Keywords
Pediatric ERAS, Magnesium Sulfate, Neuroanesthesia, Brain relaxation, brain neuromonitoring
This article is included in the Oncology gateway.
Pediatric glioma surgery poses significant anesthetic challenges due to raised intracranial pressure (ICP), neurodevelopmental vulnerability, and the need to achieve optimal brain relaxation without delaying postoperative recovery. Intravenous magnesium sulfate (MgSO4), through NMDA receptor antagonism and analgesic- and hemodynamic-sparing effects, may support propofol-based total intravenous anesthesia (TIVA) within pediatric ERAS pathways.
A 10-year-old girl with a left temporoparietal glioma presented with seizures, progressive right hemiparesis, and papilledema. She underwent craniotomy under propofol-based TIVA augmented with MgSO4 (loading 30 mg/kg; maintenance 10–15 mg/kg/h). Individualized cerebral physiology was guided using real-time optic nerve sheath diameter (ONSD) and transcranial Doppler (TCD) measurements. After induction and MgSO4 infusion, ONSD values and middle cerebral artery pulsatility indices decreased toward more favorable ranges, consistent with improved intracranial compliance. Intraoperatively, brain relaxation was excellent, hemodynamics were stable, and only minimal rescue opioids and neuromuscular blockers were required. The patient was extubated within 24 hours, demonstrated low postoperative pain scores without agitation or PONV, and had no magnesium-related toxicity. A transient episode of diabetes insipidus occurred postoperatively, attributed to tumor-related hypothalamic–pituitary involvement, and resolved with vasopressin therapy.
This case highlights the feasibility of MgSO4 as an adjunct to propofol-based TIVA in pediatric neuro-oncologic surgery. Real-time ONSD and TCD monitoring provided a practical, non-invasive method to tailor ICP and cerebral perfusion management.
MgSO4 may be a safe and effective adjuvant during pediatric glioma resection when combined with vigilant monitoring and ultrasound-guided cerebral physiology assessment. Prospective studies are needed to validate standardized dosing and ultrasound-guided ICP management protocols.
Pediatric ERAS, Magnesium Sulfate, Neuroanesthesia, Brain relaxation, brain neuromonitoring
Pediatric gliomas are rare, yet they represent some of the most challenging cases in neuro-oncology, accounting for less than 5% of childhood brain tumors. These tumors often present with nonspecific symptoms such as seizures, progressive hemiparesis, and signs of raised intracranial pressure (ICP), which can rapidly lead to neurological deterioration.1 Anesthetic management in these cases demands meticulous attention to the unique anatomical and physiological characteristics of the pediatric brain, including heightened sensitivity to anesthetic agents, greater risk of cerebral ischemia, and neurodevelopmental vulnerability.2–4 Therefore, neuroanesthesia in children must not only ensure hemodynamic stability and adequate analgesia but also aim to minimize secondary neuronal injury and support favorable cognitive outcomes.
Total intravenous anesthesia (TIVA) with propofol has gained significant acceptance in pediatric neurosurgery, mainly due to its neuroprotective properties, ability to suppress cerebral metabolic demand, and reduced effects on intracranial pressure (ICP)2,5,6 Concurrently, magnesium sulfate (MgSO4) is emerging as a valuable adjunct to anesthetics. Its effectiveness stems from its capacity to block N-methyl-D-aspartate (NMDA) receptors, regulate calcium influx, and promote cerebral vasodilation5,7–9 These mechanisms contribute to decreased central sensitization, improved brain relaxation, and a reduced requirement for opioids and neuromuscular blockers. While MgSO4 has been widely researched in obstetric anesthesia, its application in pediatric neuroanesthesia remains relatively unexplored, particularly concerning real-time, noninvasive ICP monitoring.
This case report describes the use of intravenous MgSO4 as an intraoperative adjuvant to propofol-based TIVA in a child undergoing craniotomy for glioma resection. It highlights the benefits of MgSO4 in improving brain relaxation and hemodynamic stability, while reducing opioid use and facilitating early postoperative recovery. Additionally, the case demonstrates the integration of real-time neurosonography specifically optic nerve sheath diameter (ONSD) and transcranial Doppler (TCD) as a noninvasive tool to guide individualized anesthetic management. This approach aligns with Enhanced Recovery After Surgery (ERAS) protocols, offering a multimodal and patient-centered strategy in pediatric neuro-oncologic care.
A 10-year-old Melanesian girl from Papua, Indonesia (weight: 26 kg, height: 132 cm), presented with a one-day history of decreased consciousness. Her symptoms began seven months earlier with intermittent headaches that progressively worsened, followed by weakness in her right arm that developed five months ago and weakness in her right leg that started two weeks ago.
Initially, she received treatment in Papua for her seizures with paracetamol and anti-seizure, although her mother forgot to administer the antiseizure medication. Although advised to seek referral to Java, the family chose to remain in Papua due to observed clinical improvements during initial treatment, as well as concerns about distance and cost. During this period, she was treated with steroids and paracetamol, leading to gradual improvement of her symptoms.
At the age of five, she experienced febrile seizures lasting over five minutes but resolved without recurrence. There were no reports of vomiting, trauma, or constitutional symptoms. The girl was delivered via cesarean section, weighing 3,100 grams, and her Apgar scores were 7 at one minute and 9 at five minutes. She has shown normal growth and developmental progress, and there is no documented family history of malignant diseases.
Initial evaluation revealed a Glasgow Coma Scale score of E4V5M6. The neurological examination revealed right-sided hemiparesis (3/5 strength) and bilateral papilledema. Fundoscopy confirmed signs of increased intracranial pressure (ICP). A series of neuroimaging studies, including a non-contrast head CT and a contrast-enhanced brain MRI, demonstrated a left temporoparietal mass with calcifications, perifocal edema, and a midline shift greater than 5 mm to the right. Preoperative brain MRI demonstrated a left temporoparietal mass with associated edema and a rightward midline shift, as shown in Figure 4. Based on clinical and radiological findings, the differential diagnosis included a supratentorial space-occupying lesion (SOL), with consideration of high-grade glioma, PNET, or pleomorphic xanthoastrocytoma.
Preoperative labs were essentially within normal limits, except for mild anemia (Hb 11.3 g/dL). Serum electrolytes, renal and liver function, and coagulation profiles were normal ( Table 1).
Written informed consent for surgery and publication was obtained from the patient’s mother. The patient was scheduled for elective craniotomy and tumor resection under total intravenous anesthesia (TIVA). Pre-surgery treatment are steroid and mannitol. Intraoperatively, standard monitors were applied. A loading dose of intravenous magnesium sulfate (MgSO4) 30 mg/kg was administered pre-induction, followed by a maintenance infusion at 10–15 mg/kg/h. Anesthesia induction used propofol, fentanyl, and atracurium. Endotracheal intubation was smooth, with no hemodynamic disturbances.
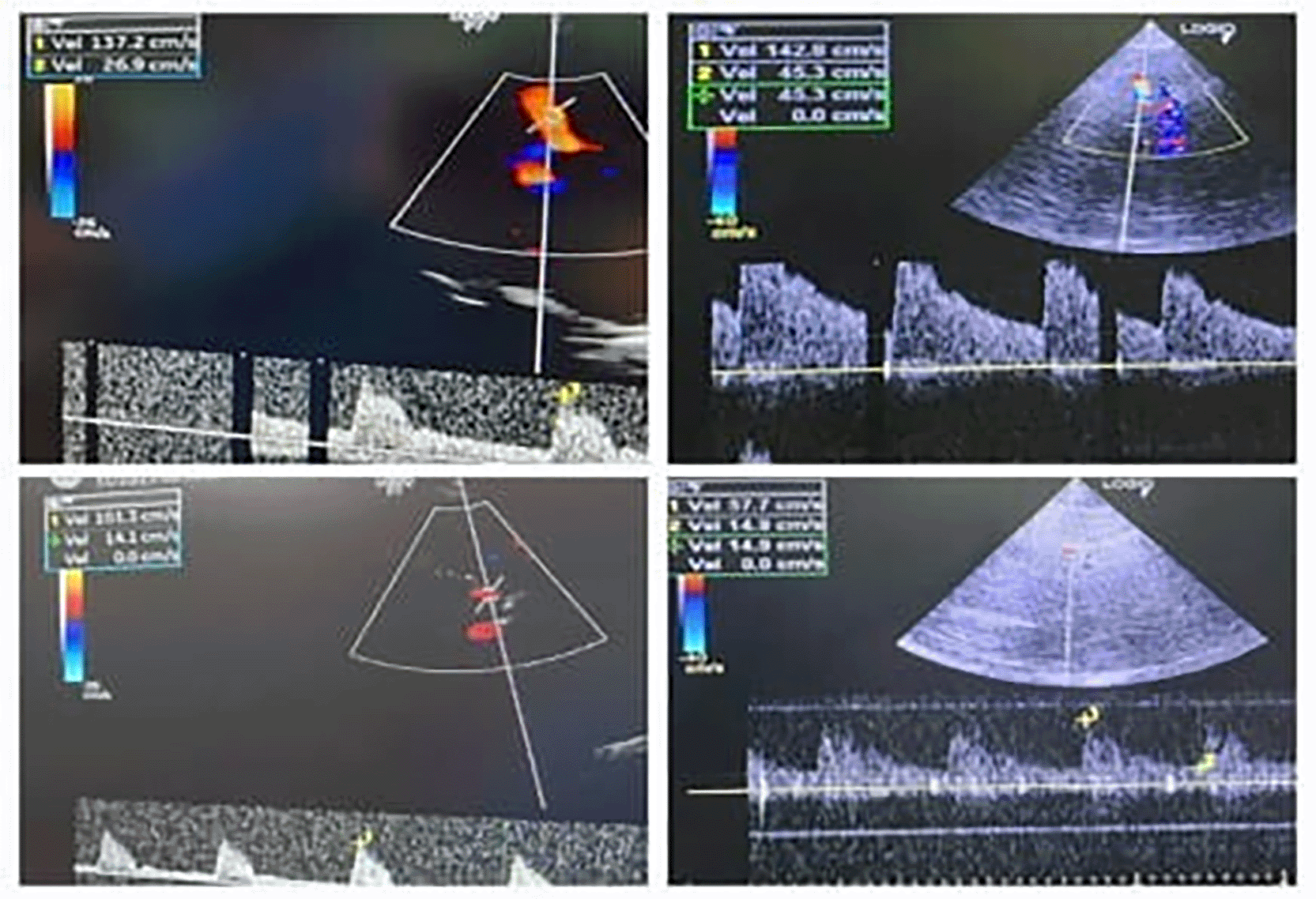
Perioperative ICP was monitored via bedside ultrasound using optic nerve sheath diameter (ONSD) and transcranial Doppler (TCD). Baseline ONSD values were elevated (OD: 0.62 mm, OS: 0.55 mm), and TCD showed high pulsatility index (PI of MCA) values 1.73 on the right (~ ICP 17.63) and 2.02 on the left (~ ICP 20.79). After induction and magnesium infusion, ONSD decreased (OD: 0.42 mm, OS: 0.46 mm), and PI improved (right: 1.2; left: 1.4), reflecting reduced ICP and improved cerebral compliance ( Table 2).
| Measurement | Before surgery | After surgery |
|---|---|---|
| ONSD Right (OD) | 0.62 mm | 0.42 mm |
| ONSD Left (OS) | 0.55 mm | 0.46 mm |
| PI Right MCA | 1.73 | 1.2 |
| PI Left MCA | 2.02 | 1.4 |
In the operating room, standard monitoring was established. Induction was achieved using intravenous propofol, fentanyl, and atracurium. A loading dose of MgSO4 30 mg/kg was administered before induction, followed by maintenance at 10–15 mg/kg/h. The patient was intubated smoothly without hemodynamic fluctuation. Maintenance included propofol (100-200 mcg/kg/min), fentanyl (0.2 mcg/kg/h), and MgSO4 infusion. Atracurium was reserved for breakthrough spontaneous respiration.
Real-time ultrasound monitoring of ICP was performed using optic nerve sheath diameter (ONSD) and transcranial Doppler (TCD). Initial ONSD values were elevated (OD 0.62 mm; OS 0.55 mm), and pulsatility indices from the middle cerebral arteries indicated raised ICP (PI: right 1.73; left 2.02). Post-MgSO4 administration, ONSD decreased (OD 0.42 mm; OS 0.46 mm), and PI improved.
Intraoperatively, brain relaxation was rated as “good” by both the anesthesiologist and neurosurgeon. A jelly-like tumor was successfully resected, with an estimated blood loss of 200 mL. The patient developed polyuria (up to 11 cc/kg/h) with hypernatremia and elevated plasma osmolarity (309 mOsm/kg), suggestive of diabetes insipidus (DI), which was managed with vasopressin infusion and fluid-electrolyte replacement. No signs of magnesium toxicity were observed.
The patient was extubated within 24 hours, alert and oriented, with minimal postoperative pain (VAS=1), managed with paracetamol alone. No postoperative complications occurred, and the patient was transferred to the pediatric intensive care unit in stable condition.
This case illustrates the intraoperative integration of intravenous magnesium sulfate as an adjuvant to propofol-based TIVA during pediatric glioma resection with suspected raised ICP, emphasizing individualized cerebral physiology rather than relying solely on conventional fixed-target paradigms. Preoperative magnetic resonance imaging (MRI) demonstrated a left temporoparietal mass with edema and midline shift, providing pathophysiological context for the patient’s elevated intracranial pressure ( Figure 4). Magnesium infusion contributed to excellent intraoperative brain relaxation, stable systemic hemodynamics, and markedly reduced requirements for rescue opioids and neuromuscular blockers, supporting an early, non-delayed recovery trajectory. Importantly, the real-time use of optic nerve sheath diameter trends and transcranial Doppler to target MCA flow dynamics enabled bedside, physiology-concordant titration of ICP surrogates and cerebral perfusion conditions without invasive monitors, providing a pragmatic route to “personalized ICP-CPP surrogate management” during neuro-oncologic surgery in resource-limited pediatric settings. Directional improvement in ultrasound-derived ICP surrogates following MgSO4 administration is summarized in Table 2, emphasizing trend-based rather than absolute numeric interpretation.
Magnesium sulfate has well-established neuro-modulatory properties relevant to children with suspected elevated ICP undergoing high-risk brain tumor resection. By providing non-competitive antagonism at the NMDA receptor, MgSO4 reduces glutamate-mediated calcium influx and protects mitochondrial integrity. At the same time, its anti-inflammatory and antioxidant effects stabilize the blood-brain barrier and suppress perioperative oxidative stress cascades commonly accentuated in the immature brain. Importantly, pediatric data demonstrate that within recommended perioperative ranges, magnesium preserves cerebrovascular autoregulation and improves cerebral compliance, a property that supports safer, individualized titration of ICP and perfusion surrogates when invasive monitors are unavailable.10–12
Propofol-based TIVA provides predictable cerebral metabolic rate reduction, preserved flow-metabolism coupling, limited disturbance on autoregulation, and favorable early wake-up profiles in pediatric supratentorial tumor surgery. Magnesium complements this by blunting sympathetic variability and reducing rescue opioid and non-depolarizing NMB top-ups via presynaptic acetylcholine suppression, without altering propofol’s core recovery-preserving advantages. This combination supports a stable intraoperative neurobehavioral respiratory recovery profile and avoids delayed extubation.13–15
Bedside ultrasound monitoring of optic nerve sheath diameter trends has been validated as a reliable non-invasive surrogate for intracranial compliance in pediatric populations, particularly in neurosurgical and neurocritical care pathways. Representative pre- and post-induction optic nerve sheath diameter measurements illustrating directional change rather than absolute ICP quantification are shown in Figure 1. Directional reduction of pre-induction elevated ONSD values (from 0.62 mm right; 0.55 mm left to <0.5 mm range post-induction) adds physiologic coherence to suspected ICP improvement and supports individualized intraoperative titration logic, especially when interpreted as trend-based compliance directionality rather than absolute numeric diagnosis.16,17 Transcranial Doppler assessment of middle cerebral artery pulsatility indices is a validated method for reflecting changes in cerebral compliance and perfusion directionality in children, including in traumatic and neurocritical populations. Corresponding changes in middle cerebral artery flow dynamics assessed by transcranial Doppler are illustrated in Figure 2. The observed reduction from pre-induction elevated PI (2.02; 1.73) into improved ranges (1.2; 1.4) remains consistent with literature-mapped pediatric Doppler–compliance trending, reinforcing physiologic plausibility without making overstatements that transcend case-level inference.16,18,19
In the framework of Enhanced Recovery After Surgery (ERAS) for pediatric neurosurgery, the opioid-sparing and neuromuscular blocker–sparing properties of magnesium hold significant clinical importance. In addition to its analgesic effects, magnesium plays a vital role in modulating sympathetic tone and cerebrovascular reactivity, which enhances cerebral compliance and promotes optimal intraoperative brain relaxation. In this physiologically stable environment, a reduction in intraoperative opioid utilization and a decreased requirement for neuromuscular blockers were effective in maintaining suitable surgical conditions. Ultrasound-based indicators provided the primary physiological assessments of improved intracranial compliance, while favorable surgical field conditions were visually confirmed by the observed brain relaxation following dural opening ( Figure 3). This recovery-conserving anesthetic approach supports early spontaneous respiratory recovery, facilitating extubation within 24 hours and resulting in a minimal burden on the early intensive care unit (ICU) concerning pain management and postoperative nausea and vomiting.20–22 Collectively, these observations reinforce a positive trajectory for early postoperative recovery, an outcome increasingly prioritized in contemporary anesthesia reporting within the pediatric ERAS framework.21,23
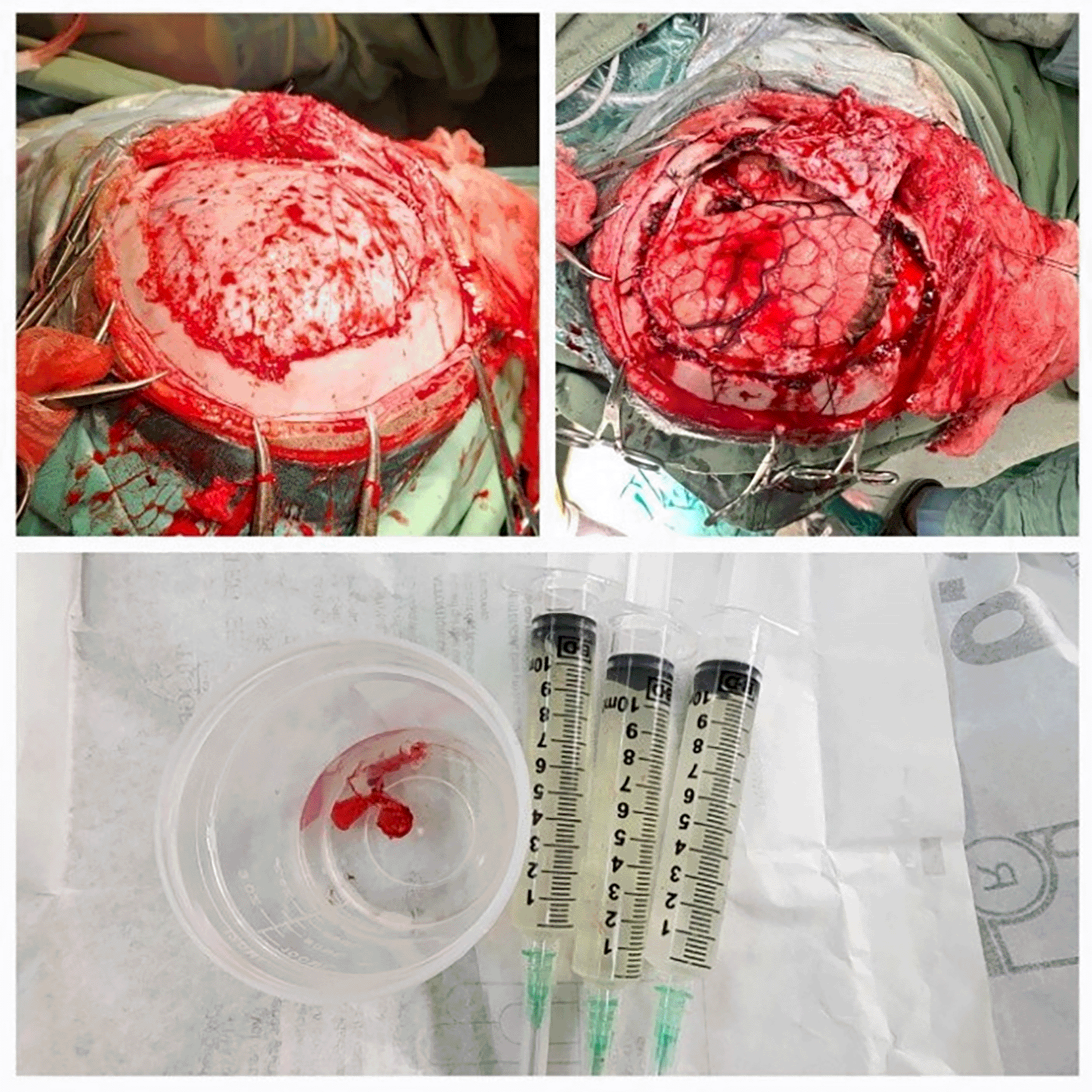
Leftside: Brain before dura opening shows mild swelling. Right side: Relaxed brain surface after dura opening post-MgSO4 administration.
In children, intraoperative MgSO4 is considered safe when administered within 30–50 mg/kg loading followed by 10–15 mg/kg/h maintenance, provided that a structured toxicity surveillance bundle is employed. This includes monitoring of deep tendon reflexes, respiratory return time, hemodynamics, and Mg-specific ECG markers such as PR prolongation or QRS widening, particularly when co-administered with TIVA and non-depolarizing NMBs. In this case, absence of hypotension, hyporeflexia, respiratory depression, or pro-arrhythmic ECG changes supports the favorable safety signal reported and meets open-research expectations for vigilant case-level toxicity transparency.10,20,21
However, despite its benefits, MgSO4 administration must be closely monitored to avoid potential complications. Clinical symptoms associated with increased plasma magnesium levels vary depending on the plasma concentration.24 High serum concentrations can lead to hypotension, bradycardia, muscle weakness, or respiratory depression, particularly in the setting of renal insufficiency or when used concomitantly with other central nervous system depressants.25,26 Therefore, monitoring plasma magnesium levels and ECG is an additional surveillance that needs to be prioritized to improve patient safety. In this case, the use of MgSO4 within recommended dosing limits (loading dose 30 mg/kg, maintenance 10–15 mg/kg/h), combined with vigilant hemodynamic and neuromuscular monitoring, allowed the anesthetic team to maximize its therapeutic advantages without compromising patient safety.
Intravenous magnesium sulfate can serve as a safe and effective adjuvant to propofol-based TIVA in pediatric glioma resection, offering immediate clinical benefits including excellent brain relaxation, systemic hemodynamic stability, and reduced requirements for rescue opioids and neuromuscular blockers. The concomitant use of real-time ultrasound surrogates (ONSD trends and TCD flow patterns) provides a pragmatic, patient-specific approach to optimize ICP and cerebral perfusion dynamics without invasive ICP monitoring, while maintaining an early recovery trajectory concordant with pediatric ERAS principles. When magnesium is administered within recommended dosing limits and under vigilant clinical observation, it demonstrates a favorable safety signal and promising “recovery-preserving” impact, warranting further validation in prospective, structured pediatric neurosurgical anesthesia protocols incorporating serum Mg monitoring, standardized ultrasound ICP-CPP surrogates, and long-term neurodevelopmental outcomes.
While this is a single case report and cannot establish causality or define numeric thresholds, it generates a strong hypothesis-forming feasibility signal for a magnesium-synergized propofol TIVA strategy using non-invasive ultrasound physiology trending to guide intraoperative ICP cerebral compliance directionality and perfusion management without delaying ICU recovery slope. Prospective validation using standardized magnesium–monitoring bundles, defined pediatric ultrasound compliance ranges, and long-term neurodevelopmental endpoints should be pursued.
Written informed consent for publication of clinical details was obtained from the patient’s legal guardian.
Tori Sepriwan: Supervision, Validation, Writing – Review & Editing; Khairunnisai Tarimah: Writing – Original Draft Preparation, Writing – Review & Editing; Dewi Yulianti Bisri: Supervision, Validation, Writing – Review & Editing; Radian Ahmad Halimi: Supervision, Validation, Writing – Review & Editing.
All data underlying the results are included within the manuscript. The completed CARE Checklist supporting this case report is publicly available as a data record in Zenodo: Sepriwan T, Tarimah K, Bisri DY, Halimi RA. (2025). Intravenous Magnesium Sulfate as an Adjuvant to Propofol-Based TIVA in Pediatric Glioma Resection: CARE Checklist. Zenodo. https://doi.org/10.5281/zenodo.17921383.27
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)