately preceding tone (1-back) or the tone presented two trials earlier (2-back).
In each trial, the onset of the pure tone was randomly set within the first 100 ms. In 10 trials per block, a white noise burst was presented 700 ms after trial onset, while in the remaining 7 trials, no noise was presented. Participants were given 1200 ms to indicate whether the tone was the same as the one presented one trial earlier (1-back) or two trials earlier (2-back).
During the n-back task, EEG was continuously recorded at 2-ms intervals from 30 sites on the scalp using a BrainAmp system (Brain Products, GmbH, Münich, Germany). EEG data were recorded using the actiCAP system from the following electrode sites: Fp1, Fp2, F7, F3, Fz, F4, F8, FT7, FC3, FCz, FC4, FT8, T7, C3, Cz, C4, T8, TP7, CP3, CPz, CP4, TP8, P7, P3, Pz, P4, P8, O1, Oz, and O2, based on the extended 10–20 international system. Eye movements were recorded from the left eye, and the left earlobe was used as the reference. BrainVision Recorder was used for data acquisition, and BrainVision Analyzer was used for data analysis.
ERPs in response to white noise were analyzed at the Fpz site. We applied a 0.5- to 30-Hz bandpass filter, eye movement correction, epoching from -100 to 700 ms after white-noise presentation, baseline correction, exclusion of epochs with artifacts, and averaging. The N100 component within 100 to 150 ms after stimulus onset was analyzed. The mean amplitude and peak latency were calculated and used for statistical analyses. Differences in task performance and ERP indices between conditions were calculated by subtracting values in the 1-back from those in the 2-back condition. Given the sample size, paired t-tests were used for comparisons between conditions, and Mann–Whitney U tests were use for analyses involving participant factors. Bonferroni correction was applied to adjust the significance level.
This study was conducted with the approval of the University of Tsukuba’s Ethics Review Committee for Human Sciences (Approval No. Tsukuba 2024-43 A, approved on 16 May 2024). Written informed consent to participate in the study and consent to publish were obtained from all participants. For participants who were minors, written informed consent was obtained from their parents or legal guardians.
Prevalence of sensory hypersensitivities
Eight participants (61.5%) in the ASD group and four (18.2%) in the control group self-reported DST. Fisher’s exact test revealed a significant between-group difference in the percentage of self-reported DST (p < .05). Of the twelve participants with DST, ten had ASD, another developmental disorder, or a psychiatric condition. Within this group, there were nine participants with ASD, four with ADHD, five with anxiety disorders, and one with a mood disorder, counting comorbid conditions. Of the 23 participants without DST, seven had ASD, another developmental disorder, or a psychiatric condition, including 4 with ASD, 3 with ADHD, 1 with an anxiety disorder, and 2 with mood disorders, also counting comorbid conditions.
Ten of the twelve participants with DST also self-reported other sensory hypersensitivities, and all five participants reporting gustatory sensitivity were in the ASD group. Among the 23 participants without DST, one reported visual hypersensitivity and one reported gustatory hypersensitivity.
Correlations among the subscales are as follows. To control for Type I error inflation due to the 15 combinations, the significance level was adjusted using the Bonferroni correction (adjusted α = 0.05/15 = 0.003). Attentional control was significantly correlated with attention to detail (r = .63, p < .001, power[1-β] = .99), annoyance (r = .62, power = .99), and loudness (r = .57, power = .96) (ps < .001). Attention to detail was significantly correlated with annoyance (r = .64, power = .99) and loudness (r = .68, power = .997). Fear was significantly correlated with loudness (r = .55, power = .94) (ps < .001).
Group comparison
Scores are presented as z-scores. To examine differences in subscale scores between participants with and without DST ( Figure 2), and between those with and without ASD ( Figure 3), Mann–Whitney U tests were conducted. Bonferroni correction was applied to adjust the significance level. No significant differences were observed for any of the subscales.
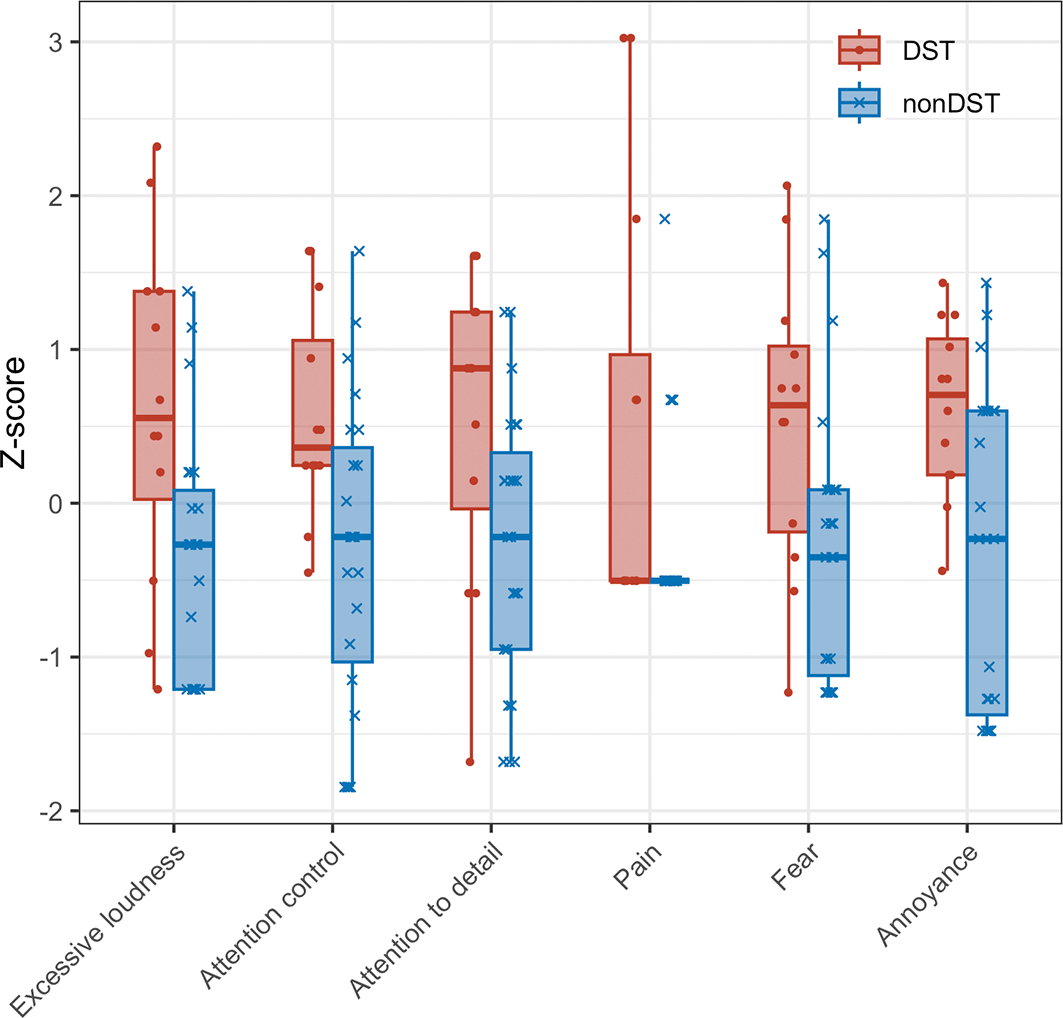
The x-axis represents the subscales of the DST questionnaire. The y-axis represents z-scores of the DST questionnaire scores. Red boxes indicate the DST self-identified group, and blue boxes indicate the non–DST self-identified group. Whiskers represent the range from the minimum to the maximum values, boxes represent the interquartile range (from the first to the third quartile), and the line inside each box represents the median.
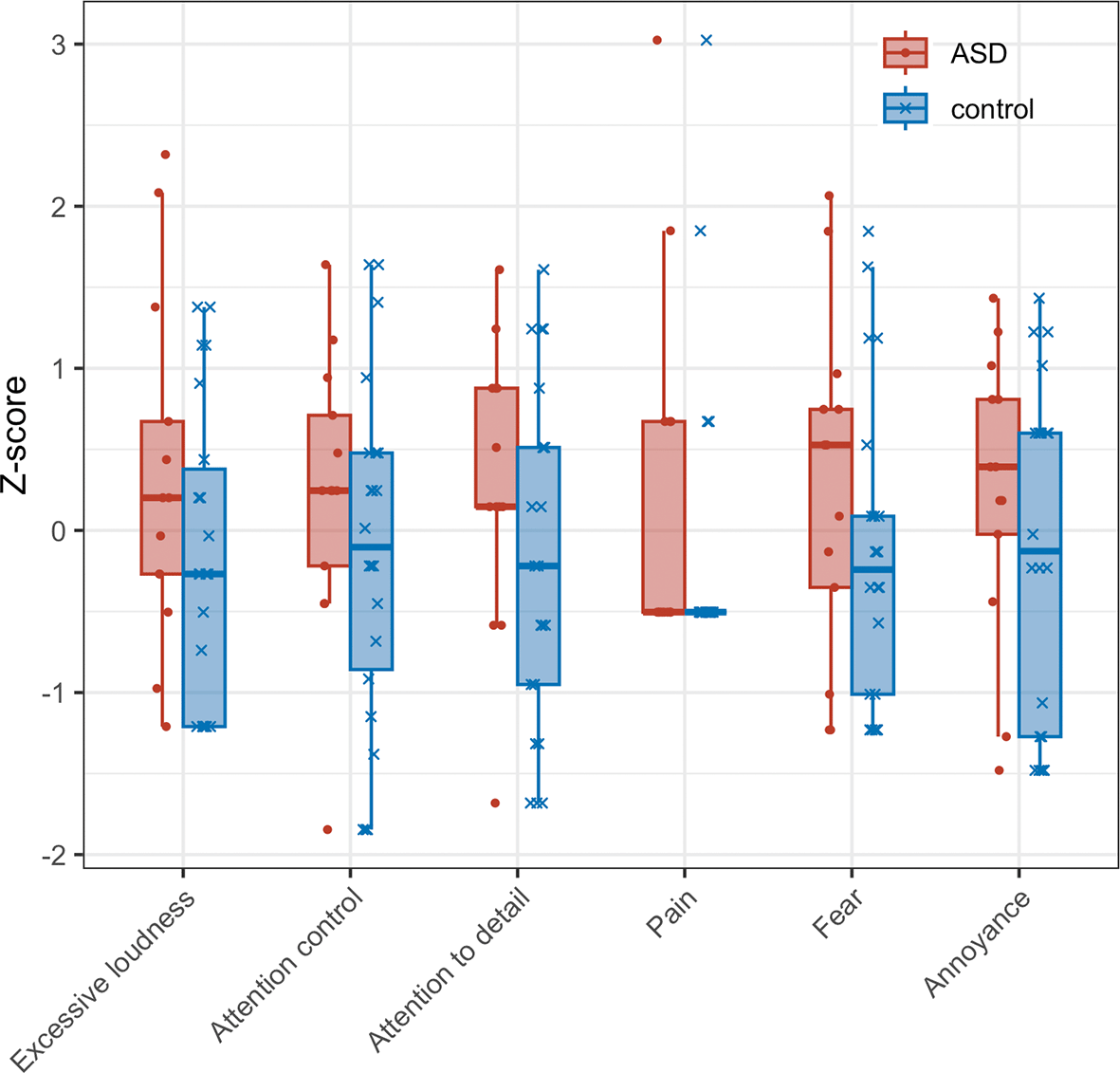
The x-axis represents the subscales of the DST questionnaire. The y-axis represents z-scores of the DST questionnaire scores. Red boxes indicate participants with an ASD diagnosis, and blue boxes indicate participants without an ASD diagnosis. Whiskers represent the range from the minimum to the maximum values, boxes represent the interquartile range (from the first to the third quartile), and the line inside each box represents the median.
The mean percentage of correct responses was 94.3% (±standard deviation [SD] = 7.2) in the 1-back task and 78.6% (SD = 10.1) in the 2-back task. The mean reaction time was 641.5 ms (SD = 113.9) in the 1-back task and 888.5 ms (SD = 168.3) in the 2-back task. A paired t-test across all participants revealed a significant difference in accuracies between the 1-back and 2-back conditions (t34 = 13.23, p < .01, Cohen’s d = 2.24, 95% confidence interval [CI] = [1.61, 2.86]), with significantly higher accuracy in the 1-back task ( Figure 5).
A paired t-test of reaction times across task difficulty also revealed a significant difference between the 1-back and 2-back conditions (t34 = -11.77, p < .01, d = -1.99, 95% CI = [-2.56, -1.41]), with significantly shorter reaction times in the 1-back task ( Figure 4).
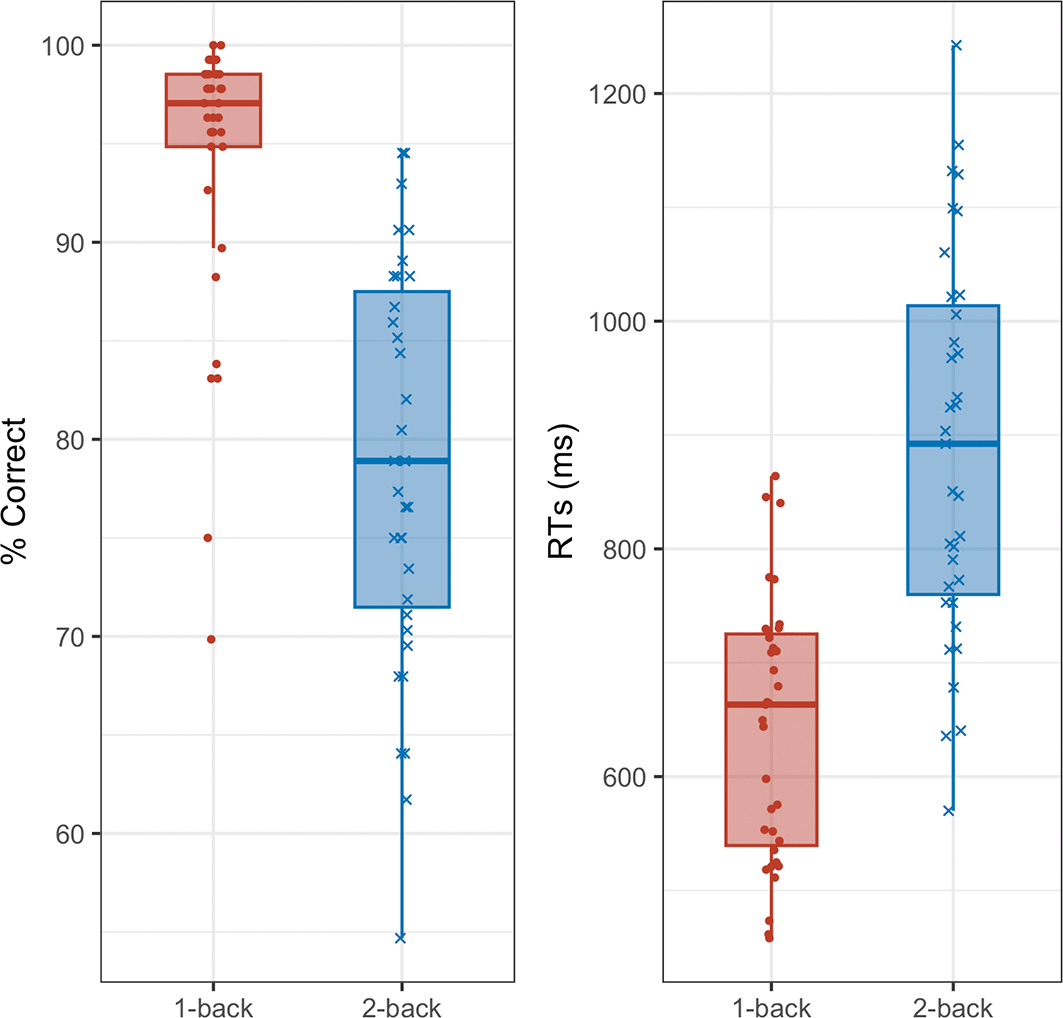
The left panel shows accuracy, and the right panel shows reaction time (ms). Red boxes indicate the 1-back condition, and blue boxes indicate the 2-back condition. Whiskers represent the range from the minimum to the maximum values, boxes represent the interquartile range (from the first to the third quartile), and the line inside each box represents the median.
Reaction times for all participants are plotted against the percentage of correct responses for each task condition. The shaded areas represent 95% CIs.
Significant correlations were observed between accuracy and reaction time (RT) in both the 1-back (r = -.58, power[1-β] = .97) and 2-back tasks (r = -.55, power = .94) when analyzed across the full dataset (ps < .01) ( Figure 5). In addition, significant correlations were found between the 1-back and 2-back tasks for both accuracy and RT (rs = [.71, .68], ps < .01, powers > .99, respectively).
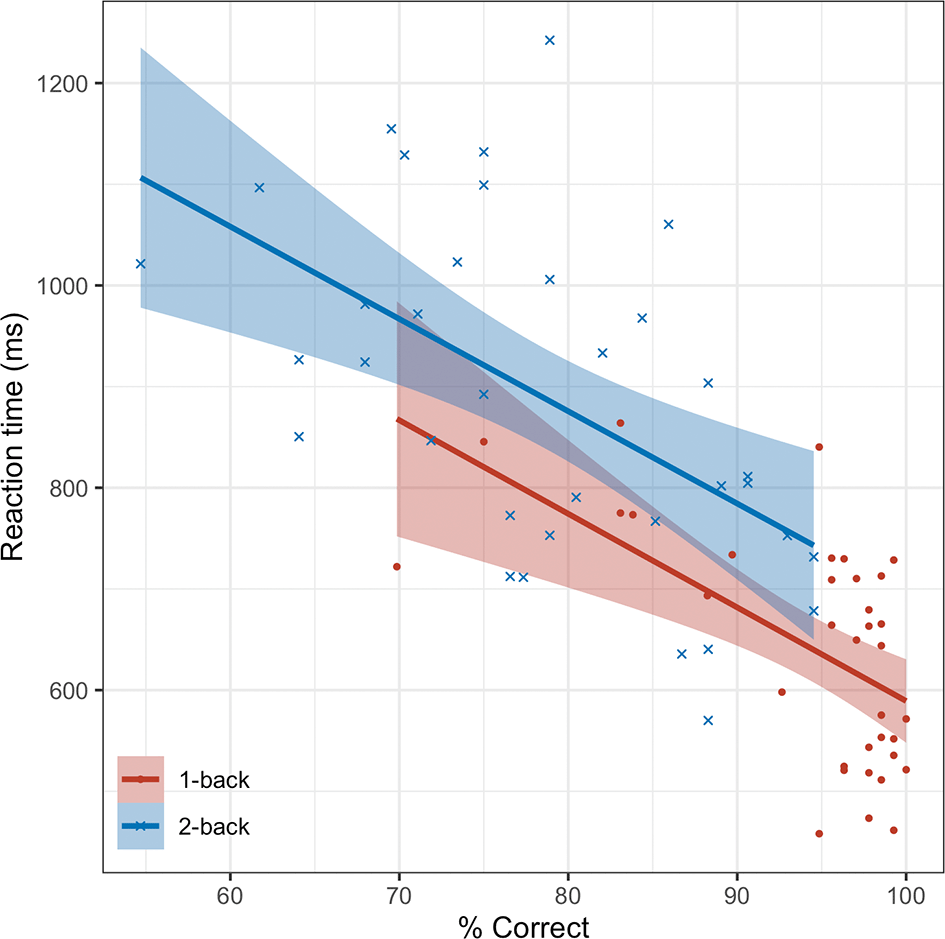
The horizontal axis represents accuracy, and the vertical axis represents reaction time. The regression line shows the relationship between these variables. The shaded area indicates the 95% confidence interval of the regression line.
Group comparison
Tables 2 and 3 present accuracy rates and RTs by ASD status and DST status. Independent t-tests and Mann–Whitney U tests revealed no significant differences in accuracy rates or RTs based on the presence or absence of ASD or DST. Moreover, the results of the t-tests and Mann–Whitney U tests indicated that the differences in task-load contrasts for accuracy rates and reaction times did not vary according to ASD or DST status.
| 1-back | 2-back | |||
|---|---|---|---|---|
| Accuracy (%) | RT (ms) | Accuracy (%) | RT (ms) | |
| ASD | 91.01 (9.97) | 699.79 (129.02) | 75.30 (10.56) | 944.90 (184.76) |
| Control | 96.32 (3.98) | 607.07 (90.30) | 80.54 (9.45) | 855.13 (152.39) |
| 1-back | 2-back | |||
|---|---|---|---|---|
| Accuracy (%) | RT (ms) | Accuracy (%) | RT (ms) | |
| DST | 93.69 (7.42) | 664.72 (129.61) | 75.39 (10.60) | 936.63 (207.13) |
| non-DST | 94.69 (7.21) | 629.40 (105.87) | 80.27 (9.57) | 863.35 (142.85) |
There was no significant correlation between each subscale of the DST score and accuracy, RT, or effects of task load on these indices.
Basic analysis of ERP
Figure 6 shows the grand-average waveforms at FCz across all participants, and Figure 7 shows the scalp distribution of the mean amplitude values in the 100- to 150-ms interval after stimulus onset. Negative waves, corresponding to N100, were observed around FCz in both the 1-back and 2-back tasks.
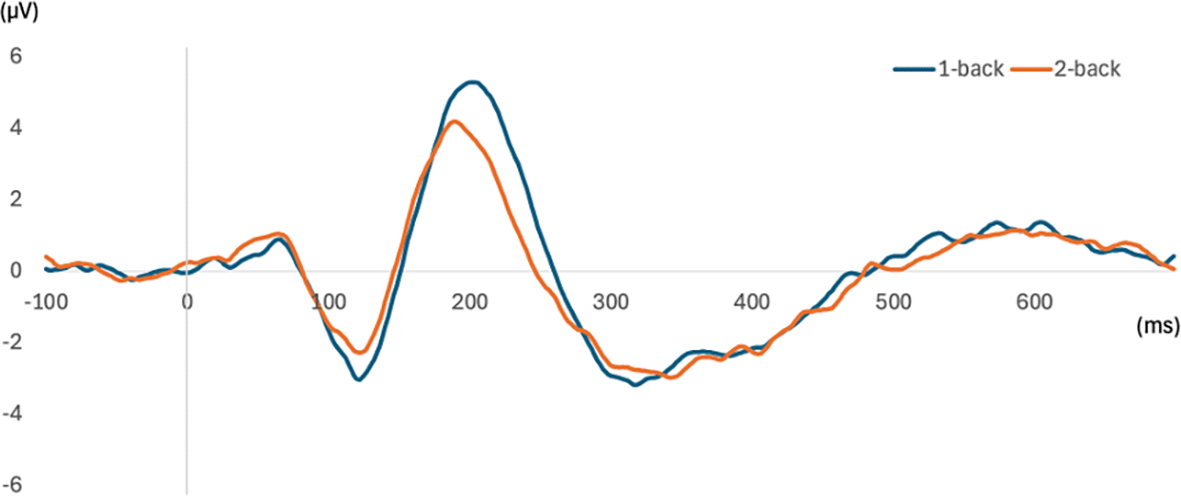
ERP waveforms at FCz are shown. The x-axis represents time (ms) relative to the onset of the white noise stimulus (0 ms), and the y-axis represents amplitude. The blue line represents the waveform for the 1-back task, and the orange line represents the waveform for the 2-back task.
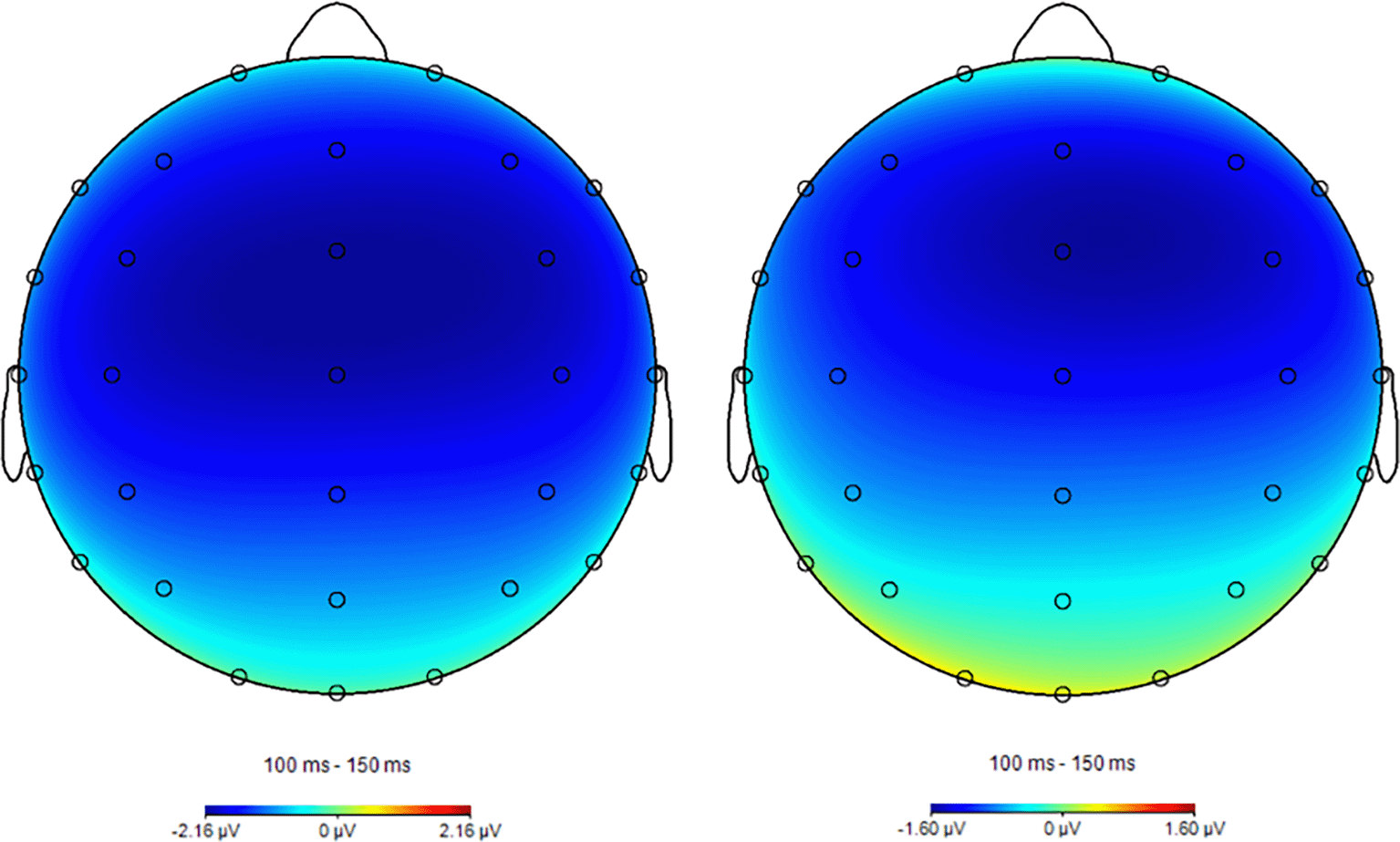
Left: Topographic map for 1-back task. Right: 2-back task. Color bars represent EEG amplitude (μV).
A t-test of N100 mean amplitudes across load conditions revealed no significant differences (1-back: mean = -2.08, ±SD = 2.19; 2-back: mean = -1.48, SD = 1.59, t34 = -1.62, p = .12, d = -.27, CI = [-.61, .07]). Similarly, a t-test between task load conditions for N100 peak latency showed no significant differences (1-back: mean = 122.29, SD = 13.54; 2-back: mean = 121.60, SD = 14.45, t34 = .23, p = .82, d = .04, CI = [-.29, .37]).
Figure 8 shows the distribution of N100 amplitudes and peak latencies for participants with and without ASD, and DST. Figure 9 shows the differences between the 2-back and 1-back task conditions for accuracy and RT, respectively, plotted according to the presence or absence of DST and ASD. Independent t-tests were conducted for the measures shown in Figures 8 and 9 to examine group differences; however, no significant differences were observed for any comparison. DST scores did not significantly correlate with ERPs, and behavioral performance did not significantly correlate with ERPs.
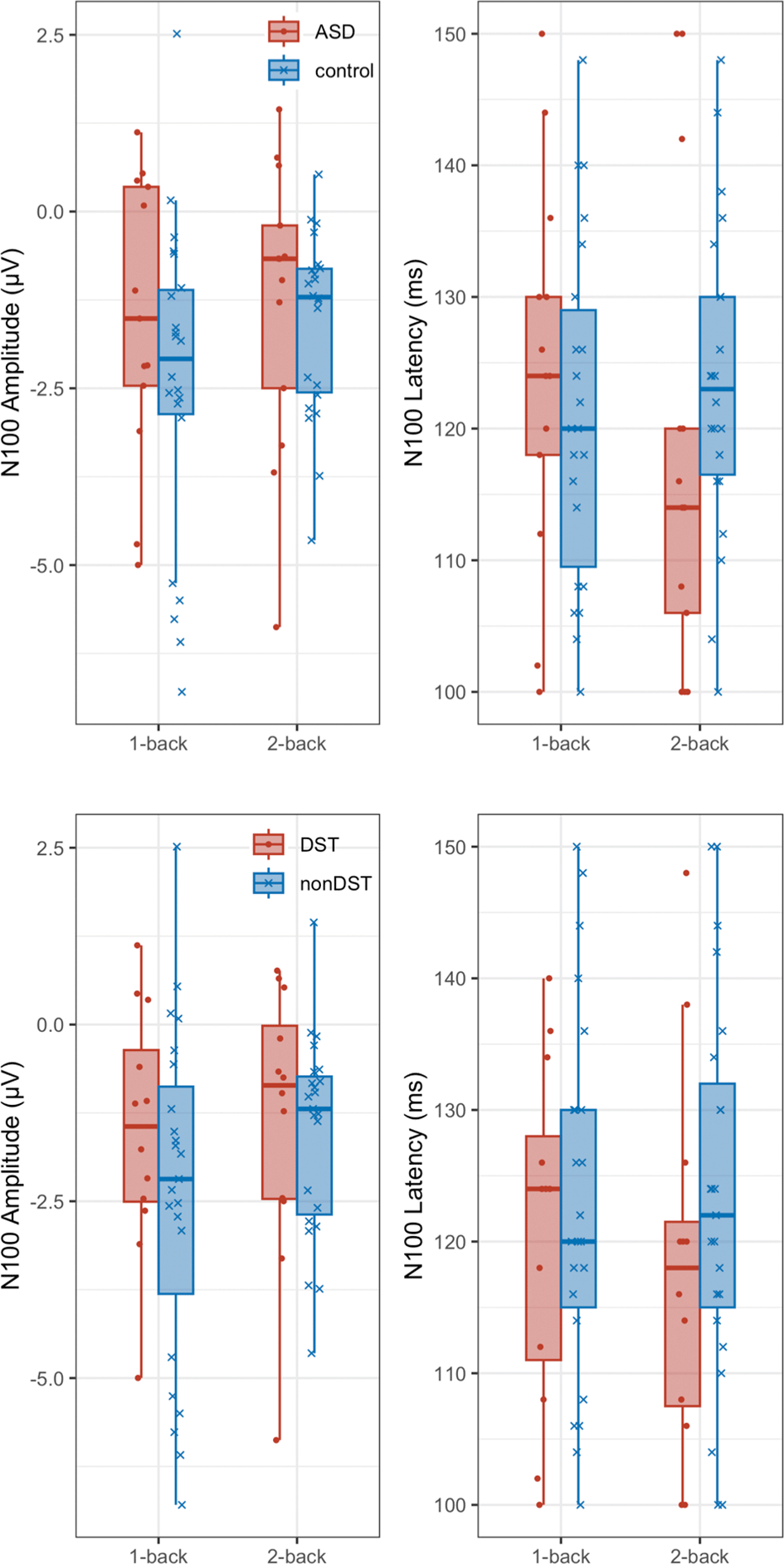
In the upper panels, red indicates the ASD group and blue indicates the non-ASD group. In the lower panels, red indicates the DST group and blue indicates the non-DST group.
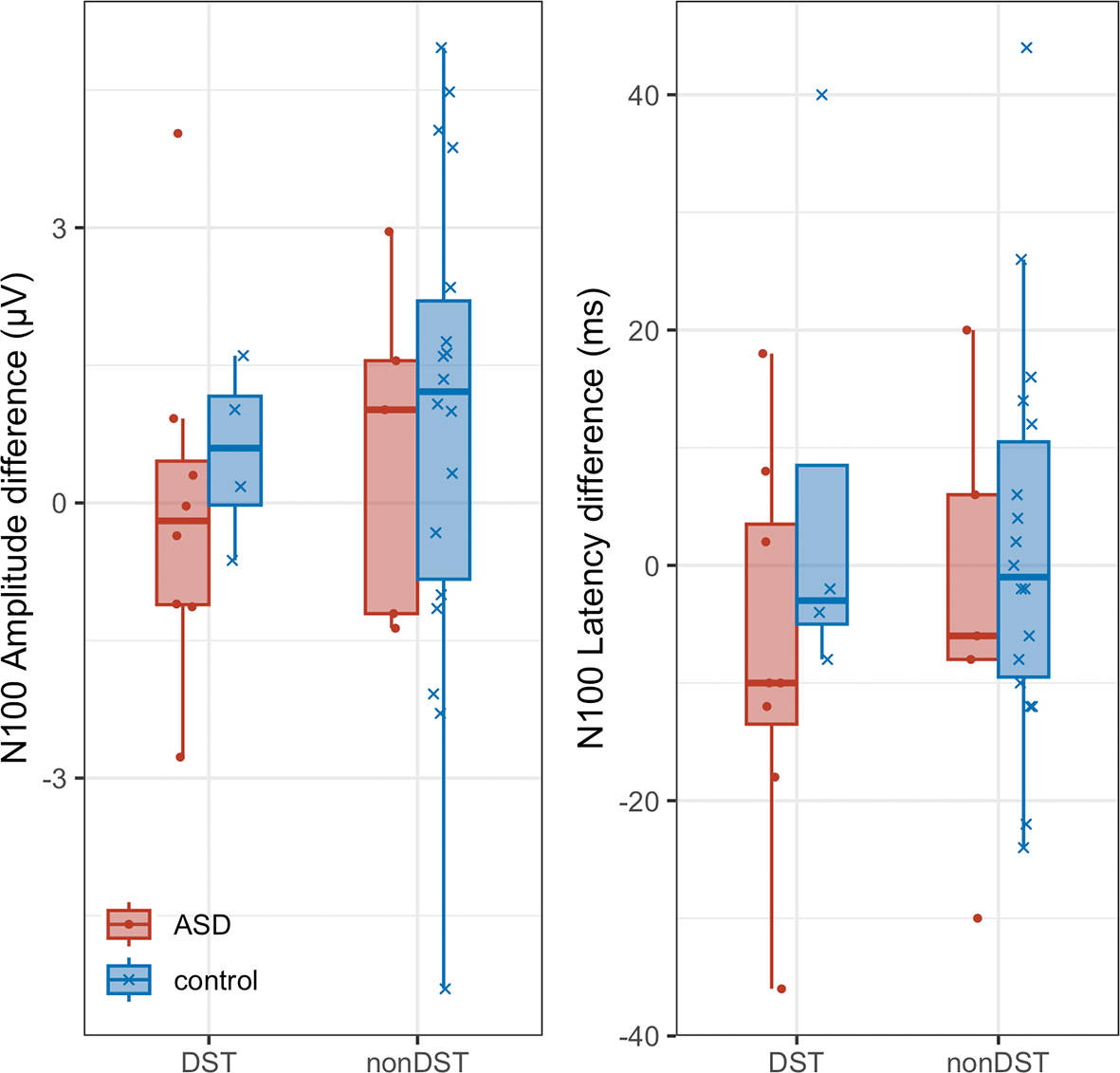
Differences were calculated by subtracting the 1-back condition from the 2-back condition. Box-and-whisker plots for amplitude and latency are shown for the four groups based on DST and ASD status.
Exploratory analysis
The positive component observed 150 to 250 ms after stimulus onset was defined as the P200, and analyses were conducted on its mean amplitude and peak latency. P200 amplitude was significantly larger in the 1-back condition (M = 3.39 μV, ±SD = 2.50) than in the 2-back condition (M = 2.43 μV, SD = 2.14, t34 = 2.80, p < .005). No significant differences in P200 latency were observed between load conditions.
Further t-tests revealed no significant differences in P200 amplitude or latency between load conditions based on the presence or absence of ASD or DST. For P200 amplitude and latency, we conducted t-tests on the differences between conditions for each participant attribute group (ASD vs. the control, DST vs. non-DST). There were no significant differences between groups.
This study sought to characterize the subjective symptoms of DST using a questionnaire that allows symptom-specific assessment. We examined the hypothesis that the task load during the cognitive task would exert a stronger influence on noise processing in individuals with DST. To this end, we performed an auditory ERP experiment using an n-back task. The results showed that the prevalence of DST was significantly higher in the ASD group than in the control group. Attentional processes appeared to influence fear, annoyance, and perceived loudness of auditory stimuli. However, difficulties in ignoring sounds in daily life did not correspond to performance on experimental tasks. ERP components reflecting attention did not differ according to the presence or absence of DST. Although self-report questionnaires suggested an association between attentional difficulties and auditory hypersensitivity, this relationship was not supported by the objective data, as neither behavioral measures nor ERP results demonstrated such an association.
There was a higher prevalence of DST in the ASD than in the control group, consistent with previous reports (Danesh et al., 2021). Developmental and psychiatric conditions were more frequent in the DST group (10/12) than in the non-DST group (7/23), also in line with earlier findings. However, in this study, both groups included a higher proportion of developmental and psychiatric conditions than would be expected in the general population. Most participants who self-reported DST also experienced other sensory sensitivities, whereas only two of the 23 participants without DST reported other sensory sensitivities. Another study also reported comorbidity with other sensory hypersensitivities (Ke et al., 2020). These findings suggest that the mechanisms underlying DST may not be limited to auditory processing but may also involve cross-modal components.
Previous studies have reported that conditions other than ASD that are likely to be comorbid with DST include hearing impairment, tinnitus, migraine, post-traumatic stress disorder (PTSD), depression, attention-deficit/hyperactivity disorder (ADHD), anxiety symptoms, and sleep disorders (Danesh et al., 2021; Kumagaya et al., 2013). Therefore, DST may be broadly classified into forms primarily related to auditory system dysfunction (Nishiyama et al., 2019) and forms associated with psychiatric symptoms (Jüris et al., 2013). The fact that attention is impaired as a common symptom of many psychiatric disorders (Trivedi, 2006) suggests that there may be some relationship between the latter type of DST and attention.
Loudness perception is strongly associated with auditory cortex activity, and auditory cortex function is known to be modulated by attention (Kaya & Elhilali, 2017). In addition, misophonia and phonophobia involve emotional reactions, and emotion and attention are known to be closely linked and mutually reinforcing (Dolcos et al., 2011). Thus, the involvement of attention is expected to differ depending on the specific subjective symptom of DST. Although possible mechanisms underlying distinct DST symptom subgroups have been suggested (Williams et al., 2021), future studies should address how cross-modal sensory hypersensitivity develops and what mechanisms mediate these effects across modalities. As previously proposed, the interaction between emotion and attention in sensory processing may provide important insight into these mechanisms (Khalfa et al., 2002).
To date, questionnaire-based assessments that comprehensively capture the subjective symptoms of DST are extremely limited; thus, the present study is distinctive in its focus on capturing subjective symptoms. The results showed that there were no correlations between ear pain and any of the other subscales, suggesting that ear pain may involve mechanisms distinct from those underlying other DST symptoms. Fear correlated only with loudness, which may indicate that fear causes sounds to be perceived as louder, or conversely, that louder sounds elicit fear. Attention control and attention to detail correlated with all subscales except pain and fear, raising the possibility that attentional factors may underlie loudness and annoyance. These aspects have not been sufficiently examined in previous studies, and the findings of the present study therefore provide novel contributions.
Even though the items were drawn from existing questionnaires, the results of the DST questionnaire should be interpreted with caution. No significant differences were observed between the self-reported DST-present and -absent groups on any of the subscale scores. One possible explanation is that it may be due to the combination of subjective symptoms. Another possible source of this discrepancy was the participants’ individual differences in self-identification of DST. In this study, participants were classified as having or not having DST based on self-report. Future studies should investigate the factors that contribute to self-perception and reporting of DST.
In our analysis of behavioral measures, there was a significant difference between the 1-back and 2-back tasks in both accuracy and RT, confirming the validity of the cognitive load manipulation (Lamichhane et al., 2020). Both tasks showed a significant negative correlation between accuracy and RT, suggesting that individual differences in response style, such as a tendency to respond carefully versus quickly, might influence performance. Neither the presence of ASD nor DST affected accuracy or RT, and these participant factors also did not influence changes in behavioral performance associated with the task load. This finding appears somewhat inconsistent with previous evidence. A meta-analysis reported that individuals with ASD show lower performance in both phonological and visuospatial working memory (Habib et al., 2019). Similarly, Barendse et al. (2018) conducted a visual n-back task and found no group differences under the 1-back condition, whereas the ASD group showed a higher error rate than controls under the more demanding 2-back condition. One possible interpretation is that the task load in the present study may not have been sufficiently demanding to reveal group differences.
There was no correlation between DST scores and task performance (either mean performance or differences across difficulty levels). Of particular interest is the lack of association between performance on the n-back task (a working memory measure) and “attentional control” scores on the DST questionnaire, which reflect perceived difficulty focusing on tasks in daily life due to environmental sounds. Assessing participants’ perceived interference from task-irrelevant stimuli in the n-back task may help clarify whether this discrepancy arises from a mismatch between actual performance and subjective evaluation, or from differences between experimental settings and real-life situations.
Sabri et al. (2014) reported greater N100 amplitudes in response to task-irrelevant stimuli under high cognitive-control load conditions than under low-load conditions. In contrast, in the present study, although the N100 component was observed, the amplitudes did not differ between task difficulty levels. Sabri et al. used headphones to present the n-back task to the attentive ear and task-irrelevant syllables to the contralateral ear, whereas we presented sound stimuli without positional information via speakers. Therefore, in our study, the attentive ear was not spatially divided by the task-irrelevant stimulus. The noise burst appeared in a limited number of trials (10/17 per block), making habituation effects unlikely. It is possible that the processing of irrelevant stimuli varies depending on whether the stimuli to which attention should be directed and those to which it should be ignored were spatially coincident or distant.
The N100 component is thought to reflect activity in the primary and secondary auditory cortex (Modi & Sahin, 2017), and attention to sound stimuli modulates neural activity in the auditory cortex (Kaya & Elhilali, 2017). Accordingly, it has been suggested that the N100 component originating in the auditory cortex is affected by attention (Remijn et al., 2014). However, we found no increase in auditory cortex activity in response to the failure of attentional inhibition. Given that the literature examining the effects of cognitive load on N100 amplitudes for task-irrelevant stimuli is limited, further studies are warranted.
We also examined the effects of task load on N100 amplitude and latency in each group but found no significant effects. This indicates that auditory cortical responses to task-irrelevant stimuli were not greater in the group with self-reported DST. Previous studies have reported that individuals with ASD show a reduction in N100 amplitude and a prolongation of latency (Takahashi & Kamio, 2018). Although these findings reflect individual characteristics of the N100 component, ASD-specific tendencies in the effects of cognitive load might also account for the present results.
There was no correlation between DST scores and N100 amplitude or the difference in N100 between task difficulty levels. A previous study reported that individuals with misophonia show smaller mean N100 amplitudes to deviant stimuli in an oddball task (Savard & Coffey, 2025). Although the pure tones and white noise in our study share features with an oddball paradigm, we did not observe this relationship when comparing participants with and without self-reported DST. There was no effect of cognitive load as estimated by the N100 difference. We found no relationship between DST or attentional control ability and N100 metrics in response to task-irrelevant stimuli.
Observation of the ERP waveforms revealed differences in P200 amplitude between task conditions, with larger amplitudes in the 1-back than in the 2-back task. While the N100 component is often used to evaluate selective attention, P200 amplitude is known to vary depending on acoustic features such as intensity, frequency, and prosody (Remijn et al., 2014). The difference in P200 amplitude may therefore reflect a shift of participants’ attention toward the acoustic differences between the pure tone and noise stimuli under low load. There were no differences in amplitude across participant factors, and neither DST nor ASD prevalence appeared to modulate ERP components reflecting enhanced attention to noise. These results suggest that the influence of DST or ASD was not evident in either selective attention or acoustic processing.
The absence of associations between DST tendencies and ERP indices of task-irrelevant noise processing suggests that attentional influences on DST may not be adequately captured by laboratory-based cognitive load manipulations alone. Although load theory predicts reduced inhibitory control over irrelevant stimuli under high attentional load (Lavie, 2005), such effects may depend critically on the ecological validity of the auditory context. In daily life, auditory hypersensitivity is often triggered by unpredictable, emotionally salient, or personally meaningful sounds. Consequently, the mismatch between subjective reports of attentional difficulty in everyday environments and the lack of corresponding neural effects in the present experiment may reflect differences between real-world sensory experiences and controlled experimental paradigms.
Studies based on the load theory of attention have examined noise processing using ERPs; however, they have not investigated its relation to DST. The present study is a unique attempt to examine DST in terms of neural responses to noise and to analyze DST by subdividing its components. Another novel aspect is the employment of a questionnaire that comprehensively assesses subjective symptoms and examines the interrelationships among the subscales of excessive loudness, pain, annoyance, fear, attention control, and attention to detail. Examining the links between subjective symptoms of atypical auditory responses and their neurocognitive basis is therefore crucial.
Nevertheless, several limitations should be noted. First, the sample size was small. Only 13 participants had a diagnosis of ASD, and only 12 participants across both groups self-reported as having DST. It is also necessary to take into account that a considerable number of participants in both groups had comorbid psychiatric disorders. Future studies should recruit larger samples and conduct group comparisons while adequately controlling for participant characteristics such as age, sex, and comorbidities.
Finally, the questionnaire used in this study combined items from existing instruments. While this approach allowed assessment of symptoms across different domains, the appropriateness of the selected items has not yet been examined. There is a need to develop a questionnaire that can measure a broad range of subjective symptoms while ensuring reliability and validity.
The datasets generated and/or analyzed during the current study are available in the Zenodo repository and are published under the Creative Commons Attribution 4.0 International license (CC-BY). https://doi.org/10.5281/zenodo.17970219 (Ito et al., 2025)
This project includes the following basic datasets.
group.csv
questionnaire_en.csv
behavior.csv
ERP_1back.csv
ERP_2back.csv
Use of Artificial Intelligence Tools
ChatGPT (GPT-5, OpenAI) was used as a tool for language assistance, including support with English translation and phrasing.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)