Keywords
long COVID, diet quality, fatigue, inflammation, physical function, aging, nutrition intervention, randomized trial
This article is included in the Coronavirus (COVID-19) collection.
Long COVID, or post-acute sequelae of SARS-CoV-2 infection (PASC), is characterized by persistent or new symptoms lasting three months or more after acute infection. Approximately 4% of U.S. adults are affected, with fatigue and muscle weakness among the most common symptoms. Chronic inflammation and nutritional imbalances are thought to contribute to long-COVID symptomatology. The Long-COVIDiet Study was designed to test the effectiveness of a whole-diet approach—grounded in the Dietary Guidelines for Americans, 2020–2025—that emphasizes nutrient-dense, anti-inflammatory foods and select supplements. The trial evaluates the impact of this dietary intervention, quantified through a diet quality score, on fatigue, muscle strength, physical function, and biomarkers of nutritional and inflammatory status in older adults with long COVID.
Fifty-six participants aged ≥50 years with long COVID, moderate-to-severe fatigue, and suboptimal diet quality will be recruited from the Baltimore, Maryland area. Participants will be randomized 1:1 to either a dietary intervention arm or an attention-control arm. The intervention includes individualized dietary counseling (eight biweekly sessions) and 16 weekly online group sessions with a registered dietitian, emphasizing anti-inflammatory and nutrient-rich foods. The control group attends matched weekly sessions on non-dietary healthy aging topics. Primary outcomes are fatigue and diet quality, assessed at baseline, 12 weeks, and 16 weeks. Secondary outcomes include muscle mass, grip strength, and physical function; exploratory outcomes include nutritional and inflammatory biomarkers. Analyses will follow the intention-to-treat principle, with linear regression and mixed-effects models evaluating changes across timepoints.
The study was approved by the University of Maryland, Baltimore Institutional Review Board (HP-00106745). Written informed consent will be obtained from all participants. Results will be disseminated through peer-reviewed publications, scientific conferences, and plain-language summaries on the study website.
Trial registration number
ClinicalTrials.gov Identifier: NCT05977179.
long COVID, diet quality, fatigue, inflammation, physical function, aging, nutrition intervention, randomized trial
This trial is registered with ClinicalTrials.gov under identifier NCT05977179.
Registered title: The Long-COVIDiet Study – A Diet Intervention Study to Mitigate Fatigue Symptoms and to Improve Muscle and Physical Function in Older Adults with Post-Acute COVID-19 Syndrome.
Protocol version:
Protocol Version 7.0, dated October 24, 2025.
As the acute phase of the COVID-19 pandemic has receded—driven by widespread vaccination, population-level immunity, and the emergence of less virulent variants—attention has shifted to a persistent and complex public health challenge: long COVID, also known as post-acute sequelae of SARS-CoV-2 infection (PASC). Approximately 10–30% of individuals infected with SARS-CoV-2 develop long COVID, which manifests as a heterogeneous constellation of symptoms that may persist for months or even years. Common symptoms include fatigue, muscle weakness, brain fog, gastrointestinal disturbances, and respiratory dysfunction, often independent of the severity of the initial illness.1–4 According to a 2025 global meta-analysis of 429 studies involving more than two million participants, approximately 36% of individuals with confirmed COVID-19 developed long COVID, representing a cumulative global burden of nearly 400 million affected individuals worldwide.4–7
Individuals with long COVID often experience compromised nutritional status as a result of multisystem physiological disruptions. Viral injury to the gastrointestinal tract, liver, and vasculature can impair nutrient absorption, alter metabolism, and increase energy demands.8–11 These nutritional disturbances are further exacerbated by chronic inflammation, immune dysregulation, and gut microbiota imbalance—pathophysiological features strongly implicated in long COVID.12 Moreover, persistent symptoms such as altered taste and smell,13 dysphagia, chronic cough, and gastrointestinal discomfort may reduce appetite and food intake, leading to nutrient deficiencies. Among frail older adults, who are especially susceptible to both long COVID and age-related nutritional deficiencies, poor diet quality—characterized by inadequate intake of fruits, vegetables, and whole grains and high consumption of pro-inflammatory foods21–23—may hinder recovery, intensify fatigue and muscle weakness, and accelerate the trajectory toward functional decline and disability.11,14
Early in the pandemic, nutritional research focused primarily on immune-supportive micronutrients—particularly vitamins A, C, D, and K, as well as zinc and selenium—with the main goal of mitigating the severity of acute SARS-CoV-2 infection.15–17 However, accumulating evidence suggests that long-term recovery requires a broader nutritional framework—one that encompasses multi-nutrient strategies and whole-dietary patterns.11 A recent comprehensive scoping review on diet and long COVID reported that approximately 76% of studies observed improvements in long COVID–related symptoms—most notably fatigue, mood disturbances, physical function, and inflammatory markers—following nutritional interventions. Notable benefits were found for multi-nutrient formulations, amino acid–based metabolic support such as L-arginine and creatine, and microbiota-targeted therapies including probiotics, synbiotics, and paraprobiotics. Moreover, adherence to Mediterranean and plant-based dietary patterns—both rich in anti-inflammatory and microbiome-supportive foods—was associated with symptom relief and enhanced functional outcomes.11
Due to the nutritional alterations observed in individuals with long COVID, there is a growing need for targeted nutritional recovery strategies. Whole-diet approaches emphasizing metabolically balanced, nutrient-dense, and anti-inflammatory eating patterns may offer a biologically plausible and scalable solution. Evidence from related conditions supports this premise: similar dietary strategies have been shown to reduce cancer-related fatigue,18 enhance muscle strength and physical performance in older adults,19 and improve post-infection recovery.20 Nevertheless, despite the growing recognition of nutrition’s potential role in recovery, rigorously designed clinical trials investigating comprehensive dietary interventions for long COVID remain limited.24,25 The Long-COVIDiet study seeks to address this critical gap by testing whether adherence to a dietary pattern consistent with the Dietary Guidelines for Americans (DGA)—which emphasizes fruits, vegetables, whole grains, lean proteins, and healthy fats—combined with targeted nutritional supplementation, can alleviate long COVID symptoms and promote recovery in older adults.
To evaluate the effectiveness of a 16-week whole-diet intervention in alleviating fatigue and improving muscle mass, strength, and physical function among older adults with long COVID.
Specific Aims:
• Aim 1: Determine whether the 16-week dietary intervention improves overall diet quality and reduces fatigue severity compared with the attention control group.
• Aim 2: Evaluate the effects of the intervention on muscle mass, strength, and physical function relative to the control group.
• Aim 3: Explore potential biological mechanisms underlying these effects by examining changes in nutritional and inflammatory biomarkers from baseline to 16 weeks.
Patients or the public were not involved in the design, conduct, or reporting of this trial. However, participants provided feedback on session content at the end of the trial, which informed refinements to the intervention materials for future implementation.
Trial design and setting
The Long-COVIDiet study is a non-blinded, parallel-group, randomized controlled trial conducted under a superiority framework. Fifty-six eligible participants are randomized in a 1:1 ratio to either a 16-week dietary intervention or an attention-control arm. The trial is conducted at the University of Maryland, Baltimore (UMB). All research procedures take place at UMB facilities, including the University of Maryland Medical Center – General Clinical Research Center (GCRC), the Center for Vaccine Development and Global Health, and the Division of Gerontology, with weekly sessions delivered online
Eligibility criteria
Eligible participants include adults aged 50 years or older with either a physician-confirmed diagnosis of COVID-19—verified by PCR, rapid antigen test, or positive anti-nucleocapsid antibodies—or a clinical diagnosis of long COVID/post-acute COVID-19 syndrome based on ICD-10-CM code U09.9. Inclusion criteria are as follows: fatigue persisting for more than 12 weeks after COVID-19 onset; a Brief Fatigue Inventory (BFI) score of 4 or higher, indicating moderate to severe fatigue26; a Mini-Eat score below 70, indicating suboptimal diet quality27; absence of any active infectious disease (e.g., COVID-19, tuberculosis, influenza, or Lyme disease); independent ambulation with or without assistive devices; reliable internet access enabling active participation in online sessions; and the ability to understand and sign informed consent. Participants are excluded if they have major medical conditions that could compromise safety or the interpretation of results, including uncontrolled diabetes, significant heart failure, dependence on home oxygen or chronic ventilation, severe hypertension, active cancer, liver disease, or severe kidney impairment, or any condition judged by the study physician to increase risk or confound outcomes. Additional exclusion criteria include diet-related restrictions or metabolic conditions affecting adherence (e.g., use of Warfarin or medically prescribed diets), active drug or alcohol dependence, planned surgical procedures within six months, concurrent participation in another clinical trial involving an active intervention, or inability to attend study visits or comply with study procedures. During the informed-consent process, participants’ English comprehension is verified to ensure understanding of study activities. Because several study assessments require reading and written responses, individuals who are illiterate are not eligible for enrollment.
Intervention and comparator
Intervention Arm (Diet Modification)
Participants randomized to the intervention arm receive a 16-week whole-diet modification program designed to promote adherence to a U.S. Healthy Dietary Pattern consistent with the Dietary Guidelines for Americans, 2020–2025 (DGA 2025).28 The program emphasizes nutrient-dense, anti-inflammatory foods—such as fruits, vegetables, whole grains, legumes, nuts, and high-quality protein sources—while limiting saturated fats, added sugars, sodium, and ultra-processed foods. Topics covered throughout the program are outlined in Table 1.
Intervention Components
1. Weekly Group Education Sessions:
Participants attend 16 weekly online sessions (30–40 minutes each) led by the Registered Dietitian (RD) or Principal Investigator. Sessions are adapted from the National Institute on Aging’s What’s On Your Plate? program,31–36 emphasizing practical strategies for improving diet quality and fostering sustainable dietary habits.
2. Personalized Dietary Counseling:
Each participant meets individually with an RD at baseline and every two weeks (eight sessions in total) to tailor dietary recommendations and enhance anti-inflammatory diet quality. Total energy expenditure (TEE) is estimated using indirect calorimetry via the ReeVue™ metabolic cart, which measures resting energy expenditure (REE); TEE is then calculated as REE × physical activity factor.30
3. Targeted Nutritional Supplementation:
Nutritional supplements are provided as needed to help participants meet individualized nutrient and protein targets. Supplementation follows evidence-based clinical nutrition guidelines and is monitored to ensure safety, adherence, and equity11,29:
4. Educational Materials and Tools:
Participants have access to the study website ( https://longcovidiet.com ) for recipes, educational materials, and self-monitoring resources, along with periodic digital reminders to support engagement and adherence.
Comparator Arm (Attention Control – No Diet Modification)
Participants randomized to the attention-control group also attend 16 weekly online sessions. These sessions address non-dietary aspects of healthy aging, adapted from the National Institute on Aging (NIA) booklet “What Do We Know About Healthy Aging?” (NIH Publication No. 22-AG-8188, 2022).37 All study materials—including slides, readings, and videos—are hosted on the study’s secure website (https://longcovidiet.com). Topics covered throughout the program are outlined in Table 1. Each session consists of approximately 15 minutes of educational content and video presentations, followed by a brief interactive quiz, for a total duration of about 30 minutes per session
Rationale for comparator
The attention-control condition is designed to match the intervention arm in duration, frequency, and level of interaction, thereby isolating the specific effects of dietary modification and supplementation from the general benefits of social engagement and attention received during the sessions. This design minimizes expectancy bias and ensures comparable participant contact across groups. Although control participants may independently modify their diets, this reflects real-world conditions given the current absence of an established nutrition-based treatment for long COVID. To account for such changes, diet quality is assessed in both groups at baseline and 16 weeks, allowing evaluation of the added benefit of a structured dietary intervention beyond nonspecific behavioral effects.
Intervention Delivery and Personnel
All intervention and control sessions are conducted online via Zoom and facilitated by a Registered Dietitian (RD) or the Principal Investigator (PI), both trained in behavioral nutrition and gerontology. The RD oversees supplement provision and adherence monitoring under the PI’s supervision. All study staff complete protocol-specific training, including the use of standardized scripts and fidelity checklists, to ensure consistent and reproducible intervention delivery. Additional materials are available upon request or through the study website via passcode access ( https://longcovidiet.com ).
Criteria for discontinuing or modifying allocated interventions
Participants may withdraw from the study at any time without providing a reason, and withdrawal does not affect their medical care. The Principal Investigator (PI) may also discontinue a participant’s involvement if safety or protocol integrity is compromised. Reasons for discontinuation include noncompliance, development of exclusion criteria, adverse events, contraindicating medical findings, or study termination by the sponsor, investigator, Institutional Review Board (IRB), or study site. Withdrawn participants receive appropriate guidance on discontinuing study activities, and those in the intervention arm are provided with a safe transition plan to resume their usual diet.
Strategies to improve adherence to interventions
To promote adherence, participants complete a study orientation before enrollment that covers study procedures, randomization, and expectations. The study team, led by the Registered Dietitian (RD) or Principal Investigator (PI), maintains regular contact through reminder texts, follow-up calls, and weekly group sessions. Adherence is assessed based on session attendance (≥80%) and 24-hour dietary recalls collected at two months, reflecting gradual improvements in diet quality. Participants receive financial compensation ($100 total) upon study completion. At the end of the trial, all participants are granted access—via secure website passcode—to the educational materials from both the diet intervention and control programs
Concomitant care permitted or prohibited during the trial
All recommended medical treatments are permitted during the study. Prescription and over-the-counter medications, supplement use, and COVID-19 vaccinations are recorded at baseline and study completion, as dosage changes may act as confounders. Monitoring these factors helps minimize bias and preserve study integrity.
Primary outcomes
Fatigue: Fatigue is assessed using the Brief Fatigue Inventory (BFI)26 and the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F).38 The BFI includes 10 items evaluating fatigue severity and its interference with daily activities over the past week, rated from 0 (none) to 10 (severe); scores ≥4 indicate moderate-to-severe fatigue. The FACIT-F measures the impact of fatigue on physical, emotional, and functional well-being and is widely used in clinical trials.
Diet Quality: Dietary intake is assessed using the Automated Self-Administered 24-hour (ASA24®) Dietary Assessment Tool, 39 which records all foods, beverages, and supplements consumed from midnight to midnight. Diet quality is evaluated using the Healthy Eating Index 2020 (HEI-2020),40,41 with scores ranging from 0–100; higher scores reflect closer alignment with the Dietary Guidelines for Americans, 2020–2025.
Secondary outcomes
Physical Function:
• Grip Strength: Measured using the Jamar Digital Dynamometer42; three trials per hand are recorded, and the mean of the dominant hand represents final grip strength.
• Short Physical Performance Battery (SPPB): Assesses gait speed, balance, and lower-body strength43,44; total scores range from 0–12, with higher scores indicating better function.
• 6-Minute Walk Test (6-MWT): Measures aerobic capacity based on the total distance walked in six minutes.45
• Body Composition: Assessed using bioelectrical impedance analysis (BIA) on the seca mBCA Alpha device, a method validated against whole-body MRI for estimating skeletal muscle mass.46
Tertiary outcomes
Inflammatory and nutritional biomarkers are assessed from fasting blood samples to examine their relationships with dietary intake (e.g., leafy green vegetable consumption49) and study outcomes, thereby elucidating potential biological mechanisms.50,51
Additional measures explore potential mediators and modifiers of the intervention effect, including sleep patterns (ASA24 Sleep Module: duration, quality, timing, and use of sleep aids)47; depressive symptoms (Patient Health Questionnaire–9, PHQ-9; scores ≥4 indicate mild-to-severe symptoms)48; and body size indices (weight, height, and BMI [kg/m2]). Demographic information, supplement and medication use, and medical history are also recorded. Physical activity is assessed using the Global Physical Activity Questionnaire (GPAQ),52 and quality of life is measured using the 36-item Short Form Survey (SF-36), which evaluates physical, social, emotional, and general health domains.53
The Long-COVIDiet study involves greater than minimal risk due to physical function testing, blood draws, and nutritional supplementation. Physical performance assessments (e.g., SPPB) pose minimal risk. Blood draws may cause mild discomfort, bruising, or swelling, which is minimized by employing trained phlebotomists. Vitamin D (1,000–2,000 IU/day) and omega-3 fatty acids (1,000 mg/day) are provided within recommended intake levels. Participants taking anticoagulant medications are excluded to minimize potential adverse effects. Participants are instructed to report any complaints to the study dietitian, who notifies the Principal Investigator (PI). Adverse events—including dizziness, fatigue, or gastrointestinal symptoms—are monitored for severity, timing, duration, and possible causality, and all events are documented and reported to the UMB Data and Safety Monitoring Board (DSMB) as appropriate.
Anticipated benefits and potential harms
The intervention is expected to improve nutritional intake, reduce fatigue, and help prevent declines in muscle mass, strength, and physical function among older adults with long COVID. The anticipated risks are minimal and may include mild gastrointestinal discomfort or intolerance to specific foods or supplements. All participants are closely monitored for any adverse effects throughout the study to ensure participant safety.
The study procedures and timeline are illustrated in the flow diagram ( Figure 1) and the SPIRIT schedule ( Table 2).
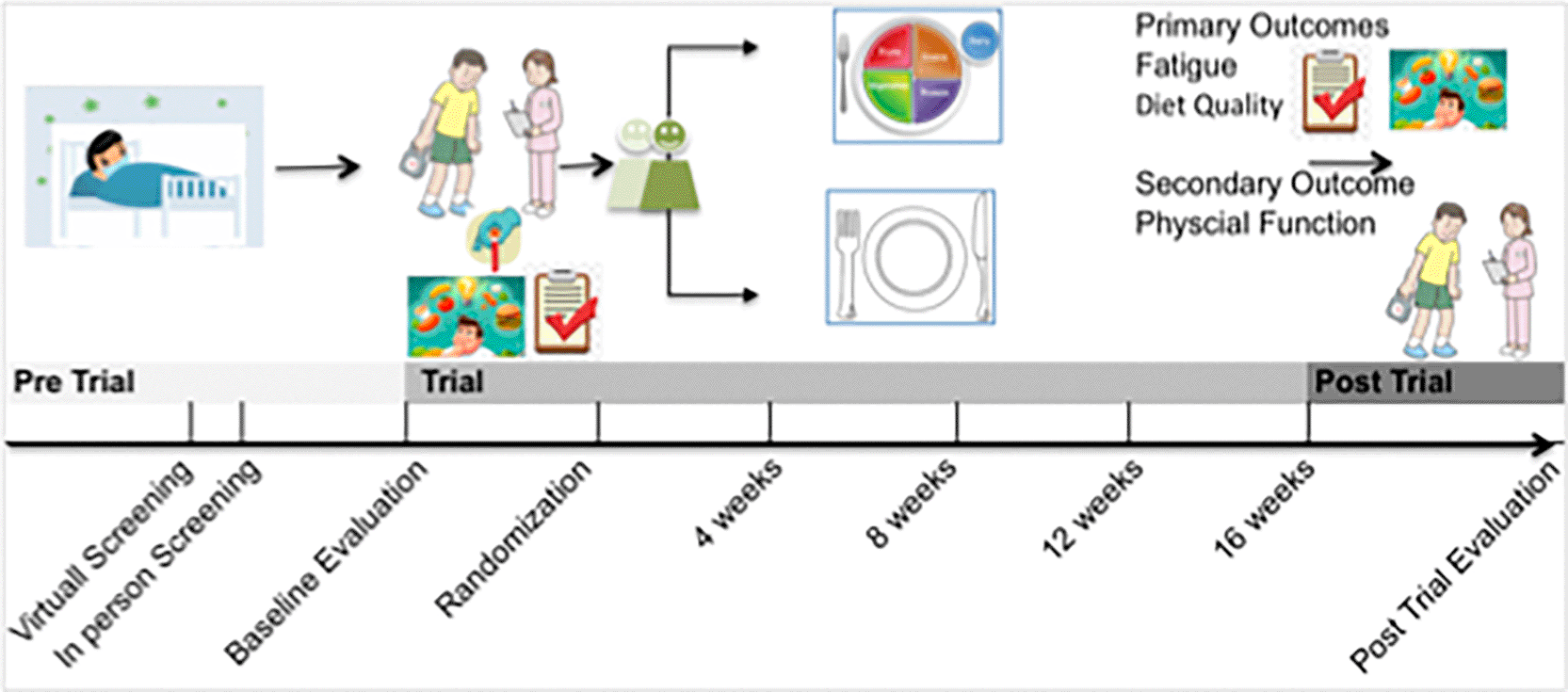
This figure presents the overall flow of the Long-COVIDiet randomized controlled trial, including participant screening and eligibility determination, baseline assessments, randomization, intervention and control activities, and the timing of outcome measurements across the study timeline. It visually summarizes the structure of study procedures and key assessments at baseline and follow-up.
Preliminary data from 21 long-COVID patients showed an HEI score difference of 14.6 points (SD = 11.2) between those with mild versus moderate/severe fatigue. To detect this difference with 90% power and α = 0.01, 44 participants (22 per arm) are needed. For the secondary aim—reducing BFI fatigue scores by 1.7 points (SD = 1.42)—24 participants (12 per arm) are required to achieve 80% power at α = 0.05. To meet both aims and allow for 20% attrition, we will enroll 56 participants (28 per arm).
Participants are recruited through multiple strategies, including:
• University of Maryland Medical System (UMMS): Leveraging electronic health records (EHR) and referrals from long-COVID clinics in Baltimore.
• Johns Hopkins Long-COVID Clinics: Collaborating with clinical teams to identify eligible participants and facilitate referrals.
• Advertisements and Media: Conducting outreach through newsletters (e.g., The Elm and The Beacon), study flyers, and social media.
• Community-Based Recruitment: Posting flyers and advertisements in senior centers, community organizations, and other public locations within a 50-mile radius of the University of Maryland, Baltimore.
Sequence generation
Participants are randomized using the REDCap randomization module, which ensures allocation concealment and automated assignment. The study biostatistician generates the randomization sequence using permuted blocks with randomly varying sizes of four to maintain group balance and prevent predictability. Participants are allocated in a 1:1 ratio to either the dietary intervention or attention-control group, with randomization activated in REDCap only after completion of baseline assessments to ensure unbiased allocation.
The randomization sequence is embedded within the REDCap randomization module to maintain allocation concealment. Eligible participants who complete baseline assessments and provide consent are assigned a unique study ID. The REDCap system withholds group assignment until randomization is triggered, ensuring that neither participants nor research staff are aware of the allocation in advance.
After randomization is triggered in REDCap, a designated research team member informs participants of their group allocation.
Due to the nature of the dietary intervention, participants and study staff delivering the intervention cannot be blinded. To minimize bias, however, outcome assessors and data analysts remain blinded. Research personnel conducting physical assessments and blood draws are not informed of participants’ group assignments, and the study biostatistician remains blinded to group allocation until completion of all primary analyses. This approach ensures objective outcome assessment and unbiased statistical interpretation.
Unblinding is not anticipated. However, if required for safety or protocol-related reasons, it is approved by the Principal Investigator (PI), documented, and reported to the Institutional Review Board (IRB).
Plans for assessment and collection of outcomes
Baseline and post-intervention assessments are conducted at the clinic by trained research staff using standardized protocols. These include measures of physical function, body composition via bioelectrical impedance analysis (BIA), and fasting blood biomarkers. Surveys on demographics, clinical characteristics, psychosocial factors, and quality of life are completed on-site through self-administered questionnaires in REDCap. Dietary intake is assessed using the Automated Self-Administered 24-hour (ASA24®) Dietary Assessment Tool. The first recall is conducted by the PI or research staff, and the remaining two recalls are completed by participants independently, as long as they feel comfortable using the system. If needed, study staff assist in completing the recalls. A total of three recalls—two on weekdays and one on a weekend day—are collected.
Plans for collection, laboratory evaluation and storage of biological specimens for genetic or molecular analysis in this trial/future.
Blood samples are collected at baseline and post-intervention to assess nutritional and inflammatory biomarkers. Samples are processed by Biorepository Core laboratory staff (centrifuged and aliquoted) and stored at −80°C. Inflammatory biomarker analyses are conducted at the University of Maryland, and nutritional analyses at Tufts University, following standardized laboratory protocols. All specimens are labeled with study ID codes and contain no direct personal identifiers. With participant consent, de-identified remaining samples may be stored for future research related to diet, aging, and long COVID, subject to IRB approval. No genetic or genomic analyses are planned for this study.
Study data are managed in REDCap with built-in range and logic checks to ensure data quality. Personal identifiers are stored separately from study data on secure, password-protected servers with restricted access. Paper records are maintained in locked cabinets, and all biospecimens are labeled with unique study IDs. Data from questionnaires, body composition assessments, and biospecimen analyses are merged using a predefined coding system. Statistical analyses are performed in SAS on secure University of Maryland servers with daily backups and a 10-year data retention policy. Only de-identified, aggregated results are reported, in full compliance with HIPAA and NIH data security standards.
Primary and Secondary Outcomes
Analyses will follow the intention-to-treat (ITT) principle, with additional per-protocol analyses including participants who attend ≥80% of sessions. Statistical significance will be set at p < 0.05 (two-tailed). Descriptive statistics will summarize baseline characteristics. Between-group differences will be tested using t-tests or Chi-square tests, and non-parametric alternatives as appropriate. Multivariable linear regression will evaluate intervention effects on changes in fatigue, adjusting for baseline fatigue, age, sex, race/ethnicity, and relevant confounders (e.g., sleep quality, emotional distress). Similar models will be used for secondary outcomes such as grip strength and physical function. For exploratory aims, regression models will examine whether changes in inflammatory biomarkers (e.g., IL-6) mediate the effect of the intervention on fatigue, including an interaction term (intervention × ΔIL-6). Covariate selection will follow the rule of one variable per 10–20 participants to prevent overfitting.
Interim Analyses
No interim analyses are planned. To maintain data integrity, outcomes will be analyzed only after completion of all final assessments. Safety and adherence will be monitored continuously by the PI and reported to the IRB as required.
Additional Analyses
Subgroup analyses will be performed by race/ethnicity and BMI. Longitudinal intervention effects will be assessed using mixed-effects models on repeated monthly measures.
Handling of Non-Adherence and Missing Data
Primary analyses will follow the ITT approach. Per-protocol analyses will include participants with ≥80% session attendance. Missing data will be handled using multiple imputation under the assumption of data missing at random to reduce bias.
Plans to give access to the full protocol, participant level-data and statistical code
The full study protocol, anonymized participant-level dataset, and statistical code will be available upon reasonable request to the corresponding author, subject to institutional and ethical approvals. Direct access for third-party organizations is not planned.
Dr. Galya Bigman (Principal Investigator) provides overall scientific leadership and oversight of the study, including trial design, implementation, data monitoring, IRB compliance, and protocol updates at the University of Maryland sites. Dr. Alice S. Ryan (Primary Mentor) advises on trial design and implementation, assists with data collection oversight, and supports IRB-related processes. Dr. Brock A. Beamer (Co-Mentor) contributes to refining eligibility criteria, recruitment procedures, participant enrollment, and human subjects protection. Dr. John D. Sorkin (Co-Mentor and Study Biostatistician) develops research questions, analysis plans, and sample size calculations, conducts longitudinal analyses, and provides ongoing input on data management. Dr. Kyla M. Shea (Collaborator, Tufts University) oversees nutritional biomarker measurement, ensures data quality, and supports laboratory analyses.
Composition of the Data Monitoring Committee, Its Role, and Reporting Structure
Participant safety is overseen by an Internal Safety Monitoring Board (ISMB) at the University of Maryland School of Medicine, which includes independent experts and meets semi-annually or as needed. The ISMB operates independently from the study sponsor and funder (NIA) and follows procedures outlined in the study’s Data and Safety Monitoring Plan (DSMP), available upon request. The Principal Investigator (PI) and mentors provide ongoing safety oversight and reporting. The DSMP, required by the UMB IRB for studies involving greater than minimal risk, guides all safety monitoring and reporting procedures.
Frequency and Plans for Auditing Trial Conduct
Formal external auditing is not planned. Trial conduct is overseen by the Principal Investigator (PI) and study team through regular meetings, internal monitoring of data quality and protocol adherence, and periodic review by the University of Maryland IRB. Any protocol deviations or adverse events are reported in accordance with IRB requirements.
All protocol amendments are reviewed and approved by the University of Maryland IRB prior to implementation. Updates are also submitted to ClinicalTrials.gov. If modifications substantially alter study procedures, participants are informed promptly, and re-consent is obtained by study staff as needed.
This study was approved by the University of Maryland, Baltimore Institutional Review Board (IRB; HP-00106745). All procedures will be conducted in accordance with the ethical standards of the University of Maryland, the Declaration of Helsinki, and relevant national regulations.
Any important protocol modifications—such as changes to eligibility criteria, outcomes, or study procedures—will be submitted to the University of Maryland, Baltimore IRB for approval prior to implementation. Approved amendments will be updated on ClinicalTrials.gov and communicated to all relevant parties, including study personnel and, when applicable, participants.
During the initial appointment, trained study staff review the study details with each potential participant using clear, understandable language. The discussion includes the study purpose, procedures, potential risks, and participant responsibilities. Once eligibility is confirmed and full understanding is demonstrated, both the participant and a study team member sign the informed consent form, either on paper or via secure electronic signature in REDCap. A signed copy is provided to each participant for their records.
Additional Consent Provisions for Use of Data and Specimens
The main informed consent form includes language allowing data and, where applicable, biological samples collected during the study to be stored and used for future research on diet, aging, and long COVID.
Participant confidentiality is maintained in accordance with HIPAA regulations. Unique barcodes link specimens and data without direct identifiers. Identifiable information is stored separately on secure, password-protected University of Maryland servers with encrypted transfers and restricted access. Paper records are kept in locked cabinets, accessible only to authorized personnel. All study staff complete HIPAA and IRB training. Data shared for NIH reporting or collaborations are fully de-identified.
Participants do not receive ongoing care or supplements after study completion. Study-provided supplements are discontinued at 16 weeks. Participants who wish to continue supplementation are advised to consult their healthcare providers. Any adverse findings identified during the study are referred to appropriate clinical services. Participants are allowed continued access to study materials through the study website if they wish to do so.
The Long-COVIDiet study is designed to ensure methodological rigor and reproducibility. All participants meet consistent inclusion and exclusion criteria, enhancing the reliability of findings and minimizing participant risk. Standardized procedures are implemented for all assessments, with detailed protocols for administering screening instruments and collecting data in a consistent order across visits. To promote adherence, make-up sessions are offered as needed. Nutritional biomarkers are used to validate self-reported dietary intake. The study design incorporates randomization, standardized eligibility criteria, and statistical control for confounding factors to ensure a robust and unbiased evaluation of the intervention’s effects.
The study team identifies potential challenges and corresponding mitigation strategies. If recruitment slows due to declining COVID-19 incidence, additional sites within the UMMS are engaged. If participant dropout exceeds 20%, linear mixed-effects models address missing data under the assumption of data missing at random, using repeated measures of fatigue and diet quality. Participants unable to complete the 6-MWT perform the validated 2-Minute Walk Test (2-MWT). For those unable to tolerate daily supplementation, dosing is adjusted to alternate days with an emphasis on food-based nutrient sources. If no significant between-group differences are observed, analyses explore potential influences such as adherence, subgroup effects, or nutrient-specific responses.
Successful implementation of the intervention is expected to result in high participant engagement, with at least 80% of participants completing the full 16-week program. Participants are anticipated to improve their diet quality substantially, achieving an average HEI score increase of 15 points and demonstrating at least 70% adherence to the U.S. Healthy Eating Pattern. These dietary changes are expected to correspond with meaningful reductions in fatigue, bringing symptom levels down to mild or none. Improvements in functional outcomes are anticipated as well, with participants resuming daily life activities such as walking and working. Additionally, the study aims to identify novel nutritional biomarkers linked to fatigue, muscle strength, and physical function.
Findings from this pilot study will inform the development of a larger, R01-funded trial aimed at scaling the intervention and deepening the investigation into the role of diet in long-COVID recovery and aging. Future work will examine the specific contributions of individual nutrients—such as fiber, vitamin K, and polyphenols—as components of the Whole-Diet Approach in mitigating fatigue and muscle loss. Particular attention will be given to the gut microbiome as a potential mediator of dietary effects, exploring how microbiota-derived metabolites influence inflammation, energy metabolism, and muscle function. Broader pathways linking diet, microbiome composition, fatigue, depression, sleep, muscle aging, and physical resilience will also be investigated to provide a more comprehensive understanding of how nutrition supports recovery and healthy aging.
The Long-COVIDiet study is among the first randomized trials to evaluate a structured dietary intervention in older adults with long COVID. By employing standardized procedures, validated outcome measures, and biomarker verification, the study aims to generate rigorous and reproducible evidence on the relationship between diet quality and post-viral recovery. If successful, the findings could inform evidence-based dietary guidelines and scalable public health strategies to reduce fatigue, enhance physical function, and support recovery in this high-risk population.
This study is supported by the National Institute on Aging (NIA) of the National Institutes of Health (NIH) under Award Number K01AG078545.
Principal Investigator: Dr. Galya Bigman
Institution: University of Maryland School of Medicine, Division of Gerontology, Department of Epidemiology and Public Health, Baltimore, MD 21201, USA
This study is funded through an NIA K01 career development award. The sponsor and funder have no role in study design, data collection, management, analysis, interpretation, or publication decisions.
This trial is registered at ClinicalTrials.gov under identifier NCT05977179. The initial registration was completed prior to participant enrollment, with summary results to be posted within one year of study completion.
This study protocol adheres to the SPIRIT 2025 statement (Standard Protocol Items: Recommendations for Interventional Trials). The completed SPIRIT 2025 checklist is available in the Open Science Framework (OSF) repository (DOI: 10.17605/OSF.IO/KT6BC).
The trial will be registered and updated on ClinicalTrials.gov within 21 days of enrolling the first participant, with annual updates and corrections as required. Results will be disseminated through peer-reviewed publications, scientific meetings, and presentations. Participants will receive a plain-language summary of study findings. Updates will also be posted on the study website (www.longcovidiet.com) and shared with relevant clinical and research networks
No data are associated with this article.
This is a study protocol; therefore, no participant-level data have been collected or are available at this stage. Upon completion of the trial and publication of the primary results paper, de-identified participant-level data, the data dictionary, and statistical code will be made available by the Principal Investigator upon reasonable request, subject to institutional and ethical approval. All shared data will comply with HIPAA and NIH data-sharing requirements. De-identified data will be provided under a data-use agreement and will become available approximately 12 months after publication of the primary results for a minimum of 5 years.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)