Keywords
acute leukemia, overall survival, immunophenotypes, promyelocytic leukemia, Kaplan-Meier, Peru
This article is included in the Oncology gateway.
This article is included in the Public Health and Environmental Health collection.
To determine the overall survival and immunophenotypes of patients with acute leukemia from a hospital in Chiclayo, Peru, during the period 2015-2019.
Observational research was conducted, analysing a total of 168 samples from patients diagnosed with acute leukemia, evaluating overall survival in the different types of leukemia at 5 years of follow-up using Kaplan-Meier curves.
The overall survival for B-cell Acute Lymphoblastic Leukemia (B-ALL) was found to be 52.7% at 5 years of follow-up, while for T-Acute Lymphoblastic Leukemia (T-ALL), it was 50% at 2 years. The overall survival for non-promyelocytic Acute Myeloid Leukemia (AML) was 53.3% at 3 years of follow-up, and for promyelocytic leukemia, it was 66.7% at 3 years. The predominant immunophenotype was B-ALL, which represented 77.38%, while AML represented the remaining cases.
The overall survival of lymphoid leukemia was superior to that of myeloid leukemia. The most frequent aberration in B-ALL was the hyperexpression of CD10, while the absence of antigens was noted in T-ALL. The most frequent aberration in promyelocytic leukemia was the hyperexpression of CD13, and for non-promyelocytic leukemia, asynchronisms and lineage infidelity were observed.
acute leukemia, overall survival, immunophenotypes, promyelocytic leukemia, Kaplan-Meier, Peru
Malignant diseases of the hematopoietic system are among the most common and aggressive neoplasms. In particular, acute leukemia (AL) represents a heterogeneous and genetically complex group of disorders,1 characterized by distinct clinical, morphological, immunological, and molecular features that primarily differentiate between lymphoid and myeloid leukemia.2 Flow cytometry immunophenotyping is the diagnostic method of choice for the simultaneous analysis of multiple parameters in mixed cell populations in acute leukemia.3
The most prevalent form of AL is acute lymphoblastic leukemia (ALL), which primarily affects children and young adults, with peak incidence between ages 2 and 5. Conversely, acute myeloid leukemia (AML) is the most common cancer in adults, accounting for approximately 1.3% of new cancer cases in the United States.4
According to the WHO, the GLOBOCAN 2020 report estimates a total of 474,519 new leukemia cases and 311,594 deaths worldwide. In Peru, an incidence of 2,532 new cases and 1,776 deaths (70.0%) due to leukemia was estimated.5 In the Peruvian pediatric population, 4% of neoplasms are affected, with males being more frequently affected. The age group most susceptible is 2 to 9 years old.6 In the metropolitan area of Lima, the incidence of cancer among children aged 0 to 4 years is 59.9 cases per million; in those aged 5 to 9, 32.5 cases per million; in adolescents aged 10 to 14, 31.5 cases per million; and in those aged 15 to 19, 28.4 cases per million.7
A systematic review and meta-analysis titled “Aberrant phenotypes in acute myeloid leukemia and their association with prognosis and survival” found that abnormal CD56 expression is a marker of poor prognosis and worse outcome, leading to a reduced overall survival (OS) of 28 months in AML patients.8
Another study, “Characteristics and survival of 927 Moroccan adults with AML,” indicated that the most common French American British (FAB) classification subtype was M2 (27%), followed by M1 (24.8%) and M4 (13.4%). Cytogenetic analysis revealed that t(8;21) was the most frequently detected balanced translocation, with the intermediate cytogenetic risk group being the most represented (65.4%). Median survival was 41.58 months for the favourable-risk group versus 29.07 months for the unfavourable-risk group.9
Between January 2014 and August 2017, a retrospective analysis was conducted on the spectrum and immunophenotypic profile of acute leukemia via flow cytometry in a tertiary care center in India. It was found that AML accounted for 32.1%, with recurrent genetic abnormalities. Mutations in the FLT3/NPM1c genes were present in 9.0%. The subtypes AML-M4 and M5 represented 14.7%, M6 1.8%, and M7 3.7%. Additionally, lineage infidelity antigens such as CD19 were expressed in 16.3%, and CD7 in 11.9% of cases.10
AL is a clonal hematopoietic stem cell disease characterized by an increased number of immature cells in peripheral blood or bone marrow.11 AML is a group of clonal, genetic, and phenotypically heterogeneous malignant diseases that progress rapidly. They are characterized by the clonal proliferation of immature cells, resulting in the loss of their differentiation capacity and the accumulation of dysfunctional myeloblasts.12,13
The FAB classification of AML, established in 1976, includes eight subgroups (M0 to M7), based on cell morphology and cytochemistry.14,15 In 2008, the WHO introduced a revised classification system. By the end of 2016, this classification was expanded to incorporate genetic information, morphology, immunophenotype, and clinical features, aiming for a more precise definition of AML.16
ALL remains the most prevalent childhood cancer. It arises from hematopoietic progenitors of B- or T-cell lineage.17 The disease involves excessive, uncontrolled proliferation and accumulation of lymphocytes or leukemic blasts.18
According to morphological criteria defined by the FAB group, B-ALL is classified into three subtypes: L1, L2, and L3, based on cell size, cytoplasm features, nuclear vacuolization, and basophilia. The European Group for the Immunological Classification of Leukemia (EGIL) further classifies B-ALL into four subgroups based on the expression of CD10, μ chains, and light chains.19
Despite significant advances in treatment, the etiology of acute leukemia remains largely unknown. Multiple factors, including pre-existing and acquired genetic mutations, radiation exposure, and various chemicals during preconception, pregnancy, and throughout life, have been investigated but remain inadequately explained.20–22
Flow cytometry measures many physical characteristics of cells, such as size and granularity, while in suspension. It allows for the identification of a broad range of cytoplasmic and cell surface antigens, making it a crucial tool in hematologic malignancy diagnosis.23,24
The applications of flow cytometry are extensive, encompassing virtually all hematological neoplasms, including acute leukemia, B-, T-, and NK-cell neoplasms, multiple myeloma, and other hematologic disorders. It is an invaluable tool for biomarker assessment, disease risk stratification, and case screening for molecular genetic testing.25
In the pediatric population, ALL accounts for about 80% of all cases of acute leukemia, with five-year disease-free survival rates reaching or exceeding 90% in developed countries.26 Approximately 60% of cases occur in children and adolescents under 20 years of age. ALL is broadly classified into B-ALL and T-ALL based on immunophenotyping; B-ALL constitutes approximately 85% of cases, although this ratio varies with age, ethnicity, and race.27 The five-year OS ranges from 60% to 70%, with substantial variation influenced by age at diagnosis, morphological features, cytogenetics, sex, and socioeconomic factors.28
T-ALL accounts for 10–15% of pediatric cases and up to 25% of adult cases, with a five-year OS of approximately 80% in children. However, in adults, OS rates are less than 50%, mainly due to higher treatment-related toxicity.29
Data on the incidence of acute leukemia in northern Peru are limited. Given the disease’s significant negative impact, the present study aims to provide regional data to serve as a reference and as a basis for future research. Specifically, it seeks to determine OS and immunophenotypic profiles of patients with acute leukemia treated at a hospital in Chiclayo, Peru.
A retrospective, observational, and non-interventional study was conducted.
The study population comprised a total of 200 clinical reports of patients diagnosed with acute leukemia, of both sexes and across all age groups, who were referred to the Flow Cytometry and Molecular Biology Laboratory at Almanzor Aguinaga Asenjo National Hospital in Chiclayo, Peru, for immunophenotypic analysis between January 2015 and December 2019.
The sampling was designed as a non-probabilistic census, including 168 reports of patients with confirmed diagnoses of ALL or AML, selected based on predefined inclusion and exclusion criteria as documented in the flow cytometry reports. All patients had received treatment within the study period.
Inclusion criteria: Reports of patients diagnosed with AML or ALL within the study period, with complete clinical, laboratory, and flow cytometry data available.
Exclusion criteria: Reports of patients who did not initiate chemotherapy following diagnosis or who abandoned their treatment protocol during the course of evaluation.
The methodology employed was a documentary analysis of flow cytometry immunophenotyping diagnostic reports, which served as the primary source of data. The patient’s vital status (deceased or alive) was ascertained through the database of the National Registry of Identification and Civil Status (RENIEC).
A structured data collection form was developed to include the variables of interest, facilitating the achievement of the study objectives. The data extracted from patients diagnosed with myeloid and lymphoid leukemias were entered into Microsoft Excel 2017 spreadsheets.72
Statistical analyses and graphical representations were conducted using SPSS version 25. OS estimates were calculated by the Kaplan-Meier method, a non-parametric approach. Comparative analyses of survival functions were performed using the log-rank test, 29 with a confidence level 95% and significance threshold at p < 0.05.
Table 1 summarizes the characteristics of 168 patients with acute leukemia, evaluated from January 2015 to December 2019. The median age was 20 years (range: 0–60). The cohort comprised 83 males (49.40%). Lymphoid immunophenotype was identified in 130 cases (77.4%), with a female predominance of 37.50% (63 cases). Among these, T-ALL accounted for 10 cases (5.95%), and B-ALL for 120 cases (71.43%). The myeloid immunophenotype was observed in 38 cases (22.62%), with 22 females (13.10%). Acute non-promyelocytic leukemia was the most frequent subtype within this group, comprising 35 cases (20.83%), whereas acute promyelocytic leukemia (APL) was identified in 3 cases (1.79%).
Table 2 shows the distribution of patients with acute leukemia across age groups. B-ALL patients were categorized into four age groups: ≤14 years (n = 78), with 40 (51.3%) females; 15–36 years (n = 24), with 17 (70.83%) males; 37–59 years (n = 11), with 11 (84.61%) females; and ≥60 years (n = 5), with 3 (60%) females. For T-ALL, patients were similarly stratified: ≤14 years (n = 5), with 4 (80%) males; 15–36 years (n = 4), with 3 (75%) males; 37–59 years (n = 1), a single male patient; and no cases were reported in the ≥60 years age group.
B-ALL: B cell Acute Lymphoblastic Leukemia, T-ALL: T-Acute Lymphoblastic Leukemia.
| B - ALL | T - ALL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | Female | Male | Total | Female | Male | Total | ||||
| n | % | n | % | n | % | n | % | |||
| ≤14 | 40 | 51.28 | 38 | 48.72 | 78 | 1 | 20 | 4 | 80 | 5 |
| 15-36 | 7 | 29.17 | 17 | 70.83 | 24 | 1 | 25 | 3 | 75 | 4 |
| 37-59 | 11 | 84.62 | 2 | 15.38 | 13 | - | - | 1 | 100 | 1 |
| ≥60 | 3 | 60.00 | 2 | 40.00 | 5 | - | - | - | - | - |
| TOTAL | 61 | 50.83 | 59 | 49.17 | 120 | 2 | 20 | 8 | 80 | 10 |
Table 3 shows the distribution of patients diagnosed with promyelocytic AML (n = 3) and non-promyelocytic AML (n = 35). It was observed that 100% of patients with AML under 14 years of age were female. Similarly, 100% of patients in the 19 to 59-year age group were female. No patients over 60 years of age presented with this type of leukemia. Among patients with non-promyelocytic AML, 13 were under 14 years old, with 8 (61.54%) males. In the 19 to 59-year age group, there were 20 patients, of whom 12 (60%) were females. Patients aged 60 years and older were all females.
| PROMYELOCYTIC AML | NON - PROMYELOCYTIC AML | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | Female | Male | TOTAL | Female | Male | TOTAL | ||||
| n | % | n | % | n | % | n | % | |||
| ≤14 | 1 | 100.0 | - | - | 1 | 5 | 38.46 | 8 | 61.54 | 13 |
| 19-59 | 2 | 100.0 | - | - | 2 | 12 | 60.00 | 8 | 40 | 20 |
| ≥60 | - | - | - | - | - | 2 | 100.00 | - | - | 2 |
| TOTAL | 3 | 100.0 | - | - | 3 | 19 | 54.29 | 16 | 45.71 | 35 |
Table 4 presents the main aberrant immunophenotypic profiles observed in 120 cases of B-ALL. Notably, the most frequent phenotypic abnormalities included line infidelity with CD13+ (31.67%), CD33+ (15.83%), CD2+ (2.50%), CD15+ (2.50%), and CD117+ (0.83%). The absence of expression was observed for CD20- (28.33%), CD38- (9.17%), CD45- (5.83%), CD22- (3.33%), and HLA-DR- (0.83%). Hyperexpression of certain markers was also noted, with CD10++ in 64.17%, HLA-DR++ in 44.17%, CD19++ in 34.17%, and CD34++ in 20%. Additionally, cases of asynchronous expression included CD19+CD22+CD34+ (11.67%), CD19+CD22+CD20- (7.50%), CD20+TdT+ (3.33%), and CD34+CD20+ (1.67%). Similarly, in 10 cases of T-ALL, the main phenotypic features included line infidelity with CD117+ and CD13+ (both 10%), and absence of expression was observed for CD3- (50%), CD8- (30%), CD4- and CD2- (both 20%), and CD5- (10%).
CD: Cluster of Differentiation, HLA: Human Leukocyte Antigen.
Table 5 displays the main aberrant immunophenotypic of promyelocytic acute myeloid leukemias, consisting of 3 cases: line infidelity included CD56+ and CD2+ (both 33.3%), and hyperexpression of CD13+++ (66.7%). For non-promyelocytic myeloid leukemias (35 cases), aberrant features included lineage infidelity with CD19+ (20%), CD7+ (9%), CD10+ (3%) and CD56+ (6%). Asynchronous expression patterns involved CD34+CD33++ (20%), CD117+CD15+ and CD34+CD15+ (6%).
CD: Cluster of Differentiation.
Figure 1 displays the OS curves for all types of acute leukemias over a five-year follow-up period. For patients diagnosed with B-ALL, an estimated OS of 52.7% (n = 120) was observed at five years post-diagnosis. The OS for T-ALL was 50% (n = 10) at two years of follow-up. Additionally, non-promyelocytic acute myeloid leukemia (NP-AML) showed an OS of 54.3% (n = 35) at three years, while promyelocytic acute myeloid leukemia (P-AML) had a survival rate of 66.7% (n = 3) in the same period. The comparison among the different leukemia types did not reveal statistically significant differences (p = 0.801).
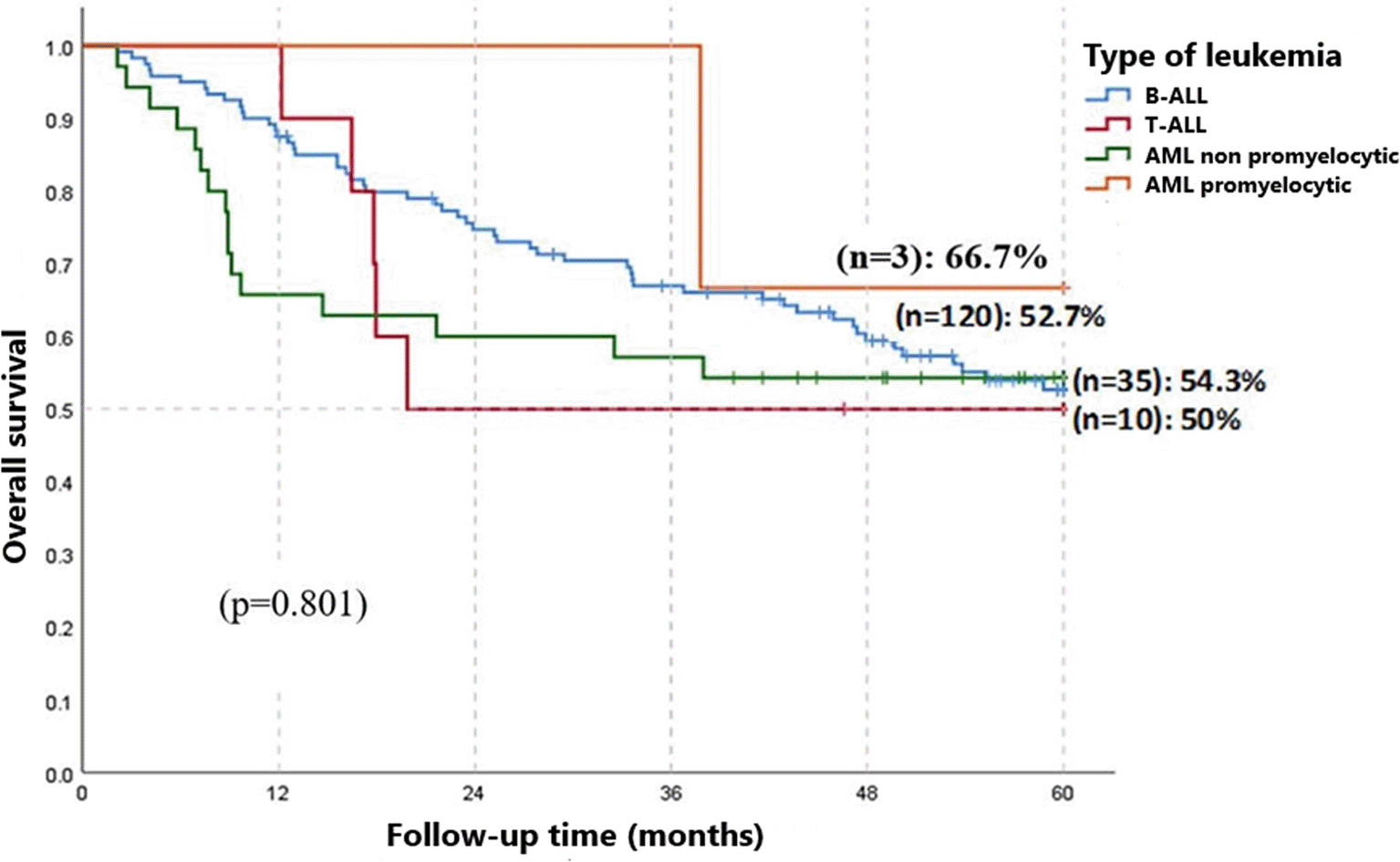
B-ALL: B cell Acute Lymphoblastic Leukemia, T-ALL: T-Acute Lymphoblastic Leukemia, AML: Acute Myeloid Leukemia.
Figure 2 categorizes patients into four age groups according to the IETSI-ESSALUD guidelines (EsSalud, 2019): 0 to 14 years, 15 to 36 years, 37 to 59 years, and over 60 years. The survival rate for patients diagnosed with B-ALL in the age group 0-14 years was 63.7% at 5 years; for those aged 15-36 years, it was 43.8% at 3 years; in the group 37-59 years, it was 30.8% at 2 years; and patients over 60 years had a 20% survival rate at 3 years. The comparison among these different age groups showed statistically significant differences (p = 0.0001).
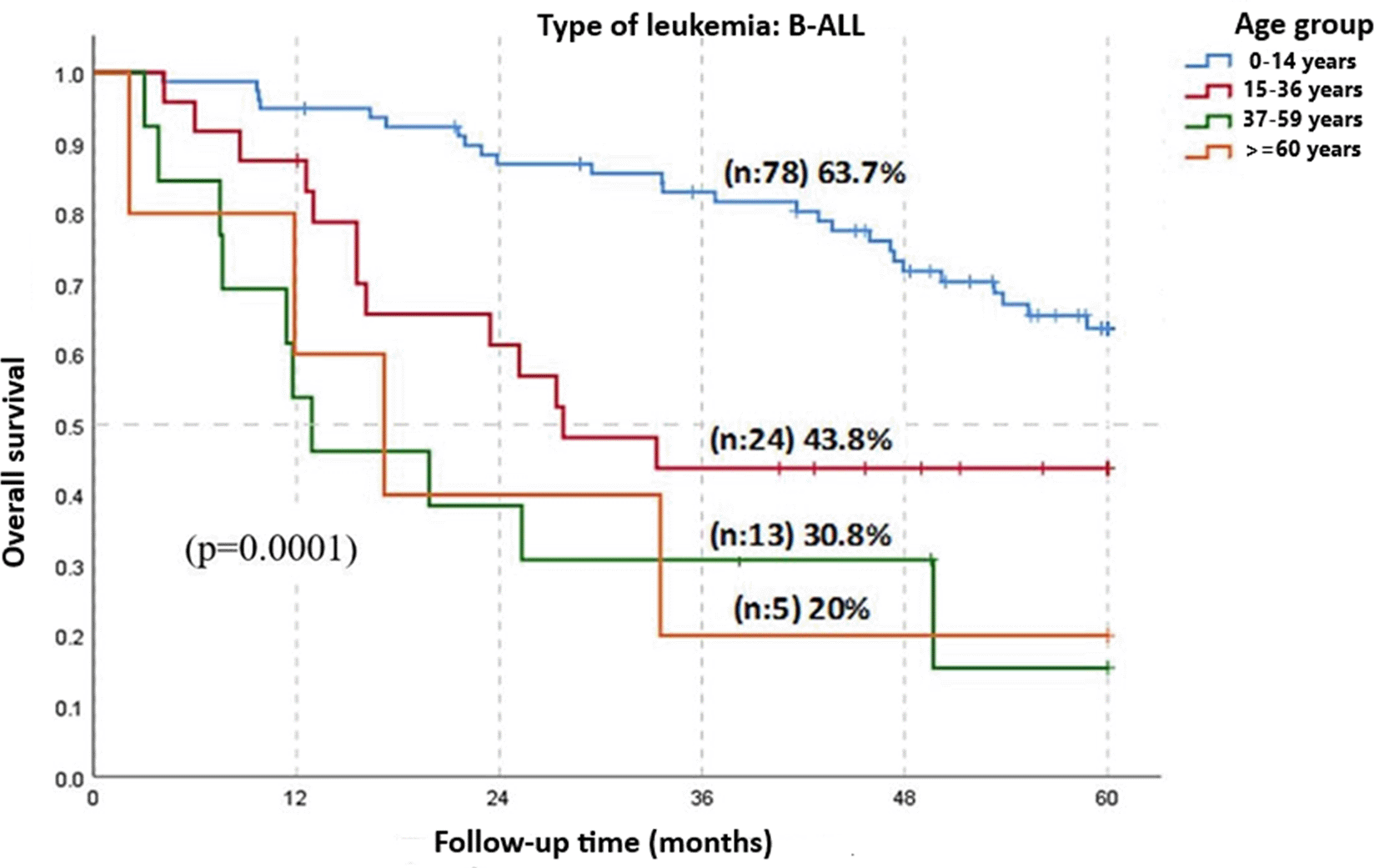
B-ALL: B cell Acute Lymphoblastic Leukemia.
Figure 3 categorizes patients diagnosed with AML into 3 age groups: 0 to 18 years, 19 to 59 years, and over 60 years, according to the guidelines of the Edgardo Rebagliati Martins National Hospital (Rebagliati, 2015). The survival rate for AML patients in the age group 0-18 was 64.3% at 3 years of follow-up; in the group 19-59 years, it was 57.1% at 3 years; and patients older than 60 years had a 33.3% OS at 7 months of follow-up. The comparison among these age groups showed statistically significant differences (p = 0.005).
Immunophenotyping provides important information for the diagnosis, classification, and monitoring of hematologic neoplasms and is essential for the identification, enumeration, and characterization of leukemic cells. In the conducted study, 168 patients with acute leukemias were reported; in 85 cases, females predominated. The most common immunophenotype was lymphoid lineage, where B-ALL predominated. Among myeloid leukemias, non-promyelocytic AML was predominant. Compared to results reported previously,30 where 116 patients were evaluated, B-ALL represented 54.31%, slightly lower than the current study’s findings, while T-ALL 4.31%, AML was expressed in 41.38%, higher than our findings, with non-promyelocytic AML dominating at 33.62%. These results vary depending on the number of patients in each study, socio-demographic characteristics, age, sex, race, clinical antecedents, laboratory studies analysing morphology, cytochemistry, immunophenotype, cytogenetics, medical attention, and appropriate management of the disease in hospital centres. According to the age group, in the study, patients with B lymphoid lineage under 14 years old were 78 patients, mainly females, with 40 cases. These results differ from those previously reported,31 where 47 patients were evaluated, with males predominating at 26 (55.32%).
Patients aged 15 to 36 years numbered 24, primarily males. Patients between 37 and 59 years were 13, with females predominating at 11 (84.61%). These results differ from those reported previously,32 where 29 patients were evaluated, of whom 16 (55.2%) were males and prevailed. Lastly, there were 5 patients over 60 years of age, with females predominating at 3 (60%). These results are consistent with those reported,32 where 15 patients were analysed, with females predominating at 12 (80%).
This distribution can be explained by the fact that B-ALL is the most common malignant neoplasm in children, accounting for 80% of all acute leukemias in childhood and 25% and 19% in those under 15 and under 19 years old, respectively.33 ALL has a bimodal age distribution, with peak incidences in children aged 2-5 years and in adults over 40 years, representing a devastating disease when it occurs in adults.34 Despite a growing number of studies, its aetiology remains not fully understood; the complex interaction between genetic predispositions (whether inherited or acquired somatically during fetal development) and postnatal environmental triggers has become a key focus of research.35
Patients with T-lymphoid lineage in T-ALL were 10, 5 were those under 14 years old, with a male predominance of 4 (80%). These results are similar to those previously reported,36 where 20 T-ALL patients were studied, with a predominance of 14 (70%) male patients. Patients aged 15 to 36 years numbered 4, with males again prevailing at 3 (75%), which differs from the findings previously reported,37 where 11 patients were evaluated and reported only 1 (9.1%) male patient. For the age group 37 to 59 years, there was one male patient (100%), consistent with the previously study,37 who reported 3 (27.3%) male patients in that age range. This is because the highest incidence of T-ALL occurs in adolescents and young adults aged 15 to 39 years male, with the overall incidence and male prevalence beginning to decline after age 40.38–40
In the case of promyelocytic AML, 3 patients were registered: one was under 14 years old, and two were between 19 and 59 years old, and there were no cases of patients over 60 years. This is consistent with the fact that in pediatric patients, the diagnosis typically occurs at an average age of 9 to 12 years.41 The disease usually manifests starting from the second decade of life and is rarely diagnosed after age 60. Conversely, in Latin America, promyelocytic AML affects young adults between 18 and 40 years, with a mean age at diagnosis of 34 years. It occurs equally among men and women, and only about 5% of cases are in patients over 60 years.42
In this study, 35 cases of non-promyelocytic AML were analysed. Of these, 13 patients were aged ≤14 years, predominantly males, with 8 (61.53%). These results are similar to those previously reported,43 who examined 219 cases, of which 140 (63.9%) were men. For patients aged 19 to 59 years, 20 cases were analysed, with females prevailing at 12 (60%). These findings differ from those previously reported,44 where studied 520 patients and found a higher prevalence among males, accounting for 52.9%. Lastly, among patients aged ≥60 years, 2 females were analysed (100%). These results differ from those previously reported,45 who analysed a total of 65 cases, with a male predominance at 43 (66%). This is evidenced by the fact that age is a very important prognostic factor and the prognosis worsens with increasing age. The incidence of AML gradually increases with age, representing 15% of childhood leukemias and about 33% of leukemias in adolescents and young adults. The high incidence of AML in older age groups, especially those over 60, is associated with prolonged exposure to environmental carcinogens and the accumulation of mutations due to genetic errors transmitted during cell division.46
Survival in ALL is predominantly observed in pediatric patients, 75% of cases occurring in children under 6 years old. The incidence shows a bimodal distribution after age 60, with improvements in survival in children under 15 reaching up to 90%, and in adolescents and young adults, increasing from 70% to 80% through the use of intensive combination chemotherapy regimens. However, in older adults, these regimens are often not tolerated, resulting in survival rates of approximately 20% or less. Poor outcomes in adults have been partly attributed to a high frequency of unfavourable genetic subtypes, pre-existing comorbidities, and limited tolerance to intensive treatment.47,48 Meanwhile, OS in T-ALL from previous studies49 has ranged between 30% and 60%. Being a highly aggressive disease, it accounts for approximately 15% to 25% of all ALL cases in children and adults.50
In this study, the 5-year OS for patients with B-ALL (n = 120) was 54.3%, and the 2-year OS for patients with T-ALL (n = 10) was 50%. These results are consistent with those previously reported,51 where was observed a 5-year OS of 45% in patients with B-ALL (n = 60), and 20% in those with T-ALL (n = 2). Contrasting also with a previously study,52 where was reported a 1-year OS rate of 35%, dropping to zero at 5 years. The higher survival rates observed in this research compared to other studies can be attributed to the availability of a flow cytometry laboratory that enables rapid determination of leukemia immunophenotype, as well as access to molecular and cytogenetic tests for risk stratification.
The 3-year OS in acute promyelocytic leukemia (n = 3) was 66.7%. These results contrast with those previously reported,53 where was documented a 2-year OS of 67% (n = 56). There is a trend toward improved survival, primarily due to lower early mortality and the promptness of treatment administered to patients. The development of targeted therapies with all-trans-retinoic acid and arsenic trioxide has transformed promyelocytic leukemia from a frequently lethal disease into a many-times curable condition,54 reaching a long-term survival of up to 80-90%.
The 3-year OS in non-promyelocytic AML (n = 35) was 54.3%. These findings differ from those previously reported,55 where was observed a 3-year OS of 31% (n = 63). By analysing other clinical and laboratory parameters, including age, hemogram at admission, peripheral blood blast rate, marrow blasts rate, and cytogenetic data of the patient, it is possible to classify into risk groups and thus achieve greater accuracy of OS in this type of leukemia. Although the OS was higher in this study, more variables should be considered. Nonetheless, AML is the most common leukemia in adults,56 representing around 80%, with an OS of less than 50% after 5 years in patients under 45 years, and less than 5% in patients over 65. In children, AML represents 15% to 20% of cases,57 with 5-year OS ranging from 60% to 70%.
The 5-year OS in the age group of patients with B-ALL (n = 78) aged 0–14 years was 63.7%. These results contrast with those previously reported,58 where was found a 5-year OS of 32.5% (n = 348); that reported in another study,31 with a 6-year OS of 46.9% (n = 47); and in another,59 where was estimated a 5-year OS of 85% (n = 7,247). The present study was conducted in a developing country and showed an OS of 63.7%, which is closer to the survival rates observed in other developing countries. This survival rate should encourage further research into this disease. Studies of children and adolescents with B-ALL in developed countries60 have shown survival rates above 90%. Meanwhile, in developing countries,61 the survival rate is around 40%.
In the age group of patients with B-ALL (n = 24) aged 15–36 years, the 3-year OS was 43.8%. This is in comparison to a previously study,62 where was reported a 2-year OS of 17% (n = 15). The difference is attributable to the fact that we are comparing with a study carried out in Africa, where resources are limited in terms of infrastructure, patient care, and access to newer and less toxic therapies. To improve this situation, targeted interventions are needed. This group includes adolescents and young adults and has a poor prognosis, with survival rates ranging from 30% to 45%, mainly due to the higher prevalence of cytogenetic alterations associated with worse prognosis of OS in this population.62
The OS of patients with B-ALL (n = 13) aged 37 to 59 years was 30.8% over 4 years of follow-up. These results differ from those previously reported,63 where a 5-year OS of 24% was documented. These findings suggest that the marked improvement in OS is mainly due to the introduction of pediatric chemotherapy in adult patients, obtaining favourable outcomes.
The 3-year OS in the age group of patients with B-ALL (n = 5) over 60 years was 20%. This contrasts with the findings in a previous study,64 where was reported a 3-year OS of 47.3% (n = 93). The contrast of both studies is in the number of patients, and also that we are comparing the results with a study conducted in a developed country where access to chemotherapy employs modified paediatric-inspired protocols, and allogeneic hematopoietic stem cell transplantation is also an important option in the treatment of this group of patients. Older adults over 60 years have the worst 5-year OS,49,65 approximately 24%. They do not respond as well to chemotherapy as younger patients and tend to die earlier during the disease course. Therefore, there is an urgent need for less intensive but more effective treatment strategies tailored for this high-risk, unstable, and vulnerable population.66
The 3-year OS in patients with AML aged 0 to 18 years (n = 14) was 64.3%. This contrasts with a previous study,67 where was reported a 36% OS at 1 year and 8 months of follow-up, and with another study68 where was documented a 5-year OS of 24.6% (n = 130). The comparison with these investigations gives us an overview in a general way that we must improve in the treatment of patients with this disease. The findings from India,67 where survival is low, differ from our longer follow-up. On the other hand,68 was achieved a longer OS period, likely due to differing protocols aimed at controlling disease proliferation. It is noteworthy that AML accounts for approximately 15–20% of childhood acute leukemias, with cure rates ranging between 60% and 70%, though these are generally lower in developing countries.57
For patients aged 19–59 years, the 3-year OS with AML (n = 21) was 57.1%. These findings differ from previous studies,12 where were reported a 5-year OS of 17.85% (n = 153), and another study69 where was observed a 2-year OS of 34.37% (n = 32). These results reflect the disease’s behaviour in this demographic and underscore the importance of appropriate treatment strategies to improve long-term prognosis.
In patients over 60 years with AML (n = 3), the OS was 33.3% at 7 months of follow-up. This contrasts with the OS of 12.14% (n = 55) at 5 years of follow-up, reported in a previously study.12 In addition, in another study69 was reported a 1-year OS of 22.8% (n = 11), which also differs from our findings. Increasing age is a bad prognostic factor for complete disease remission.70 Independently of treatment, the results are unsatisfactory, with an OS of 10% around 5 years.71 Despite numerous attempts to find effective and easily assessable treatments, treatment for older adults has not seen much progress in recent decades.
In summary, the 5-year OS for B-ALL was 52.7%, and for T-ALL it was 50% at 2 years. The 3-year OS for non-promyelocytic AML was 53.3%, and for promyelocytic AML was 66.7%. For B-ALL patients, in terms of age groups, the 0–14-year had a 63.7% OS at 5 years; those aged 15–36 years had a 43.8% at 3 years; patients aged 37–59 years, 30.8% at 2 years; and patients over 60, 20% at 3 years. OS in AML 0–18-years was 64.3% at 3 years, 57.1% in the 19–59 years group at 3 years, and for those older than 60 years was 33.3% at 7 months. B-ALL accounted for 92.3% of lymphoid leukemias, with aberrant expression of CD13+ in one-third of cases. T-ALL represented 7.6% of lymphoid leukemias, with aberrant markers including CD117+ and CD13+. Promyelocytic leukemia accounted for 7.89% of AML, with aberrant expression of CD56, CD2 in one third of the cases, and hyperexpression of the CD13+++ marker in more than half. Non-promyelocytic AML represented the highest percentage of AML, expressing CD19+ as a lineage infidelity marker.
It is concluded that the overall survival of lymphoid leukemias was superior to that of myeloid leukemias. The most frequent aberration in B-ALL was CD10 hyperexpression, whereas in T-ALL, the absence of antigens was common. The most frequent abnormality in promyelocytic leukemia was hyperexpression of CD13, while in non-promyelocytic AML, asynchrony and lineage infidelity were characteristic.
In conclusion, the overall survival of lymphoid leukemias was superior to that of myeloid leukemias. The most frequent aberration in B-ALL was hyperexpression of CD10, whereas the absence of antigens was most common in T-ALL. In promyelocytic leukemia, the predominant abnormality was hyperexpression of CD13, while in non-promyelocytic leukemias, asynchrony and lineage infidelity were the most characteristic findings.
We confirm adherence to the Journal’s ethical publication standards. The study was approved by the Research Committee of the Hospital Almanzor Aguinaga Asenjo (NOTE 093-IEAI-GRALA -JAV-ESSALUD 2020). As the study involved retrospective analysis, the consent of the participants was waived off from the institutional committee.
The data that support the findings of this study are available in Zenodo Research Data (Dataset_studio).
Data repository: https://doi.org/10.5281/zenodo.17971441.72
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
This work has been reported in line with the STROBE guidelines. The STROBE checklist has been uploaded to zenodo and is available at https://doi.org/10.5281/zenodo.17971441.72
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We are very grateful to the Almanzor Aguinaga Asenjo National Hospital for providing us with the necessary facilities.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Hemato-oncology, immunophenotyping, flow-cytometry
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 06 Jan 26 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)