Keywords
Breast cancer; IL-17; IL-34; CXCL12;Cytokines.
This article is included in the Oncology gateway.
This article is included in the Fallujah Multidisciplinary Science and Innovation gateway.
Immune dysregulation—particularly involving cytokines and chemokines—contributes to breast cancer progression and metastasis. To compare circulating levels of interleukin-17 (IL-17), interleukin-34 (IL-34), and C-X-C motif chemokine ligand 12 (CXCL12) between premenopausal and postmenopausal women with breast cancer and age-matched healthy controls.
In this case–control study, 130 women were enrolled: 90 patients with breast cancer (45 premenopausal; 45 postmenopausal) and 40 healthy controls (20 premenopausal; 20 postmenopausal). Serum IL-17, IL-34, and CXCL12 concentrations were quantified by ELISA. Group differences were assessed using independent-samples t-tests.
Mean serum levels of IL-17, IL-34, and CXCL12 were significantly higher in patients than in controls across both menopausal strata (all p < 0.001). The pattern of elevation was consistent in premenopausal and postmenopausal subgroups, indicating that the observed immune perturbations are not restricted by menopausal status.
IL-17, IL-34, and CXCL12 are markedly elevated in women with breast cancer irrespective of menopausal state and may serve as accessible immune biomarkers for disease detection and monitoring. Validation in larger, longitudinal cohorts is warranted to evaluate diagnostic performance and potential therapeutic relevance.
Breast cancer; IL-17; IL-34; CXCL12;Cytokines.
Breast cancer (BC) remains one of the most frequently diagnosed malignancies among women and a leading cause of cancer-related mortality worldwide. In 2020, an estimated 685,000 women died from the disease, representing a substantial global health burden.1
Although early detection and advances in therapy have improved survival, outcomes vary significantly across populations, with higher incidence and mortality observed among White women and lower rates among Asian women.2,3
Beyond hormonal and genetic factors, increasing evidence highlights the central role of the immune system in breast cancer initiation, progression, and therapeutic response. The tumor microenvironment is a dynamic ecosystem in which immune cells, cytokines, and chemokines interact to either restrain or promote tumor growth. Dysregulated immune signaling—particularly involving pro-inflammatory mediators—has been implicated in tumor proliferation, angiogenesis, and metastasis. Recent research has underscored the importance of immune-related biomarkers in understanding disease heterogeneity and improving risk stratification. Boivin et al. (2020) reported that breast cancer treatment can significantly alter immunological markers, suggesting a close link between cytokine dynamics and patient outcomes.4
Similarly, Glushkov et al. (2021) identified disruptions in the immuno-hormonal network of postmenopausal women with breast cancer, further emphasizing the influence of immune-endocrine interactions on disease behavior.5,6
Among the numerous immune mediators implicated in breast cancer, the cytokines interleukin-17 (IL-17), interleukin-34 (IL-34), and the chemokine C-X-C motif ligand 12 (CXCL12) have attracted considerable attention.7–9 IL-17, primarily secreted by T helper 17 (Th17) cells, is a potent pro-inflammatory cytokine that can facilitate tumor growth, angiogenesis, and immune evasion. IL-34, a pleiotropic cytokine involved in monocyte and macrophage differentiation, has shown dual behavior—acting as both a tumor promoter and an immune modulator depending on the molecular subtype of breast cancer.10 CXCL12 and its receptor CXCR4 form a critical chemotactic axis that supports tumor cell migration, organ-specific metastasis, and the formation of an immunosuppressive niche within the tumor microenvironment. Genetic studies reinforce these mechanistic insights. For example, Alijanpour et al. (2024) reported a significant association between the CXCL12 rs1801157 polymorphism and increased breast cancer risk, particularly among hormone receptor–positive subtypes.11 Similarly, Zins et al. (2018) found that IL-34 expression patterns differ across breast cancer subtypes, correlating with prognosis and immune infiltration profiles.12 Elevated CXCL12 and IL-17 signaling have also been linked to enhanced tumor invasiveness and reduced responsiveness to immunotherapy.13
Despite mounting evidence connecting cytokine profiles with tumor behavior, comparative analyses of these immune mediators across menopausal states remain limited. Menopause induces substantial hormonal and immunological changes that may influence cancer pathophysiology and response to treatment. Understanding whether cytokine and chemokine levels differ between premenopausal and postmenopausal patients could provide valuable insight into the immune mechanisms underpinning breast cancer.14
Accordingly, the present study aims to evaluate serum levels of IL-17, IL-34, and CXCL12 in premenopausal and postmenopausal women with breast cancer relative to healthy controls. By clarifying the immunological landscape across menopausal status, this work seeks to identify potential biomarkers for diagnosis, prognosis, and immunotherapeutic targeting in breast cancer.7,8
This case–control study was conducted between December 2023 and June 2024 at the Specialized Oncology Center, Salah al-Din Governorate, Iraq. Eligible participants included premenopausal and postmenopausal women newly diagnosed with breast cancer. Exclusion criteria were prior exposure to immunotherapy, a history of autoimmune disorders, previous malignancies, or unwillingness to participate. All participants provided written informed consent before enrollment, and the study protocol adhered to the ethical standards of the institutional review board of Tikrit University, College of Science.
A total of 130 women were enrolled and categorized into four subgroups:
• Premenopausal patients (n = 45)
• Postmenopausal patients (n = 45)
• Premenopausal healthy controls (n = 20)
• Postmenopausal healthy controls (n = 20)
Menopausal status was defined according to standard clinical criteria (cessation of menses for ≥12 months without other pathological or physiological causes).
Breast cancer diagnosis was confirmed using a combination of clinical examination, mammography, ultrasonography, biopsy, histopathology, and immunohistochemistry. Tumor classification followed established pathological criteria. None of the participants had received chemotherapy or radiotherapy prior to sample collection to avoid confounding immune responses.
From each participant, 5 mL of peripheral venous blood was collected into plane tubes. Samples were centrifuged at 2700 rpm for 10 minutes to separate sera, which was aliquoted and stored at −20°C until analysis.
Serum concentrations of IL-17, IL-34, and CXCL12 were determined using enzyme-linked immunosorbent assay (ELISA) kits (protocol based on M. Saxton et al., 2014) by SunLong Biotech Co., LTD.15 Assays were performed in duplicate following the manufacturer’s instructions. Procedures included coating with capture antibodies, incubation with detection antibodies, substrate development, and measurement of optical density (OD) using a microplate reader. Cytokine concentrations were calculated from standard curves and expressed in pg/mL. Data were initially recorded in Excel spreadsheets for statistical processing.
This study was conducted in accordance with the ethical standards of Tikrit university-college of science by Scientific research ethics committee which provided approval and reference/permit numbers (SCITUT 00023) and the principles of the Declaration of Helsinki. Verbal informed consent was obtained from all adult participants prior to data collection. Verbal consent was chosen instead of written consent due to cultural considerations, limited literacy and field conditions. All participants were informed about the study’s objectives, procedures, and their right to withdraw at any time without any consequences. Confidentiality and anonymity were strictly maintained throughout the study. The study protocol was reviewed and approved by the Institutional Review Board (IRB) of Tikrit university, ensuring that no harm came to the participants during the research process.
Data were analyzed using IBM SPSS Statistics (version 26). Continuous variables were expressed as mean ± standard deviation (SD). Between-group comparisons were performed using independent-samples t-tests, and statistical significance was defined as p < 0.05. Graphical visualizations were generated to depict mean cytokine levels with standard deviation error bars.
A total of 130 women participated in the study, including 90 patients with breast cancer (45 premenopausal and 45 postmenopausal) and 40 age-matched healthy controls (20 premenopausal and 20 postmenopausal). All participants successfully completed blood sampling and cytokine analysis.
Serum concentrations of IL-17, IL-34, and CXCL12 were markedly elevated in women with breast cancer compared with healthy controls. These elevations were observed consistently across both premenopausal and postmenopausal subgroups ( Table 1). Independent-samples t-tests confirmed statistically significant differences between patient and control groups for all measured parameters (p < 0.001 for each comparison).
Data are presented for all patients and controls, as well as stratified by menopausal status (premenopausal and postmenopausal). P values indicate statistical significance between patients and controls for each cytokine/chemokine. CXCL12, C-X-C motif chemokine ligand 12; IL-34, interleukin-34; IL-17, interleukin-17.
Among breast cancer patients, premenopausal women exhibited slightly higher mean values of all three analytes than postmenopausal counterparts; however, these within-patient subgroup differences were not statistically significant (p > 0.05).
Figures 2 and 3 illustrate the distribution of mean serum levels of CXCL12, IL-34, and IL-17 across all subgroups, with standard-deviation error bars highlighting interindividual variability. The graphical trends corroborate the numerical results, showing a consistent up-regulation of the studied cytokines in both premenopausal and postmenopausal patients relative to controls.
Figures 1–3 illustrate the mean cytokine levels with standard deviation error bars across groups.
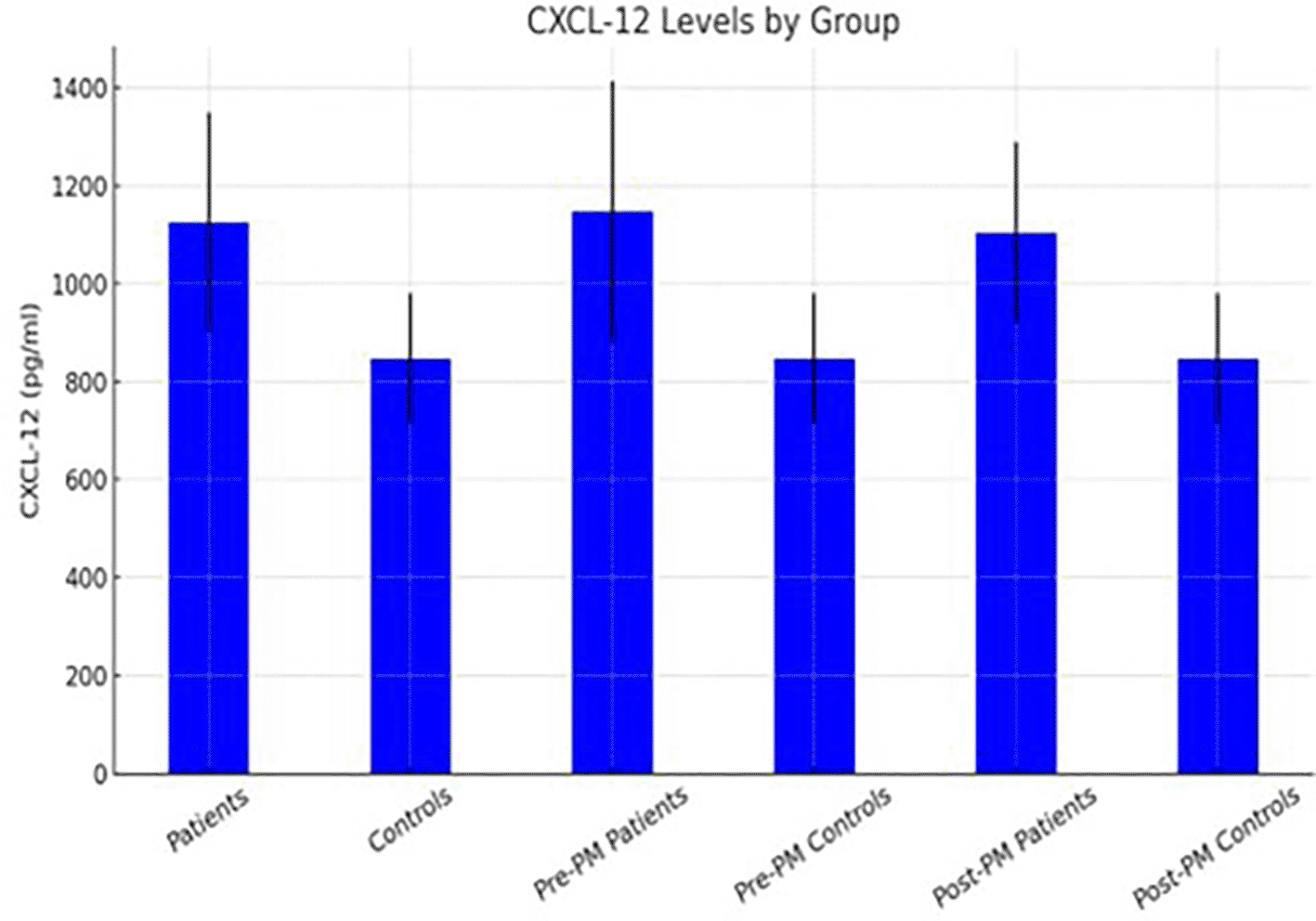
Data are presented as mean ± SD. Comparisons between groups were performed using appropriate statistical tests. *p < 0.05 versus control group.
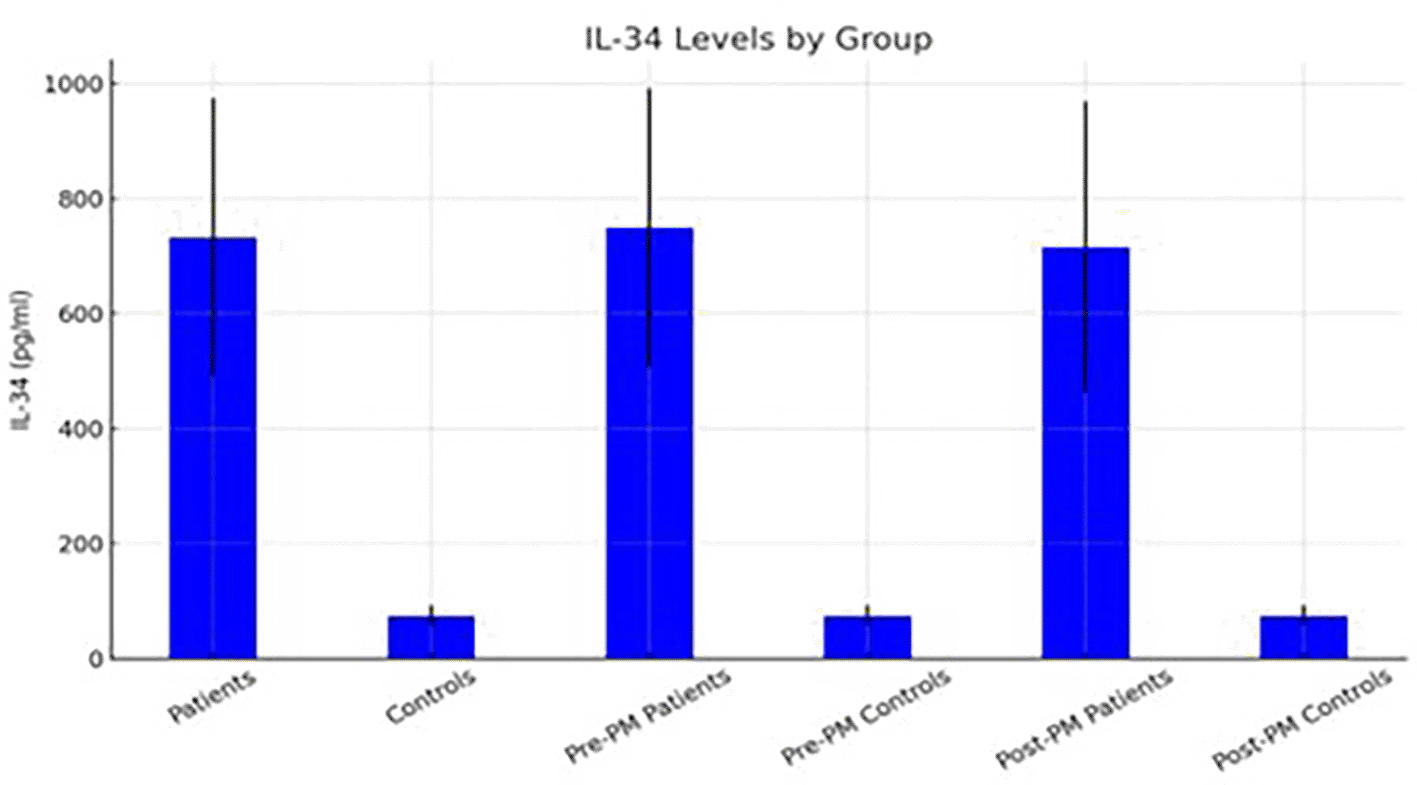
Data are presented as mean ± SD. Statistical significance is indicated for comparisons between patients and controls. *p < 0.05.
Collectively, these results demonstrate a uniform elevation of IL-17, IL-34, and CXCL12 in the circulation of women with breast cancer, independent of menopausal status. The data suggest a shared immune activation or dysregulation pattern that may reflect tumor-associated inflammatory signaling rather than hormonal differences.
The present study demonstrates that serum concentrations of IL-17, IL-34, and CXCL12 are significantly elevated in women with breast cancer compared to healthy controls. This elevation was consistent across both premenopausal and postmenopausal subgroups, indicating that immune dysregulation associated with breast cancer is not confined to hormonal status. Collectively, these findings reinforce the concept that cytokine and chemokine signaling networks play central roles in breast tumorigenesis, progression, and metastasis.
The observed increase in IL-17 aligns with previous evidence describing its tumor-promoting properties. IL-17, a proinflammatory cytokine primarily secreted by T helper 17 (Th17) cells, can stimulate angiogenesis, enhance tumor cell proliferation, and suppress antitumor immunity Studies such as those by El-Batal et al. (2024) and Yang et al. (2014) reported similar trends, where high circulating IL-17A levels correlated with disease severity and unfavorable prognosis, particularly in aggressive subtypes such as triple-negative breast cancer. Elevated IL-17 may therefore serve as both a biomarker of immune activation and a mediator of chronic inflammation that sustains tumor growth.13,16
IL-34 also displayed a marked rise among patients, supporting its emerging recognition as a multifunctional cytokine in the tumor microenvironment. Previous reports, including Franzè et al. (2019) and Zins et al. (2018), have emphasized IL-34’s dualistic behavior—acting as a pro-tumorigenic factor in some contexts and an immune modulator in others. IL-34 facilitates the recruitment and polarization of tumor-associated macrophages, which in turn foster immunosuppression and angiogenesis. The current findings strengthen the hypothesis that IL-34 contributes to the establishment of a permissive immune microenvironment in breast cancer, particularly through macrophage-driven pathways.10,12
The chemokine CXCL12 and its receptor CXCR4 are well-established mediators of tumor cell migration and metastasis. Our data reveal pronounced elevations in CXCL12 levels among both premenopausal and postmenopausal patients, consistent with findings from Boimel et al. (2012) and Luker and Luker (2006) demonstrating that the CXCL12–CXCR4 axis directs breast cancer cells toward metastatic sites such as bone, lymph nodes, and lungs. Moreover, Alijanpour et al. (2024) identified a significant association between the CXCL12 rs1801157 polymorphism and increased breast cancer susceptibility, lending genetic support to the functional role of this pathway. Taken together, the persistent CXCL12 upregulation observed in this study underscores its value as a potential biomarker and therapeutic target for metastasis prevention.7,8,11
Although estrogen levels and hormonal fluctuations influence immune regulation, the lack of significant differences between premenopausal and postmenopausal patients in our dataset suggests that cytokine-mediated immune activation may operate independently of menopausal hormonal status. This finding aligns with prior observations by Glushkov et al. (2021) and Cairat et al. (2022), who reported that systemic inflammation and cytokine dysregulation persist regardless of estrogen depletion. Therefore, the immune system’s contribution to breast cancer appears to transcend menopausal boundaries, reflecting fundamental immunopathogenic processes intrinsic to the tumor microenvironment.5,2
The concurrent elevation of IL-17, IL-34, and CXCL12 highlights a convergent inflammatory network that may influence both tumor biology and patient outcomes. Functionally, IL-17 promotes tumor-promoting inflammation, IL-34 supports macrophage recruitment and immune evasion, and CXCL12 drives metastatic dissemination. These coordinated signals likely sustain the chronic inflammatory state that characterizes breast cancer progression. From a clinical perspective, quantifying these cytokines could aid in early disease detection, risk assessment, or treatment monitoring, particularly in settings where molecular profiling resources are limited.17,18
While the current study establishes strong associations between cytokine elevations and breast cancer presence, its cross-sectional design limits causal inference. Longitudinal studies with larger and more diverse cohorts are required to assess how cytokine fluctuations relate to treatment response and survival outcomes. Additionally, integrating cytokine profiling with genomic and immunophenotypic analyses may clarify the mechanistic underpinnings of immune–tumor crosstalk and support the development of targeted immunotherapies.
In conclusion, the consistent elevation of IL-17, IL-34, and CXCL12 in both premenopausal and postmenopausal women with breast cancer underscores their shared involvement in tumor-promoting inflammation and immune dysregulation. These molecules represent promising candidates for further exploration as non-invasive immune biomarkers and potential therapeutic targets in breast cancer management.
The data underlying this article are available on Figshare at the following DOI link: https://doi.org/10.6084/m9.figshare.30555995.v1.19 All datasets are released under an open licence (CC BY 4.0) permitting unrestricted reuse.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)