Keywords
Curcuma longa, Methamphetamine, Histomorphometric parameters, Reticular fibers, Seminiferous tubules, Antioxidants, Male infertility
This study evaluated the effects of Curcuma longa on histomorphometric parameters and reticular fiber expression in the testes of methamphetamine-exposed Wistar rats.
Twenty-five adult male rats were divided into five groups: Group 1 (Normal Control) received distilled water; Group 2 (Methamphetamine-Only) was administered 5 mg/kg b.w. methamphetamine; Group 3 received methamphetamine alongside a low dose of Curcuma longa extract (100 mg/kg b.w.); Group 4 was given methamphetamine with a medium dose of Curcuma longa extract (200 mg/kg b.w.); and Group 5 received methamphetamine and a high dose of Curcuma longa extract (400 mg/kg b.w.), all treatments were administered for 28 days. Histomorphometric analysis (seminiferous tubular diameter, germinal epithelial height) was conducted using ImageJ software, while reticular fibers were visualized via Gomori’s methenamine silver staining.
Methamphetamine exposure significantly increased reticular fiber density around seminiferous tubules and dilated tubular diameter compared to other groups (p < 0.05). Curcuma longa treatment dose-dependently reversed these alterations, with the High Dose Curcuma longa treated Group (400 mg/kg) showing reduced reticular fiber expression and restored tubular diameter (247.35 ± 4.40 μm), comparable to the normal control group. Germinal epithelial heights remained statistically unchanged across groups, suggesting shorter intervention periods may limit observable epithelial recovery. Tissue anti-oxidant analysis revealed a significant increase (p < 0.05) in superoxide dismutase (SOD), catalase (CAT), malondialdehyde (MDA), and glutathione peroxidase (GPx) levels in the methamphetamine group compared to the control, whereas treatment groups showed significant reductions (p< 0.05) compared to the methamphetamine group. The ameliorative effects of Curcuma longa are attributed to curcumin’s antioxidant and anti-apoptotic properties, which mitigate oxidative stress and extracellular matrix remodeling induced by methamphetamine.
Curcuma longa attenuates methamphetamine-induced testicular damage, highlighting its potential as a therapeutic adjunct for drug-associated male infertility. Further clinical studies are warranted to validate these findings in human populations.
Curcuma longa, Methamphetamine, Histomorphometric parameters, Reticular fibers, Seminiferous tubules, Antioxidants, Male infertility
The escalating global prevalence of substance dependence and abuse, particularly among adolescents and young adults at reproductive age, poses a significant threat to male fertility.1,2 Methamphetamine (METH), a potent psychostimulant with addictive and neurotoxic properties, ranks among the most abused drugs worldwide, contributing to rising rates of male infertility.3,4 In Nigeria, its abuse under local monikers like mkpuru mmiri reflects a growing public health crisis, with detrimental socioeconomic and reproductive consequences.5 Studies indicate that chronic methamphetamine use disrupts male reproductive function through oxidative stress, hormonal imbalances, and structural damage to testicular tissue, leading to reduced sperm count, motility, and viability.6,7 These alterations, including apoptosis in seminiferous tubules and impaired spermatogenesis, underscore the urgent need for interventions to mitigate drug-induced infertility.8
The resurgence of herbal medicine as a viable alternative to conventional treatments aligns with the World Health Organization’s advocacy for integrating plant-based therapies into primary healthcare.9,10 In recent times, studies have found herbal remedies useful in ameliorating infertility caused by exposure to cadmium.11,12 Antimalarial treatment using herbal remedies such as Mangifera indica has also proven a better remedy as aftermath of reproductive disorder wasn’t associated with malaria treatment using this herb.13 Even in other aspect of physiological systems, herbs have shown notable result in improving health. Ameliorating ulcer and liver disorders has been associated to ginger extract14,15 and locally prepared Tom brown weaning meal.16 Medicinal plants like Curcuma longa (turmeric), renowned for their antioxidant and anti-inflammatory properties, offer promising avenues for addressing oxidative stress-related pathologies, including reproductive dysfunction.17 Turmeric’s active compound, curcumin, demonstrates potent antioxidant activity and has been shown to enhance sperm function in vitro, improving motility and fertilization capacity.18 Despite its safety profile and therapeutic potential, its application in mitigating drug-induced testicular damage remains underexplored, particularly in the context of methamphetamine exposure.
Methamphetamine-induced oxidative stress disrupts the redox balance in gonadal tissues, overwhelming endogenous antioxidants like superoxide dismutase (SOD) and glutathione peroxidase (GPx), and triggering lipid peroxidation (LPO) and cellular damage.19,20 This oxidative milieu compromises sperm quality and testicular architecture, with histopathological changes such as reticular fiber proliferation around seminiferous tubules further impairing spermatogenesis.21 Histo-morphometric analysis of testicular tissue, including parameters like tubular diameter and epithelial height, alongside reticular fiber density, provides critical insights into structural and functional alterations caused by toxicants.22 Curcuma longa has been shown to counteract the neurotoxic effects of METH and other drug stimulants on different parts of the brain, by aiding autophagy in the cells, modulation of inflammatory cytokines, maintenance of ion homeostasis, epigenetic regulation, enhancement of antioxidant capacity, as well as the activation of the cAMP response element-binding protein (CREB) and brain-derived neurotrophic factor (BDNF) signaling pathways.23,24 Given that oxidative stress and inflammation are common underlying mechanisms of METH-induced toxicity in both brain and reproductive tissues,25,26 and considering the protective effects of Curcuma longa in the brain, it is plausible that Curcuma longa may also mitigate METH-induced oxidative stress and damage in testicular tissues, warranting further investigation in this area. Again, Curcumin, a polyphenol compound which is sourced from Curcuma longa has been found to be a good remedy for treating testicular cancer.27 Hence, there is possibility of Curcuma ameliorating any detrimental effect that may emanate from the use of Methamphetamine on the testis. Hence, the purpose of this research.
In this study, we investigate the protective effects of Curcuma longa on histo-morphometric parameters, reticular fiber expression and level of antioxidant markers in the testicular tissue of methamphetamine-exposed Wistar rats treated with graded doses of turmeric. By evaluating its capacity to counteract oxidative damage and restore testicular architecture, this research aims to advance the development of accessible herbal interventions for drug-induced male infertility.
Fresh rhizomes of Curcuma longa were purchased from Watt Market, Calabar, Cross River State, Nigeria. The plant was authenticated by Mr. Effa Effa A, a taxonomist at the Department of Botany, University of Calabar, and assigned a voucher number (Bot/Herb/UCC/318). The rhizomes were washed, air-dried at room temperature, and pulverized into fine powder using a motorized blender. A cold maceration method was employed for extraction: 1500 g of powdered material was soaked in 4.5 L of absolute ethanol for 48 hours with periodic agitation. The mixture was filtered first with muslin cloth and then through Whatman No. 1 filter paper. The filtrate was concentrated using a water bath (35–40°C) to obtain a crude extract (127.9 g), which was stored at 4°C until use.
The crystal methamphetamine used was procured with the necessary permits and approvals from the Nigeria Drug Law Enforcement Agency (NDLEA) Calabar command Cross River State, ensuring compliance with national laws and regulations governing the use of controlled substances in scientific research.
Twenty-five (25) adult male Wistar rats (150–200 g) were procured from the Animal Facility of the College of Medical Sciences, University of Calabar. Animal handling was done according to ARRIVE guidelines. The rats were housed in ventilated plastic cages under standard laboratory conditions (25°C, 50–60% humidity, 12/12-hour light-dark cycle) with ad libitum access to feed and water. Bedding material (sawdust) was replaced daily to maintain hygiene. Ethical approval (320ANA2924) was obtained from the Faculty Animal Research Ethics Committee (FAREC-FBMS), University of Calabar, in compliance with institutional and national guidelines.
The median lethal dose (LD50) of methamphetamine was determined using Lorke’s three-phase method. In the initial phase, doses of 0.05, 0.15, 0.25, and 0.50 mg/kg were administered to four groups (n = 3 rats/group). Subsequent phases tested higher doses (0.75–50.00 mg/kg). No mortality or acute toxicity (e.g., hyperactivity, defecation) was observed. The LD50 was calculated as the geometric mean of the highest non-lethal dose (10.00 mg/kg) and the lowest lethal dose (20.00 mg/kg).
After a 14-day acclimatization period, rats were randomly divided into five groups (n = 5/group) through balloting:
− Group 1 (Normal Control): Received distilled water.
− Group 2 (Methamphetamine-Only): Administered methamphetamine (5 mg/kg b.w. orally).
− Groups 3–5 (Meth + C. longa): Received methamphetamine (5 mg/kg b.w.) alongside graded doses of C. longa extract (100, 200, and 400 mg/kg b.w., respectively).
Treatments were administered daily for 28 days orally using orogastric tubes.
After fasting overnight, rats were humanely euthanized under deep anesthesia induced by intraperitoneal administration of ketamine (dose: 200 mg/kg body weight). Death was confirmed by cervical dislocation and absence of vital signs (respiratory arrest, loss of corneal reflex, and absence of heartbeat). Blood was collected via cardiac puncture and centrifuged (3000 rpm, 10 min) to separate serum, which was stored at −20°C. Testes were excised, weighed, and fixed in 10% buffered formalin for histological processing.
Testicular tissues were dehydrated through a graded ethanol series, embedded in paraffin, and sectioned (5 μm) using a rotary microtome. Sections were stained with hematoxylin and eosin (H&E) using a standard protocol. Photomicrographs with calibrated scales were captured using a light microscope equipped with a digital camera. Images were analyzed using ImageJ software (NIH, USA) to measure seminiferous tubule diameter and germinal epithelial height.
Reticular fibers were visualized using Gomori’s methenamine silver stain (Sigma-Aldrich) according to the manufacturer’s instructions. Deparaffinized sections were oxidized in 0.5% periodic acid (15 min), incubated in methenamine silver solution (60°C, 1 h), toned with gold chloride (0.2% solution, 1 min), and counterstained with nuclear fast red (5 min). Reticular fibers appeared black against a pink background. Sections were examined under a light microscope and photomicrographs were captured using a digital camera.
Superoxide Dismutase (SOD) activity was determined by its ability to inhibit the auto-oxidation of epinephrine, measured by the increase in absorbance at 480 nm.28 The reaction mixture contained 2.95 ml of 0.05 M sodium carbonate buffer (pH 10.2), 0.02 ml of the sample, and 0.03 ml of epinephrine, with hydrochloric acid used to initiate the reaction. Enzyme activity was calculated by measuring the change in absorbance at 480 nm for 5 minutes. Catalase activity was determined by measuring the decrease in absorbance at 620 nm due to H2O2 decomposition.29 The reaction mixture included 1.0 ml of 0.01 M phosphate buffer (pH 7.0), 0.1 ml of the sample, and 0.4 ml of 2 M H2O2, and was stopped with dichromate-acetic acid reagent. Lipid peroxidation was assessed via malondialdehyde (MDA) levels using the method of Buege and Aust (1978), with absorbance read at 532 nm. Glutathione peroxidase (GPx) activity was measured by adding the sample to a reaction mixture containing Tris buffer, EDTA, sodium azide, GSH, and H2O2, and expressed as μg of GPx consumed/min/mg protein.30
Methenamine Silver (Gomori PAM) staining of testicular tissue in the Control Group (Group 1) revealed moderate reticular fibre expression with intact basement membranes and minimal formation around seminiferous tubules (Figure 1), indicative of normal testicular architecture. In contrast, the Methamphetamine-Only Group (Group 2) exhibited markedly increased reticular fibre density, characterized by thickened fibres encircling the basement membrane of seminiferous tubules (Figure 2), suggesting methamphetamine-induced structural alterations. The Low Dose Group (Group 3), treated with methamphetamine and 100 mg/kg b.w. C. longa, demonstrated moderate reticular fibre expression with preserved basement membrane integrity (Figure 3), indicating partial attenuation of drug-induced changes. Further reduction in fibre density was observed in the Medium Dose Group (Group 4), where reticular fibre formation around tubules decreased significantly compared to Group 2 (Figure 4). Notably, the High Dose Group (Group 5) showed the most pronounced protective effect, with sparse reticular fibre expression and intact basement membranes resembling near-normal morphology (Figure 5).
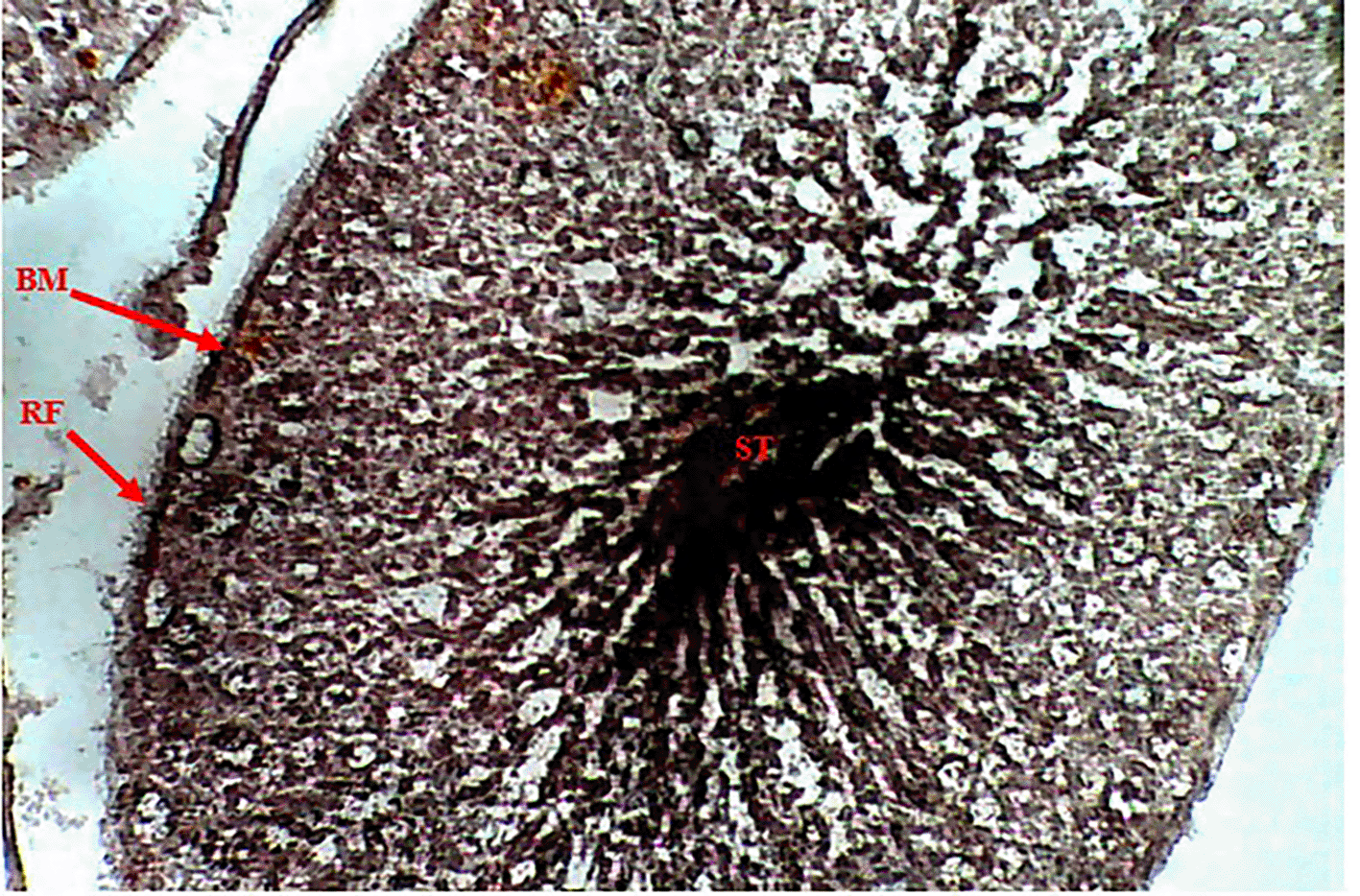
Photomicrograph testes showing moderate expression of reticular fibers (RF). Intact basement membrane (BM) and decreased formation of reticular fiber around the seminiferous tubules (ST).
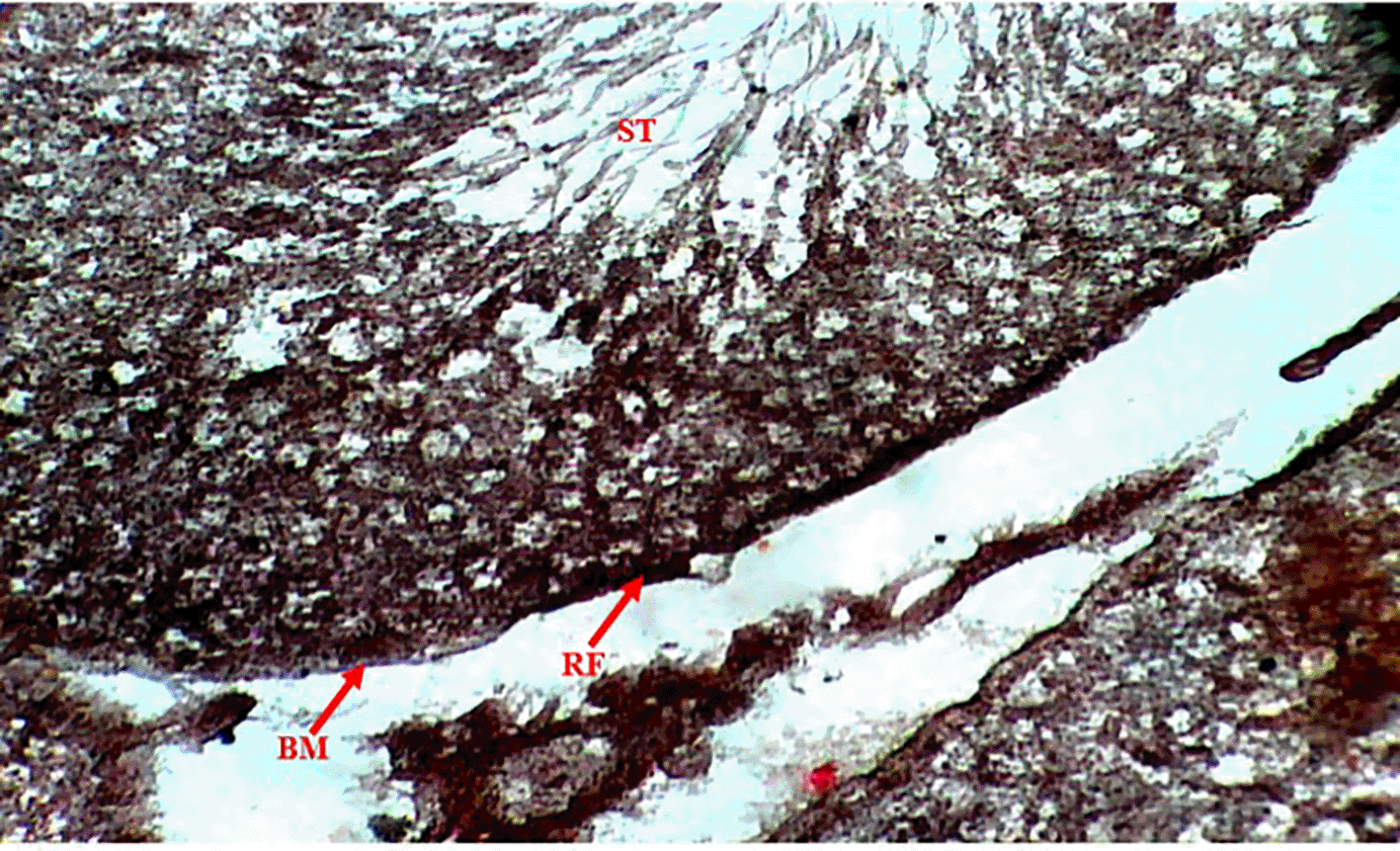
Photomicrograph of testes showing increased expression of reticular fibers (RF). Basement membrane (BM) of seminiferous tubules (SM) is surrounded by thickened reticular fiber (RF).
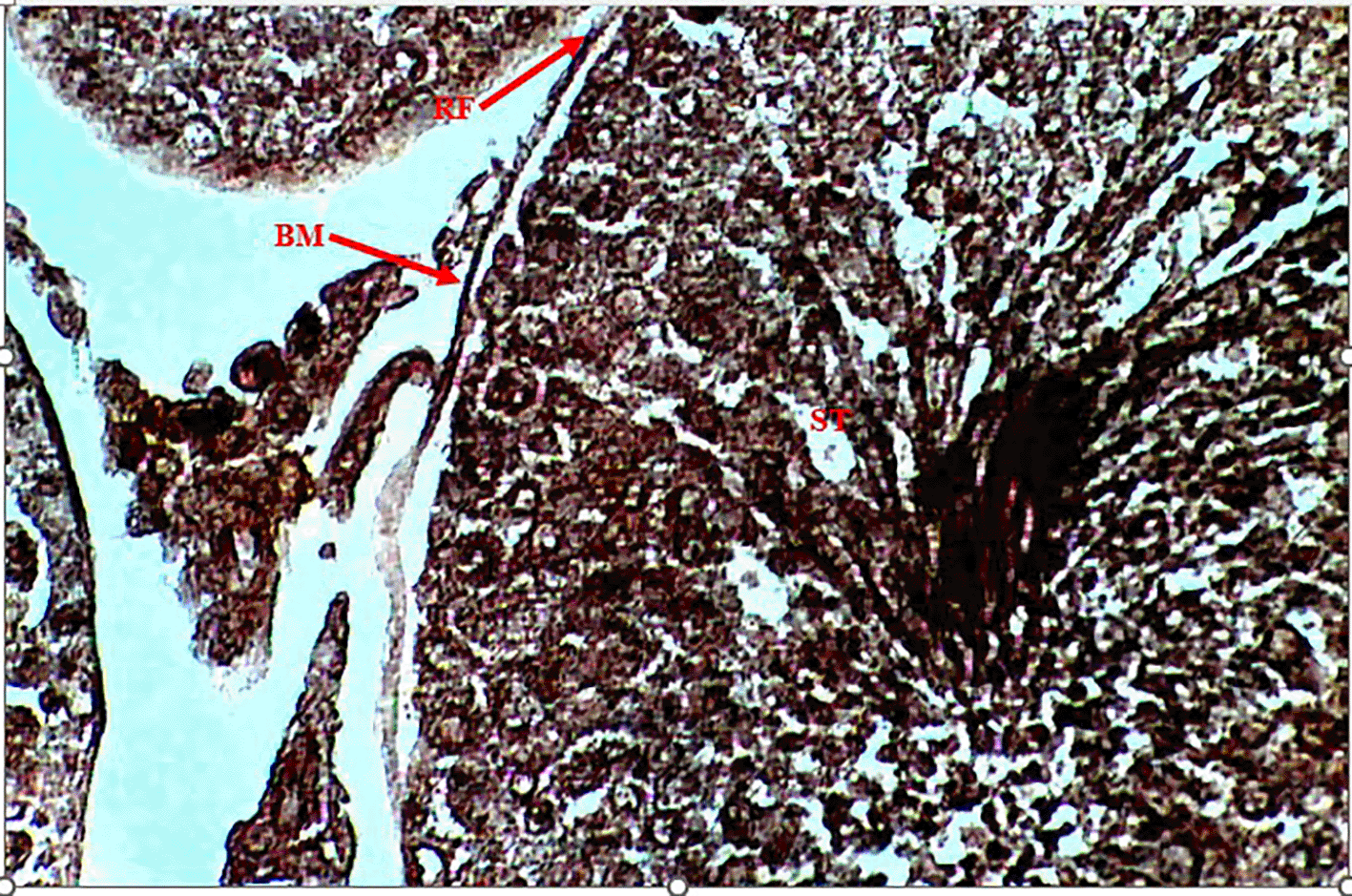
Photomicrograph of testes showing moderate expression of reticular fibers (RF). Basement membrane (BM) surrounding seminiferous tubules (ST) not intact.
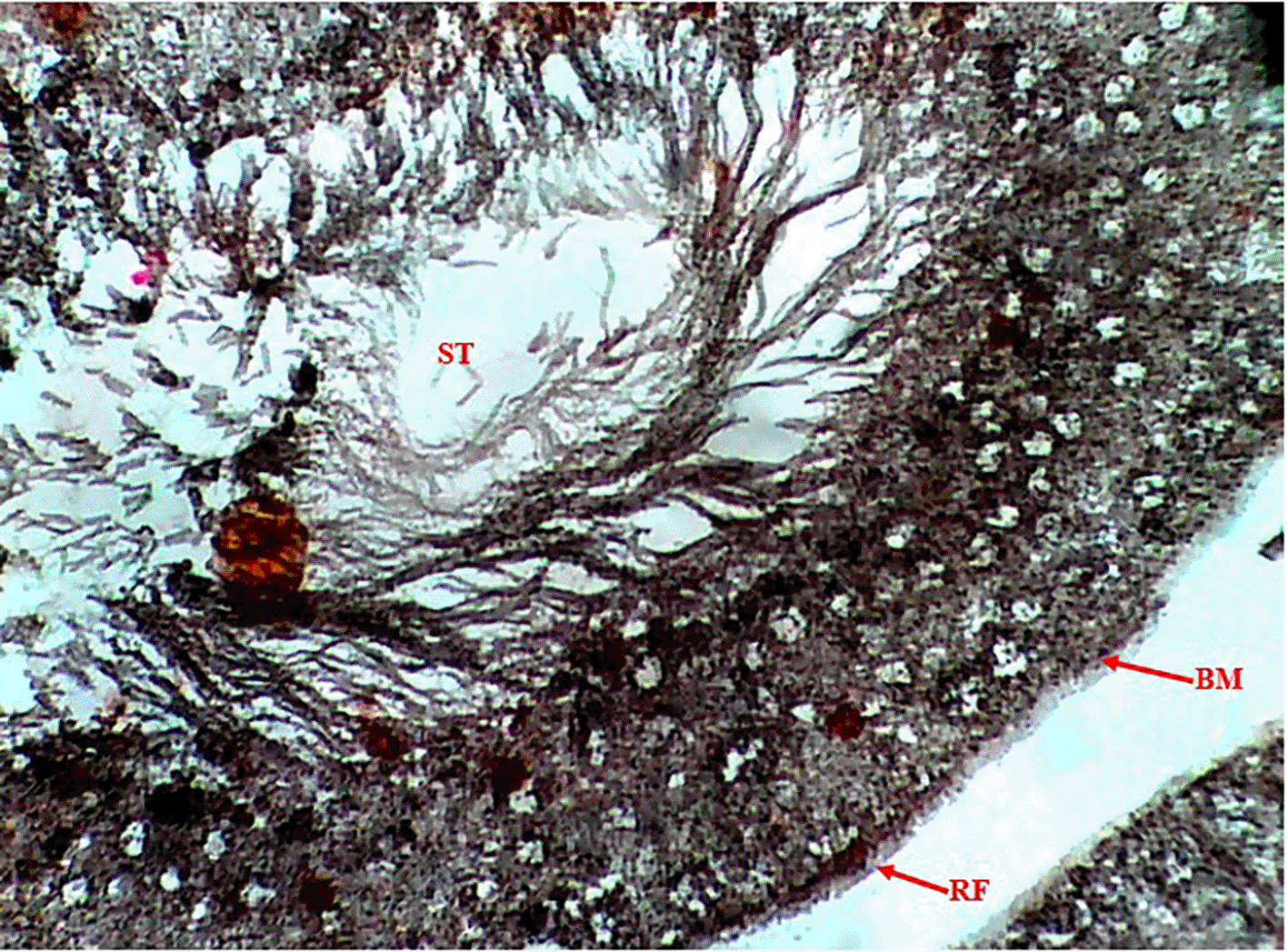
Photomicrograph of the testis, showing moderate expression of reticular fibers.
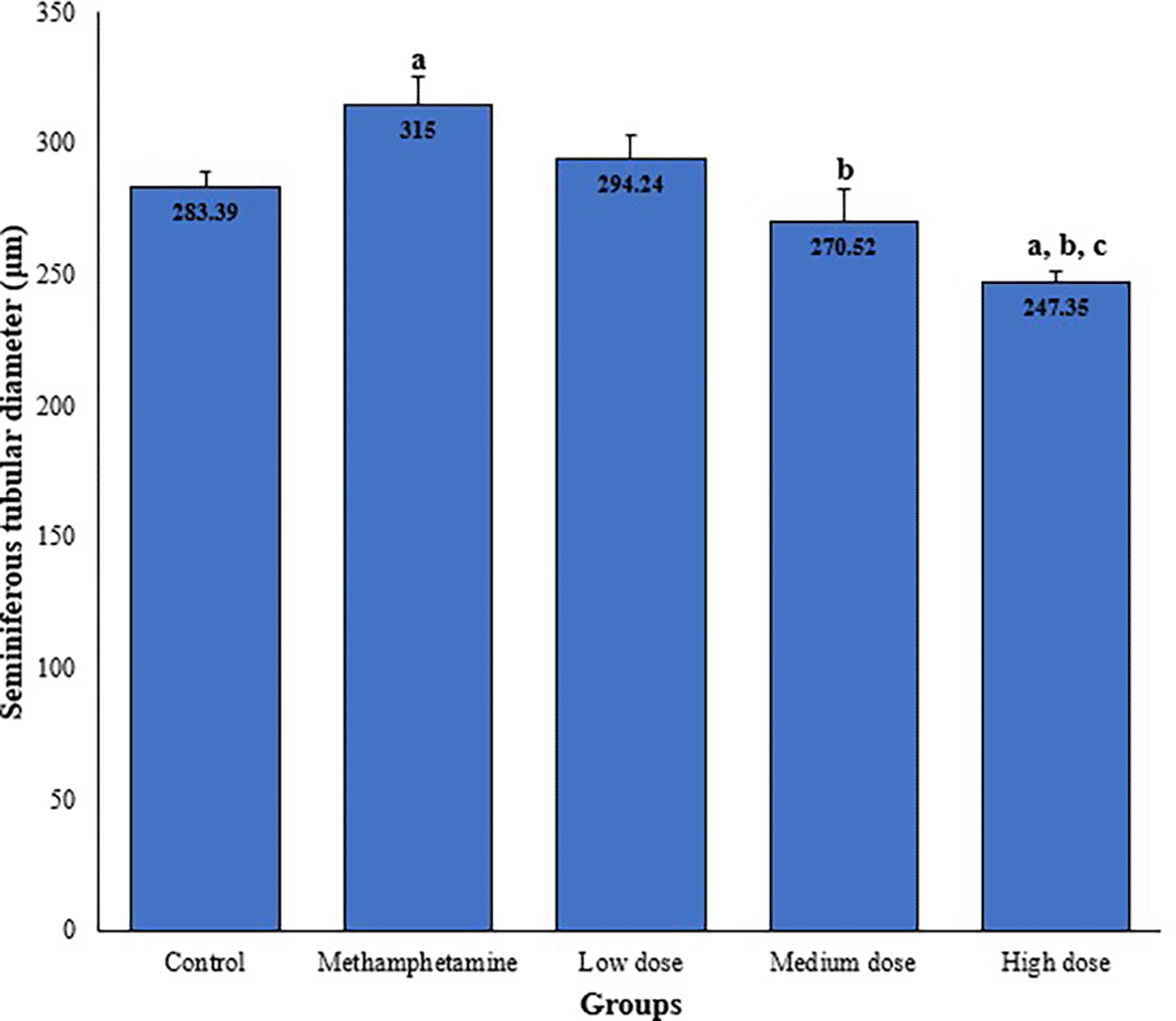
The results revealed a significant increase (p < 0.05) in seminiferous tubular diameter in the Methamphetamine-Only Group (315.00 ± 10.18 μm) compared to the Control Group (283.39 ± 5.63 μm). Tubular diameters in the treatment groups exhibited a dose-dependent reduction, with the Low Dose (294.24 ± 9.27 μm) and Medium Dose (270.52 ± 12.30 μm) groups showing a decrease, while the High Dose Group (247.35 ± 4.40 μm) demonstrated a significant decrease (p < 0.05) relative to both the Methamphetamine-Only and Control groups ( Table 1, Figure 6).
| Groups | Seminiferous tubular diameter (μm) | Seminiferous epithelial height (μm) |
|---|---|---|
| Control group (n = 50) | 283.39 ± 5.63 | 95.17 ± 4.56 |
| Methamphetamine group (n = 50) | 315.00 ± 10.18a | 98.46 ± 7.24 |
| Low dose group (n = 50) | 294.24 ± 9.27 | 97.21 ± 3.94 |
| Medium dose group (n = 50) | 270.52 ± 12.30b | 97.05 ± 5.37 |
| High dose group (n = 50) | 247.35 ± 4.40a,b,c | 81.63 ± 2.87 |
| p-value | <0.001 * | 0.099 |
Values are expressed as mean ± SEM, n = 50 for each group.
a = significantly different from control group.
b = significantly different from Methamphetamine group.
c = significantly different from low dose group.
d = significantly different from medium dose group.
From the result, the seminiferous epithelial height (μm) for the various groups were; 95.17 ± 4.56 for Control group, 98.46 ± 7.24 for Methamphetamine group, 97.21 ± 3.94 for Low dose group, 97.05 ± 5.37 for Medium dose group, and 81.63 ± 2.87 for High dose group. There was no significant (p > 0.05) differences in the seminiferous epithelial height among the various groups ( Table 1, Figure 7).
Results obtained on the level of oxidative stress markers showed a significant (p > 0.05) increase in the activities of superoxide dismutase (SOD), catalase (CAT), malondialdehyde (MDA) and glutathione peroxidase (GPx) in the methamphetamine group, compared to the normal control. On administration of the low, medium & high doses of Curcuma longa extract, the antioxidant enzymes concentrations were observed to have reduced significantly (p < 0.05) when compared to the Methamphetamine group (see Table 2).
| Groups | SOD (μmol/mL) | CAT (μmol/mL) | MDA (μmol/mL) | GPx (μmol/mL) |
|---|---|---|---|---|
| Control group (n = 5) | 26.74 ± 2.40 | 49.64 ± 0.12 | 5.01 ± 0.07 | 4.13 ± 0.04 |
| Methamphetamine group (n = 5) | 55.25 ± 7.01a | 89.22 ± 4.06 | 13.74 ± 1.49a | 5.42 ± 0.31 |
| Low dose group (n = 5) | 41.14 ± 1.49a | 69.52 ± 16.41 | 10.49 ± 1.28a | 4.25 ± 0.81 |
| Medium dose group (n = 5) | 38.72 ± 4.50b | 75.36 ± 18.02 | 8.16 ± 2.03b | 4.45 ± 0.44 |
| High dose group (n = 5) | 31.94 ± 0.33b | 55.56 ± 3.68 | 8.23 ± 0.15b | 4.16 ± 0.04 |
| p-value | 0.024 * | 0.153 | 0.032 * | 0.317 |
This study investigated the protective effects of Curcuma longa on histomorphometric parameters and reticular fiber expression in the testicular tissue of methamphetamine-exposed Wistar rats. The findings revealed that methamphetamine exposure significantly increased reticular fiber density around seminiferous tubules and altered tubular diameter, while C. longa administration attenuated these changes in a dose-dependent manner. The thickening and proliferation of reticular fibers observed in the methamphetamine-only group align with previous reports linking psychostimulants to extracellular matrix remodeling and basement membrane disruption.31 Such structural alterations likely impair spermatogenesis by destabilizing the microenvironment required for germ cell differentiation and migration.32 The reduction in reticular fiber density in C. longa-treated groups, particularly at high doses (400 mg/kg b.w.), underscores the plant’s capacity to mitigate methamphetamine-induced fibrosis. This protective effect may be attributed to curcumin, the primary bioactive compound in turmeric, which exhibits potent antioxidant and anti-inflammatory properties.33 By scavenging reactive oxygen species (ROS) and inhibiting lipid peroxidation, curcumin likely preserves basement membrane integrity and prevents collagen deposition, thereby restoring testicular architecture. Notably, methamphetamine exposure caused a significant increase in seminiferous tubular diameter, consistent with apoptosis-related pathological changes such as membrane blebbing and vesicle expansion.34 The dose-dependent reduction in tubular diameter observed in C. longa-treated groups suggests its anti-apoptotic efficacy. Curcumin’s ability to modulate pro-apoptotic pathways (e.g., caspase-3 inhibition) and enhance endogenous antioxidant defenses (e.g., SOD, GPx) may counteract methamphetamine-induced germ cell loss.35 The antioxidant effect of C. longa was further proven by the significant reduction in tissue antioxidant markers in the curcumin-treated group compared to the methamphetamine group, which caused an increase in antioxidant markers, further supporting the antioxidant effects of the plant as noted in other studies.33 However, the absence of significant differences in germinal epithelial height across groups may reflect the study’s shorter duration (28 days) compared to long-term interventions in other studies.36 The ameliorative effects of C. longa align with its historical use in traditional medicine for enhancing male fertility. Its rich phytochemical profile, including curcuminoids, flavonoids, and phenolics, synergistically combats oxidative stress and inflammation, key drivers of drug-induced testicular damage.37 These findings reinforce the potential of herbal therapies as adjunct treatments for substance abuse-related infertility.
Study was limited to assessing only histomorphometric parameters and recticular fiber density. The bioinformatics study of various extract and its subsequent effect on other reproductive parameters will be a novel approach to future research studies.
This study demonstrates that methamphetamine exposure induces detrimental structural changes in testicular tissue, including reticular fiber proliferation and seminiferous tubular dilation. Curcuma longa extract, particularly at higher doses, effectively mitigates these alterations, likely through its antioxidant and anti-apoptotic mechanisms. These results highlight turmeric’s potential as a therapeutic agent for addressing methamphetamine-associated male infertility. Further clinical studies are warranted to validate its efficacy in humans.
Ethical approval was obtained from the Faculty Animal Research Ethics Committee (FAREC-FBMS), University of Calabar, Nigeria (Certificate No. 320ANA2924), in compliance with national and institutional guidelines for the humane use of laboratory animals. The ethics approval granted by the Faculty Animal Research Ethics Committee (FAREC-FBMS), University of Calabar, Nigeria included specific restrictions on data sharing, which are detailed in the Data and Material Availability Statement. All the work in the experiment was done according to ARRIVE guidelines.
The authors declare that they have obtained necessary consent for publication from all parties involved in the study.
The data supporting the findings of this study are not publicly available due to legal and security restrictions associated with research involving controlled substances (methamphetamine). Unrestricted public release of the full datasets could raise regulatory and safety concerns. All animal experiments were reviewed and approved by the Faculty Animal Research Ethics Committee (FAREC-FBMS), University of Calabar, Nigeria. The approving body determined that unrestricted public sharing of the underlying data is not permitted under the conditions of approval. However, the data used to support the findings of this study may be granted upon reasonable request from the corresponding author, subject to verification of appropriate credentials and compliance with applicable legal, institutional and animal-research regulations. Any approved access will be provided under conditions that prohibit unauthorized redistribution or misuse of the data. All the work in the experiment was done according to ARRIVE guidelines as found in figshare repository with checklist title ‘Arrive guidelines for Testicular Histomorphemetric Parameters, Recticular Fibres Expression and antioxidant markers Assessment on Testicular Tissue of Methamphetamine-Exposed Wistar Rats Treated with Curcuma longa’, DOI ‘https://doi.org/10.6084/m9.figshare.30959549.v1’ and CCO license.38
The authors would like to acknowledge the support of their respective institutions, including the University of Calabar and Arthur Jarvis University, for providing the necessary facilities and resources to conduct this research. Special thanks to Mr. Effa Effa A, a taxonomist at the Department of Botany, University of Calabar, for authenticating the plant specimen. The authors also acknowledge the Nigeria Drug Law Enforcement Agency (NDLEA) Calabar command for providing the necessary permits and approvals for the use of controlled substances in this research.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Ph.D. ( Pharmacology) , Pharmacology, Phytochemical, Rheumatoid Arthritis, Plant-based Medicine, Animal-related study, Toxicology, Different Disease treatment, Drug Delivery, plant study, insect physiology, Mushroom-related study, Entomology, Poultry Management, Zoology, Microbiology, Expert in cosmetic product, Fisheries.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 14 Jan 26 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)