Keywords
Cross-protection, Immunological Memory, Specificity, Trained Immunity, Innate Immunity
This article is included in the Cell & Molecular Biology gateway.
Innate immune cells can acquire a form of memory through epigenetic and metabolic reprogramming following exposure to pathogen-associated molecular patterns (PAMPs), resulting in an enhanced, heterologous inflammatory response upon subsequent stimulation, a phenomenon termed trained immunity. This emerging concept challenges the traditional view that immunological memory is restricted to the adaptive immune system and is reshaping current understanding of host defense. Trained immunity is driven by long-lasting functional reprogramming of innate immune cells, particularly monocytes, macrophages, natural killer (NK) cells, dendritic cells, and their progenitors, leading to heightened responsiveness to secondary, often unrelated, stimuli. Comparable forms of innate immune memory have been documented across diverse biological systems, including systemic acquired resistance in plants, immune priming in insects, and trained immunity in mammals, highlighting its evolutionary conservation. The capacity of trained immunity to enhance immune responses provides a mechanistic basis for improved protection against reinfection and strengthened tumor surveillance. However, its dysregulated or excessive activation may also contribute to the development of autoinflammatory and autoimmune diseases, underscoring its dual and context-dependent nature. Consequently, trained immunity holds significant relevance for a wide range of clinical and translational applications, including infectious disease control, cancer immunotherapy, inflammatory disorders, and vaccine development. Harnessing trained immunity in vaccine design offers promising opportunities to achieve broader protective coverage, prolonged immune durability, and improved vaccine efficacy. Despite these advances, key challenges remain, including elucidating the precise molecular mechanisms underlying trained immunity, understanding its crosstalk with adaptive immune responses, and identifying optimal inducers and adjuvants capable of safely modulating trained immune pathways. Addressing these knowledge gaps will be essential for translating the concept of trained immunity into effective and safe therapeutic and vaccine strategies for human health.
Cross-protection, Immunological Memory, Specificity, Trained Immunity, Innate Immunity
The defense mechanism of the host against pathogenic microorganisms is facilitated by a sophisticated system that involves mucosal barriers, humoral elements, and specialized immune cells.1,2 Immune responses are conventionally categorized into two categories: innate and adaptive, which are classically distinguished by their specificity and memory capabilities.3 Immune memory is defined by the capability of antigen-specific immune cells to recognize and remember previously encountered pathogens, allowing for a faster and more robust response upon reinfection.4,5
Initially, the innate immune system, being germline-encoded, was believed to lack genetic memory through cell division.3 It was widely assumed that only the adaptive immune system could develop immunological memory. Naive B and T lymphocytes were previously believed to proliferate and produce long-lasting memory cells after their initial exposure to a pathogen, offering targeted and efficient safeguard against future infections by similar pathogens.6 This memory in the adaptive immune system arises primarily through somatic genetic rearrangements and clonal selection. As a consequence, the innate immune system was traditionally regarded as responsible only for nonspecific pathogen clearance, using mechanisms like phagocytosis or complement-mediated processes.7
Innate immune cells contain pattern recognition receptors (PRRs) that provide a degree of specificity in immune recognition by identifying pathogen-associated molecular patterns (PAMPs) found on invading microorganisms.8 Recent evidence supports the idea that cells of the innate immune system, including monocytes, macrophages, and natural killer (NK) cells, can also acquire memory from prior stimulations and modify their responses to subsequent challenges.9 These innate immune cells undergo functional modifications that advance the rate and magnitude of the secondary immune response during reinfection. This enhanced response is not necessarily pathogen- directed and may provide protection against unrelated pathogens. This phenomenon is referred to as innate immune memory or trained immunity.1,7
Trained immunity involves not only the reprogramming of intracellular signaling pathways in innate immune cells but also significant modifications in cellular metabolism, including glycolysis, oxidative phosphorylation, fatty acid metabolism, and amino acid metabolism. These metabolic alterations enhance the capability of innate immune cells to mount a more effective response upon re-exposure.7,10 The importance of trained immunity in host defenses to infectious diseases is evident. Additionally, proposed mechanistic links between trained immunity and the nonspecific effects of vaccines,11 offer promising clinical applications.12 It is important to note that, in addition to microbial ligands (PAMPs), the same pattern recognition mechanisms can be activated by non-infectious substances, particles, or danger signals known as damage-associated molecular patterns (DAMPs).13 These endogenous molecules, often linked to tissue damage, can activate the innate immune system, leading to either mild chronic or severe acute sterile inflammation. Furthermore, DAMPs are well-established modulators of adaptive immune responses.14
Understanding trained immunity has the potential to transform our comprehension of host immune defense mechanisms and the processes underlying immunological memory, potentially paving the way for the development of a new class of vaccines and immune therapies. While trained innate immunity can be beneficial in post-infectious and tumorigenic contexts, it may also have harmful effects by promoting immune responses associated with autoimmune and inflammatory diseases.15,16 The advancement of vaccines utilizing trained immunity principles offers potential advantages over conventional vaccines in certain scenarios. In the context of trained immunity, it is anticipated that immune responses may be broader and more robust, independent of antigen specificity.17,18 Therefore, this review aims to clarify the role of trained immunity as the memory component of innate host defense and its potential applications in immune therapies and vaccine development.
Vertebrate immune responses are conventionally categorized into two distinct types: innate and adaptive immunity.1,19 Adaptive immunity, which depends on specialized cells such as T-lymphocytes and B-lymphocytes, is specifically tailored to recognize pathogens and develop immunological memory against particular infections. A key property of adaptive immunity is its ability to provide enhanced defense against re-exposure by the similar microbes.20 This is achieved through the antigen specificity of lymphocytes, which is acquired by rearranging receptor genes, their capacity for clonal expansion upon encountering foreign antigens, and their development into active cells capable of destroying the infectious pathogen.21
Historically, it has been believed that, in contrast to adaptive immunity, the innate immune response lacks specificity and immunological memory. Innate host defenses encompass a variety of proteins, including those in the complement system, as well as cellular responses mediated by phagocytes and natural killer (NK) cells.20 Effector mechanisms of innate host defense, such as phagocytosis, microbial killing, and the lysis of virus-infected cells, are mediated by pattern-recognition receptor–pathogen-associated molecular pattern (PRR–PAMP) interactions and have traditionally been regarded as rapid protective responses that do not generate classical antigen-specific immunological memory; however, accumulating evidence suggests that these processes may contribute to innate immune adaptation or “trained immunity,” although the underlying mechanisms remain incompletely understood.1 Innate immune responses are rapid, activated within minutes of encountering a pathogen challenge, whereas adaptive immunity typically takes a span of days to generate a strong antibody-mediated and cell-based immune response.1
Recent discoveries challenge the traditional distinction between innate and adaptive immunity. Research has shown that the enhanced protection observed during re-exposure or cross-protection cannot be fully explained by adaptive mechanisms alone. Increasing evidence suggests that the innate immune system may also contribute to these heightened responses. The identification of pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), C-type lectin receptors, NOD-like receptors (NLRs), and RIG-I-like helicases, has demonstrated that innate immune cells can recognize specific pathogen-associated molecular patterns (PAMPs).20 As a result, the innate immune response can vary based on the particular interactions between PAMPs and PRRs,8 providing specificity to its responses. Although the concept of innate immune memory is well-established in plants and strongly supported in invertebrates, its full confirmation and mechanistic understanding in vertebrates remains an area of active investigation. Nevertheless, an increasing body of evidence suggests the presence of memory-like behavior in vertebrate innate immune responses.1
Vertebrate immune responses have traditionally been categorized into innate and adaptive arms, with immunological memory attributed solely to the adaptive component. However, organisms such as plants and invertebrates, which lack adaptive immune systems, have demonstrated the ability to mount protective responses upon re-exposure to pathogens.22 Remarkably, invertebrates have even shown evidence of graft rejection, and historical mammalian studies have observed cross-protection even when functional T and B lymphocytes are absent.1
Currently, it is understood that innate immune cells are capable of undergoing functional reprogramming, enabling them to mount faster and stronger responses upon subsequent pathogen exposure. This process, termed as trained immunity or innate immune memory, raises important questions regarding the specificity and breadth of the enhanced response.17 Experimental studies indicate that trained immunity can also provide defense against a variety of microbes ( Figure 1).10 Trained immunity can provide defense against re-infection by identical or distinct microbes and is observed in organisms without adaptive immune systems, such as plants,8 invertebrates,23 and mammals that lack functional T and B cells.24,25

Trained immunity inducers, such as live-attenuated vaccines, microbial components, or host-derived molecules, can initiate metabolic and epigenetic reprogramming. These changes promote a hematopoietic shift toward myelopoiesis and granulopoiesis (referred to as central trained immunity), leading to the generation of functionally enhanced innate immune cells, such as neutrophils and monocytes/macrophages. These trained innate immune cells exhibit heightened effector functions (peripheral trained immunity), which can provide increased protection against infections and boost antitumor responses. However, in some cases, this heightened reactivity may become maladaptive, contributing to hyperinflammatory or autoimmune conditions.10
The immunological memory associated with trained immunity differs fundamentally from that of adaptive immunity. Unlike adaptive classical memory, which relies on antigen-specific clonal expansion of lymphocytes, trained immunity is largely mediated by epigenetic and metabolic reprogramming in innate immune cells.13 A broad spectrum of cells can be involved, including monocytes, macrophages, neutrophils, basophils, NK cells, dendritic cells, and innate lymphoid cells (ILCs), each displaying unique functional characteristics that set them apart from the memory cells of the adaptive system.26
A defining feature of trained immunity is the ability to generate enhanced secondary responses, or cross-protection, against both homologous and heterologous pathogens ( Figure 1). Although these responses are triggered by prior exposure to a pathogen, they do not depend on antigen specificity derived from somatic gene rearrangement, as seen in adaptive immunity. This positions trained immunity as a unique immunological process that lies outside the conventional boundaries of the innate–adaptive paradigm.26
Molecular and Cellular Mechanisms of Trained Immunity: When PRRs recognize PAMPs, innate immune cells like macrophages and dendritic cells become activated. This interaction initiates epigenetic remodeling, leading to changes in chromatin accessibility and transcriptional profiles, effectively reprogramming the cells to respond more robustly to future stimuli. Simultaneously, trained immunity involves extensive metabolic reprogramming, encompassing shifts in glycolysis, oxidative phosphorylation, fatty acid metabolism, and amino acid metabolism. These metabolic adjustments support the increased functional activity of trained immune cells.27 Upon re-stimulation, these cells secrete elevated levels of pro-inflammatory cytokines, including IL-1, IL-12, IL-18, and IL-23. These cytokines promote the production of IL-17 and IFN-γ by innate lymphocytes, including γδ T cells, ILCs, and NKT cells, consequently boosting the host’s defense mechanisms against subsequent infections.15 The proper initiation of trained immunity processes relies on the dynamic interconnection of epigenetic and metabolic changes in innate immune cells upon activation.26
The metabolic state of immune cells is crucial in determining their functional capacity.28 Regarding trained immunity, metabolic reprogramming plays a central role in sustaining enhanced innate immune responses. The primary feature of this reprogramming is the shift from oxidative phosphorylation to aerobic glycolysis, a process essential for activating innate immune cells and producing inflammatory mediators.29 This metabolic shift is regulated by the AKT–mTOR–HIF1α pathway and can be triggered by microbial components such as β-glucan and pathogens like Candida albicans. Likewise, BCG vaccination has been shown to induce both metabolic activation and epigenetic remodeling of innate immune cells.25 The significance of glycolysis in trained immunity is underscored by the use of 2-deoxyglucose (2-DG), which, when administered during BCG-induced training, abolishes the enhanced cytokine production. Furthermore, inhibitors like rapamycin or metformin, which target key glycolytic regulators, prevent the establishment of critical histone modifications, such as H3K4me3 and H3K9me3, in response to β-glucan and BCG, effectively blocking the induction of trained immunity.30
A key metabolic adaptation in trained monocytes involves the redirection of the TCA (Krebs) cycle toward anabolic pathways that generate cholesterol and phospholipids from intermediates like citrate and acetyl-CoA. β-glucan exposure increases cholesterol biosynthesis ( Figure 2),10 whereas fluvastatin, a HMG-CoA reductase inhibitor, prevents trained immunity by suppressing H3K4me3 and reducing cytokine output. This highlights the vital function of the mevalonate pathway in sustaining trained immune responses.31 Notably, blocking glycolysis (via 2-DG), mTOR signaling (via rapamycin), or histone methylation (via methyltioadenosine) can also impair mevalonate-induced training, illustrating the tight coordination between metabolic and epigenetic networks in macrophages.31
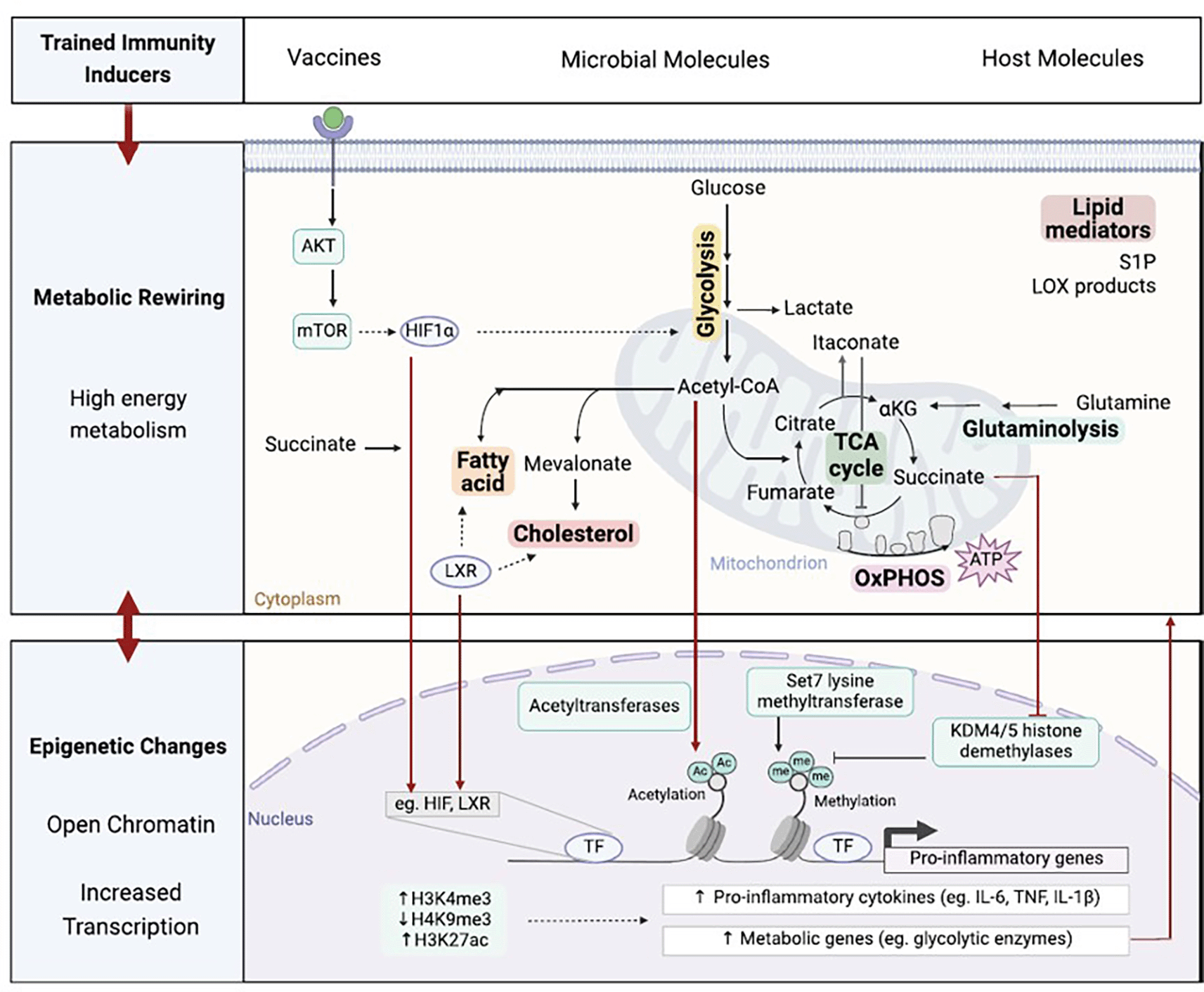
The upregulation of multiple metabolic pathways supports the development of trained immunity across various cell types by supplying energy, essential biosynthetic precursors, and by modulating protein function. Metabolites that accumulate during this process can activate transcription factors and influence the activity of epigenetic enzymes. Together, these effects establish a lasting epigenetic landscape that enhances the transcription of pro-inflammatory and metabolic genes. This, in turn, reinforces the metabolic shift and sustains elevated effector functions over time. (S1P sphingosine-1-phosphate; LOX lipoxygenase; αKG α-ketoglutarate; TCA tricarboxylic acid; OxPHOS oxidative phosphorylation; AKT protein kinase B; mTOR mammalian target of rapamycin; HIF1α hypoxia inducible factor 1α; LXR liver X receptor; TF transcription factor; Ac acetyl; me methyl).10
In parallel, glutaminolysis replenishes TCA cycle intermediates and contributes to the buildup of metabolites such as succinate and fumarate, both of which act as cofactors in epigenetic regulation. Succinate inhibits KDM6, an enzyme that demethylates H3K27, thereby increasing H3K27me3 at specific gene loci associated with anti-inflammatory (alternatively activated) macrophage states.32 Although KDM6 expression does not change in trained cells, its activity is influenced by metabolite accumulation. Meanwhile, fumarate impairs the function and expression of KDM5, which normally demethylates H3K4me3. Inhibition of KDM5 leads to the sustained presence of this active chromatin mark, thereby promoting gene transcription and stabilizing the trained phenotype.30
Epigenetics encompasses inherited gene modifications activity that happen without altering the underlying DNA sequence. These include chromatin remodeling, DNA methylation, histone modifications, and the regulatory effects of noncoding RNAs. More recently, RNA-based modifications, including methylation of mRNA, tRNA, and rRNA, have been recognized as additional layers of epigenetic control.33 Another important epigenetic mechanism is RNA editing, which can alter codons and potentially modify protein sequences by introducing post-transcriptional changes.34–36
Environmental exposures and microbial signals that activate innate immunity can drive such epigenetic transformations. While many of these changes are stable and can even be inherited through germline transmission, they are also reversible and highly responsive to environmental stimuli.37,38 In trained immunity, these epigenetic modifications are vital for “priming” innate immune cells, allowing them to mount quicker and more robust responses when re-exposed to pathogens or danger signals.38
The interaction between an organism’s genotype and environment leads to the expression of its phenotype, providing a framework for understanding the variations observed at the cellular, tissue, and organ levels. This process is known as epigenetic reprogramming.39 Epigenetic mechanisms are crucial for regulating the balance between the resting state and initiation of myeloid cells, such as monocytes and macrophages, in response to inflammatory triggers. These processes are also crucial for inducing and maintaining the polarization of innate immune cells and preserving innate immune memory following activation.40
The extensive epigenetic reprogramming of vertebrate innate immune cells upon experiencing pathogens or other stressors enables them to mount distinct transcriptional responses, inducing modifications in gene expression and cell function.21 Epigenetic changes operate at the chromatin level, regulating the access of the cell’s transcriptional machinery. This regulation occurs prior to transcription through modifications to chromatin, such as DNA and histone methylation, histone acetylation, and deacetylation. Additionally, post-transcriptional gene regulation involves micro-RNAs, which can inhibit translation or promote mRNA degradation.41
When innate immune cells are induced to adopt a trained immunity phenotype, they exhibit enhanced, faster, or fundamentally distinct transcriptional responses to frequent challenges.42 While the precise molecular processes involved altered responsiveness are not yet fully understood, they likely involve changes in chromatin organization within topologically associated domains, the involvement of long non-coding RNAs, DNA methylation, and the reprogramming of cellular metabolism. In resting myeloid cells, most pro-inflammatory gene loci remain in a repressed state,43 limiting the accessibility of the transcriptional machinery to regulatory regions that control the expression of inflammatory factors.44 Research has shown that initiation of innate immune cells leaves an “epigenetic scar” on the activated genes, which permanently alters the cells’ long-term response capabilities, ultimately resulting in functionally trained immunity programs.26 As a result, trained cells retain a permissive chromatin landscape that enables more rapid and robust transcriptional activation upon secondary stimulation, even by heterologous stimuli, thereby constituting the functional basis of trained immunity.26
A rapid, multifaceted response and the capacity to maintain lasting memory of previous infections are key characteristics of innate immunity. It is broadly understood that innate immunity also role as a function in generating a memory response following pathogenic challenges.21 Earlier studies in mammals showed cross-protection between microbes independent of T and B cells, and more recent research has identified memory functions in NK cells and macrophages, which are central to innate immunity.1 Research in vertebrates has significantly contributed to understanding immune memory and the mechanisms behind it. These studies have predominantly focused on innate immune cells, such as NK cells, monocytes/macrophages, neutrophils, basophils, dendritic cells, and innate lymphoid cells (ILCs).26
Natural Killer Cells, which are innate immune cells, are stimulated during pathogen invasions, particularly viral infections. For instance, when exposed to cytomegalovirus, specific NK cell clones with the Ly49H receptor on their surface undergo expansion and exhibit signs of immunological memory.45 When re-infected, these memory NK cells display an enhanced response, characterized by increased cytokine production through the Ly49H receptor, resulting in a more specific defense against the pathogen.46 NK cells demonstrate adaptive immune properties following infection, undergoing a secondary expansion upon re-infection and exhibiting rapid degranulation and cytokine release, leading to a more protective immune response.26 The memory properties of NK cells include a proliferation limit of about 1000 times and the capacity to self-renew for several months after a recall reaction.47 This evidence supports the notion that memory is an important characteristic of NK cell responses.47
Monocytes and macrophages are an essential components of the innate immune system, also demonstrate immune memory features during secondary immune responses against bacterial infections. Studies have shown that macrophages undergo epigenetic changes upon recognition of pathogens through PRRs. These changes prepare macrophages for subsequent encounters with the same or different pathogens recognized by the same PRR.15 Adoptive transfer of macrophages from immunized donors to naïve recipients can confer sufficient defense.48 These cells undergo epigenetic changes upon PRR ligation, preparing them for subsequent encounters with the training pathogen.15 Enhanced secondary responses can be observed against both the training pathogen and different pathogens recognized by the same PRR. Examples of this effect include stimulation by vaccines containing Bacillus Calmette-Guérin (BCG), β-glucan, or Candida albicans (C. albicans).48
Although monocytes have a short lifespan, enhanced secondary responses can still be observed months after the primary stimulus, suggesting that immune memory is established at the progenitor cell level. However, the exact mechanisms underlying this memory formation remain unknown.48 Monocytes originating from trained hematopoietic stem cells (HSCs) relocate to peripheral tissues, in which they specialize into monocyte-derived macrophages that exhibit robust effector functions and improved antimicrobial responses against a broad spectrum of pathogens.26
Neutrophils are a short-lived population of bone marrow cells primarily involved in bacterial infections. They function as phagocytes, eliminating pathogens, and generate reactive oxygen species. Additionally, they emit extracellular neutrophil traps and different types of proteases.49 After infections with certain pathogens, neutrophils undergo changes in their transcriptional profile, leading to the development of an innate immune compartment that displays long-term immunity. This trained immunity in neutrophils provides protection during secondary infections and cancer.50
Basophils another type of innate immune cell, can capture antigen-specific IgE antibodies through surface IgE receptors, enabling them to quickly capture and clear pathogens.51 The capacity of IgE antibodies to recognize specific antigens plays a crucial role in amplifying their immune response.52 Studies have shown that basophils defense against re-infection with Nippostrongylus brasiliensis in hookworms, independent of mast cells and memory T helper 2 cells.53 Although basophils themselves do not acquire a memory phenotype due to their short lifespan, the presence of pathogens induces changes in hematopoiesis,54 resulting to epigenetic and transcriptomic alterations in the progenitor cell subpopulation of basophils. This ultimately results in long-term protective innate immune memory.55
Dendritic cells (DC), specialized antigen-presenting cells, have also been found to exhibit immune memory responses. Studies have shown that dendritic cells isolated from mice exposed to specific pathogens display robust interferon-gamma production upon re-exposure with the identical microbes. This suggests the presence of immune memory at the level of dendritic cells, contributing to a more effective immune response upon secondary exposure.56
Innate Lymphoid Cells (ILCs), discovered in various tissues, have demonstrated memory-like properties. ILCs undergo expansion and exhibit transcriptional, phenotypical, and epigenetic changes following infections. This immune memory response has been observed in both ILC1s, induced by pro-inflammatory cytokines and antigen specificity, and lung-specific ILC2s, which display a memory-like phenotype after exposure to allergens.57
These studies collectively highlight the presence of immune memory in various innate immune cell types in vertebrates. The mechanisms underlying this memory formation involve epigenetic changes, including modifications to chromatin organization, DNA methylation, and alterations in gene expression. The existence of immune memory in innate immune cells provides a basis for more effective and targeted immune responses upon re-exposure to pathogens or other triggers, ultimately contributing to host defense.26
In plants, the innate immune system is capable of developing memory in response to past threats. This phenomenon, termed as Systemic Acquired Resistance (SAR), provides strong evidence that plants can respond more effectively to reinfection.58,59 The molecular mechanisms and biochemical mediators driving SAR are well understood,58 with epigenetic reprogramming of host defense playing a key role.59
When living cells or tissues are exposed to a stimulus, it can shape their response to subsequent encounters, indicating the presence of cellular memory from the initial exposure. In plants, this phenomenon is well-established. Upon recognizing herbivore-associated molecular patterns (HAMPs), damage-associated molecular patterns (DAMPs), pathogen-derived effectors, or specific xenobiotics, plants often enter a primed state.60 This priming boosts their ability to mount faster and more robust defense responses when faced with future stress or pathogen challenges.61 Defense priming occurs not only in the tissue directly subjected to the trigger but also in systemic, unharmed, or untreated areas of the plant. Once primed, plants can mount a quicker and stronger defense to even low levels of stimulation compared to unprimed plants, often accompanied by both local and systemic immunity.62 This priming is associated with SAR and chemically-induced immunity in plants.63 The capacity for inducible immunity varies with the plant’s life cycle, with perennials exhibiting a better capacity for inducible immunity than annual plants.61
In plants, the innate immune system includes both local and systemic defense mechanisms. Systemic immunity can be triggered by events such as foliar pathogen infections, root infiltration by beneficial microorganisms, or mechanical damage. When a localized infection occurs in the leaves, plants initiate a systemic immune response, which involves the upregulation of pattern recognition receptors (PRRs), the build-up of inactive signaling enzymes, and changes in chromatin structure. These modifications contribute to the establishment of immunological memory, enabling distal leaves to elicit a quicker and efficient defense to future infections.64 This process enhances the plant’s defensive capacity, effectively ‘priming’ it for upcoming threats.61
Invertebrates, despite their wide-ranging diversity in morphology, structure, behavior, lifespan, and evolutionary background, must manage infections and recognize both genetically similar and allogeneic individuals. Similar to vertebrates, invertebrates identify pathogens through the detection of specific molecular components, known as pathogen-associated molecular patterns (PAMPs).65 Additionally, DAMPs are recognized by pattern recognition receptors (PRRs) and DAMPs are also recognized by pattern recognition receptors (PRRs).66
Recent research challenges the long-standing notion that invertebrate innate immunity lacks memory capabilities. Increasing evidence demonstrates that invertebrates develop enhanced resistance to subsequent infections following an initial exposure to a pathogen.67 Some studies have observed sustained increases in antimicrobial activity after infection, which in some cases lead to heterologous enhanced resistance to future infection.68,69
| Organism | Experimental model | Biological effect | Specificity | References |
|---|---|---|---|---|
| Plants | ||||
| Large variety of plants | Viruses, bacteria, fungi | Protection against reinfection | Variable | 78 |
| Nonvertebrate | ||||
| Mealworm beetle | LPS or bacterial prechallenge | Protection against secondary infection | No | 68 |
| Drosophila | Streptococcus pneumoniae prechallenge | Protection against Streptococcus pneumoniae | Uncertain | 69 |
| Anopheles gambiae | Plasmodium prechallenge | Protection against Plasmodium | No | 73 |
| Vertebrates | ||||
| Humans | BCG | Heterologous protection to secondary | No | 79 |
| Mice | Murine CMV infection | NK-dependent protection | No | 45 |
Emerging testimony of immune memory in invertebrates is derived from research on infection resistance, natural transplantation immunity, and both individual and transgenerational immune priming.70 The concept of cross-protection suggests heterologous priming of innate immune responses, as seen in the defense of mealworm beetles against fungi after lipopolysaccharide (LPS) injection.69 Additionally, a specific form of innate immune memory has been identified in insects, such as the maternal transfer of immunity against Pasteuria ramosa in the water flea Daphnia.71 Moreover, shrimp can be vaccinated and defend against white spot syndrome through exposure to a viral protein.72
Two main mechanisms may explain the improved innate immune responses (or memory) in invertebrates: (1) quantitative improvement of the immune response during reinfection, and (2) qualitative changes driven by somatic diversification. The first mechanism involves several pathways that can lead to an increased innate immune response during reinfection. These pathways include long-term stimulation of the Toll and Imd signaling pathways, alterations in immune cell populations,73 enhanced immune functions like phagocytosis,74 and upregulation of peptidoglycan recognition proteins and lectin receptors.75
PAMPs and DAMPs released by invading pathogens are usually recognized by PRRs on innate immune cells. Upon activation by pathogen-derived antigens and the initiation of phagocytosis, these immune cells trigger signaling cascades that influence protein expression both at the transcriptional and translational levels. In response, the activated cells generate antimicrobial agents (AMFs) like antimicrobial peptides, reactive oxygen species, and melanin to eliminate pathogens. Moreover, these immune cells may undergo epigenetic modifications in their chromatin and chromosomes, which can create a ‘memory’ of the pathogen encounter and contribute to the development of innate immune memory. Importantly, epigenetic alterations in gametes, particularly in the egg, following pathogen exposure, may enable future generations’ immune cells to generate quicker and more robust immune responses to similar pathogens.22
The immunological characteristics of trained immunity have been demonstrated to persist for a period ranging from three months to one year. Additionally, defense against infections induced by live attenuated vaccines can last for five years, providing heterologous immunity over time ( Table 1).22 Monocytes from BCG-vaccinated individuals demonstrated an increased release of inflammatory cytokines for at least three months post-vaccination, and surface receptor levels remained altered for up to one year. In a similar vein, in Rag1 mice, flagellin treatment prevented rotavirus shedding for as long as 150 days after injection.76
Regarding to bacteria or fungi, an infection with one pathogen induces a memory defense that confers resistance to another, different microbes. However, for viruses, the memory response differs, being more characteristic of the adaptive immune system, particularly in terms of specificity. This distinction may be due to the involvement of NK cells in viral memory. While NK cells are essential for memory against viruses, their role in the response to other infections cannot be ruled out.77
The induction of cross-protection in non-vertebrate models suggests the nonspecific induction of innate immune responses. For instance, mealworm beetles are protected against fungi after being injected with lipopolysaccharides. However, in Drosophila infected with Streptococcus pneumoniae or Beauveria bassiana, protection is observed against re-infection with the same pathogen, but not against different microbes.69 Similarly, Anopheles gambiae mosquitoes infected with Plasmodium falciparum show partial protection from secondary infection by the same Plasmodium species.73
Trained immunity, the capacity of innate immune cells to develop memory-like responses, has transformed into a promising intervention point for a range of illnesses.80 By harnessing the innate immune system’s capacity for enhanced and long-lasting immune responses, researchers aim to develop therapeutic strategies to modulate trained immunity for intervention purposes.26 Here, the concept of trained immunity as a therapeutic target and its potential application is discuss below.
Infectious Diseases: Trained immunity can be exploited to strengthen host defense against infectious diseases. By inducing trained immunity, innate immune cells can exhibit heightened responsiveness, offering broader defense against several array of microbes.81 This approach may prove particularly advantageous in situations where traditional vaccines are limited or ineffective, such as in cases of recurrent infections or infections caused by pathogens with high mutation rates.26
Cancer Immunotherapy: Trained immunity holds promise in cancer immune treatments. Innate immune cells, such as macrophages and NK cells, serve crucial functions in tumor surveillance and elimination. By modulating trained immunity, it is possible to aamplify the anti-cancer effects of these innate immune cell.82 For example, stimulating trained immunity in macrophages can promote tumor infiltration, activation, and enhanced killing of cancer cells. This approach may complement existing immunotherapies, such as immune checkpoint inhibitors, to improve treatment outcomes.26,82
Autoimmune and Inflammatory Disorders: Trained immunity can also be targeted to modulate excessive immune responses in autoimmune and inflammatory disorders. In these conditions, the immune system incorrectly attacks the body’s own tissues, leading to prolonged inflammation and tissue harm. By regulating the trained immune response, it may be possible to dampen aberrant immune activation and restore immune homeostasis, thereby reducing disease severity. However, caution is warranted as trained immunity can have both positive and negative effects, and its modulation must be carefully balanced.26
Vaccine Development: Trained immunity can be leveraged to design novel vaccines with improved efficacy. By incorporating trained immunity inducers or adjuvants, vaccines can elicit stronger and longer-lasting immune responses. This approach may be particularly valuable against pathogens with high antigenic variability or in situations where a rapid and broad immune response is required.83 Additionally, training the immune system through vaccination may confer protection against multiple pathogens, offering a potential solution for emerging or unknown infectious diseases.26
Aging and Immunosenescence: The aging process is correlated with immune function decline, a process called immune-senescence, leading the body more prone to infections and reduced vaccine efficacy. Targeting trained immunity pathways may help rejuvenate the immune system in elderly individuals, enhancing immune responses and improving vaccine effectiveness.84 By boosting innate immune cell function and memory, trained immunity-based interventions may help overcome age-related immune deficiencies.26
Traditional vaccines primarily target the adaptive immune response, specifically by inducing the development of microbial-specific antibodies and activating antigen-specific T cells. However, vaccines designed to induce trained immunity aim to stimulate innate immune cells, including monocytes, macrophages, and NK cells, to generate a more potent and long-lasting immune response.85 The design of vaccines conferred by trained immunity is still an emerging field, and additional studies are crucial for maximizing and refine these approaches. Challenges include identifying the most effective inducers of trained immunity, understanding the mechanisms underlying the establishment of trained immunity, and ensuring the reliability and efficacy of these vaccines in diverse populations.86 Overall, exploiting the phenomenon of trained immunity in vaccine design holds significant potential for developing novel vaccines that provide broader protection, longer-lasting immunity, and improved vaccine efficacy targeting a wide array of infectious diseases.86
Trained Immunity-Based Vaccines (TIbV) for Emerging Pathogens: Trained immunity-based vaccines (TIbV) have appeared as a promising approach to enhance vaccine efficiency, particularly in response to outbreaks caused by emerging pathogens. By harnessing the innate immune system’s capacity for “memory-like” responses, TIbVs aim to provide broader and longer-lasting defense against invasions, including those caused by novel or rapidly mutating pathogens.87
Training protocols, such as those involving β-glucan or BCG (Bacillus Calmette–Guérin) vaccination, have been reported to increase the quantity of hematopoietic stem cells and multipotent progenitors in the bone marrow. This results in elevated white blood cell counts prior and throughout infection, effectively enhancing the immune system’s ability to respond to pathogen challenges.88 For example, the adoptive transfer of long-term hematopoietic stem cells or bone marrow cells from mice that have undergone training with β-glucan and BCG into naïve mice has been shown to increase the proportion of Gr1+CD11b+ bone marrow cells in the blood, providing protection against pulmonary tuberculosis.89 Several strategies have been explored in the design of vaccines conferred by trained immunity.
Trained Immunity Inducers: Researchers have investigated various compounds and molecules that can trigger trained immunity. β-glucan, a fungal cell wall component, and BCG (Bacillus Calmette-Guérin) vaccine have shown promising results in inducing trained immunity. These compounds activate PRRs on innate immune cells, leading to epigenetic and metabolic reprogramming and the establishment of a trained immune phenotype.86,90
Adjuvants: They are ingredients incorporated into vaccines to amplify the immune reaction, added to vaccines to enhance the immune response. Traditional adjuvants primarily target the adaptive immune system. However, the emergence of adjuvants that can specifically activate innate immune cells and induce trained immunity is an active area of research.90 These adjuvants can be applied to increase the effectiveness of vaccines by promoting a stronger and more durable immune response.86
Reprogramming Vaccine Platforms: Another approach in designing vaccines conferred by trained immunity involves modifying existing vaccine platforms to specifically target innate immune cells. For example, modifying viral vectors or nanoparticles to deliver immunomodulatory molecules directly to innate immune cells can trigger trained immunity and provide broad protection against various pathogens.86,91
Developing Trained Immunity-based Vaccines (TIbV) may offer advantages over conventional vaccines in specific scenarios. TIbV can elicit more extensive and potent immune reactions free from the constraints of antigen specificity. A few potential uses are: (1) Addressing pathogens causing recurrent infections, especially when conventional vaccines are unavailable.92 For instance, during the 1918 influenza pandemic, bacterial vaccines were surprisingly successful in preventing secondary infections, possibly by inducing trained immunity alongside preventing Streptococcus pneumonia infections.93 (2) Preventing diseases involving co-infections of bacteria and viruses, such as asthma exacerbations.92 (3) Targeting microbes with high mutation rates, like the influenza virus.94 TIbV’s broad spectrum can avoid the selective pressure imposed by highly specific vaccines. This approach may also prevent the emergence of new bacterial strains after conventional vaccination, as seen with pneumococcal serotype-specific vaccines.95 (4) Providing preventive measures for at-risk individuals for pathogens lacking vaccination options, such as children, the elderly, and those prone to mucosal infections.96 TIbV could be an alternative to the widespread use of antibiotics for recurrent infections, particularly in immuno-compromised individuals.97 And, (5) Restoring immune responsiveness in conditions characterized by immune paralysis or unresponsiveness, such as severe sepsis and certain malignancies.98
Several clinical studies and trials have explored the application of trained immunity-based vaccines (TIBVs) across a wide range of conditions, including infectious diseases, cancer, and autoimmune disorders. Beyond traditional vaccine targets, TIBVs are gaining attention for their ability to induce broad, non-specific protection through innate immune reprogramming. Recent research highlights their expanding potential in novel vaccine platforms and even in veterinary medicine.18
BCG Vaccination in Infectious Diseases and Aging Populations: The Bacillus Calmette–Guérin (BCG) vaccine has long been used to protect against tuberculosis, but recent clinical trials have reaffirmed its broader immunological benefits. Studies have demonstrated that BCG vaccination can reduce all-cause mortality in children and delay the onset of infections in the elderly.99 For instance, a randomized trial in Guinea-Bissau examined the effects of the BCG-Japan strain in adults over 50 years of age. The results showed increased production of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α, indicating the induction of a trained immunity phenotype. These effects were particularly pronounced in individuals with prior exposure to Mycobacterium tuberculosis, suggesting enhanced innate immune responsiveness in older populations.100 Ongoing trials are also investigating BCG’s potential to boost immunity against viral pathogens, including SARS-CoV-2.101
Trained Immunity in Cancer Immunotherapy: Trained immunity is also being harnessed in oncology. BCG remains the standard of care for superficial bladder cancer, with its therapeutic effect partly attributed to the activation of innate immune mechanisms.82,102 More recently, novel cancer vaccine platforms, including personalized mRNA vaccines, have demonstrated the ability to activate both innate and adaptive immune responses. In early-stage glioblastoma trials, these platforms have shown promising outcomes. Additionally, a personalized cancer vaccine has achieved remarkable success in an early-phase clinical trial for advanced kidney cancer.103 The treatment was well tolerated, with only minor localized and flu-like symptoms, underscoring its potential as a safe and effective therapeutic approach.103
BCG Vaccine in Autoimmune Diseases: The potential of BCG vaccination in modulating autoimmune responses has also been investigated.99 In conditions such as multiple sclerosis and type 1 diabetes, BCG has been shown to enhance glycolysis in conventional T cells, promoting the development of suppressive regulatory T cells (Tregs). This shift helps prevent neuronal damage in multiple sclerosis and improves glycemic control in type 1 diabetes, indicating that trained immunity may offer a novel immunomodulatory strategy for managing autoimmune diseases.99,104
Adenoviral Vector COVID-19 Vaccine: A recent study demonstrated that an adenoviral vector-based COVID-19 vaccine could induce trained immunity in humans. The research observed epigenetic reprogramming of monocytes and enhanced cytokine production upon secondary pathogen exposure. These findings suggest that adenoviral vaccines may offer broad, innate immune protection that extends beyond their specific antigenic targets.105
Protein-Free Vaccine Against Nosocomial Pathogens: In a previous study, researchers developed a protein-free vaccine composed of aluminum hydroxide, monophosphoryl lipid A, and mannan.106 When tested in mouse models, the vaccine induced trained immunity, evidenced by epigenetic modifications and enhanced macrophage phagocytic activity. Notably, it provided protection against a range of hospital-acquired pathogens without engaging adaptive immune responses, highlighting a novel strategy for preventing nosocomial infections.107
Measles-Mumps-Rubella (MMR) Vaccine and Trained Immunity: Emerging evidence also supports the non-specific benefits of the MMR vaccine. A previous study revealed that MMR vaccination could induce trained immunity through functional and metabolic reprogramming of γδ T cells. This suggests that MMR may provide off-target protective effects against unrelated infections by modulating innate immune cells.108
A Sublingual Poly-bacterial Vaccine for Respiratory Infections: MV130, a sublingual bacterial vaccine, has demonstrated strong potential as a TIBV for respiratory infections.81 A retrospective real-world study involving 599 patients (186 children and 413 adults) with recurrent respiratory tract infections (RTIs) showed that the median number of RTI episodes was reduced by over 70% in children and more than 80% in adults following MV130 administration. Antibiotic consumption also decreased by over 80% in both groups. These clinical outcomes suggest that MV130 effectively induces trained innate immunity and reduces both infection rates and antibiotic dependence. The vaccine’s non-specific protective effects have been especially notable in pediatric populations, where it has also been used to prevent wheezing episodes.109
Veterinary Applications of Trained Immunity: Trained immunity concepts are also being applied in veterinary medicine to improve vaccine design against emerging zoonotic diseases. These approaches emphasize metabolic reprogramming and epigenetic modifications to enhance innate immune responses in animals. By reducing dependence on antibiotics, trained immunity-based strategies in veterinary applications offer a promising avenue for managing infections in livestock and companion animals while addressing the global issue of antimicrobial resistance.110
Trained immunity has emerged as a novel strategy in immunotherapy and vaccine design. TIbVs can target specific antigens or act as adjuvants to boost conventional vaccines. While clinically promising, their use is challenged by safety concerns, mechanistic uncertainty, and potential immune overactivation. Therefore, TIbV applications require careful patient selection and monitoring, particularly in inflammatory or immunologically vulnerable populations.111
Risk of Hyperinflammation: A core characteristic of trained immunity is the amplification of the inflammatory response upon re-exposure to stimuli. While beneficial for combating infections and tumors, this heightened reactivity may also become pathogenic. Excessive production of cytokines such as IL-1β, TNF-α, and IL-6 can lead to systemic inflammation, tissue damage, or even cytokine storms, particularly in individuals with existing immune dysregulation.26 For instance, β-glucan-induced training has shown both protective effects and harmful hyperinflammatory outcomes in preclinical studies, underscoring the importance of tightly regulating inflammatory responses.112
Autoimmunity and Immune Dysregulation: Trained immunity lowers the activation threshold of innate immune cells, increasing sensitivity not only to pathogens but also to benign or self-derived signals.113 In genetically predisposed individuals, this can result in autoimmune or autoinflammatory conditions, such as systemic lupus erythematosus or rheumatoid arthritis.114 Persistent epigenetic changes may lead to chronic immune activation even in the absence of infection, raising concerns about the long-term safety of TIBV-based interventions.115
Lack of Antigen Specificity: Unlike adaptive immunity, trained immunity lacks antigen specificity. This non-selective activation can lead to off-target effects, where the immune system may attack non-harmful stimuli or healthy tissues.17 Moreover, generalized immune activation can alter host-microbiota interactions, potentially resulting in dysbiosis, chronic inflammation, or impaired immune tolerance. This lack of precision necessitates the development of targeted or combinatorial approaches to refine immune responses.17,116
Duration and Reversibility of Trained Responses: The persistence of trained immunity is not fully understood. While durable epigenetic modifications are central to its protective potential, they also risk prolonged inflammation or immune exhaustion if left unchecked.116 Current technologies do not offer fine-tuned control over the magnitude, duration, or resolution of trained immune states, limiting their clinical safety and predictability.117
Interindividual Variability: Responses to trained immunity inducers vary widely across individuals, influenced by genetic polymorphisms, age, comorbid conditions (e.g., diabetes, obesity), nutritional status, and prior microbial exposures.118 These factors complicate standardization, hinder reproducibility, and may increase the likelihood of adverse effects. Personalized approaches will be necessary to optimize therapeutic outcomes.119,120
Mechanistic Gaps and Biomarker Limitations: Despite significant progress, the molecular mechanisms driving trained immunity remain incompletely characterized, especially in humans. Key signaling pathways, epigenetic regulators, and metabolic circuits are still being uncovered. Furthermore, the lack of validated biomarkers impedes monitoring trained immune responses in clinical settings, limiting risk stratification and personalized treatment strategies.121
Clinical Trial Design and Safety Concerns: Designing robust clinical trials for trained immunity-based therapies is inherently complex. Preclinical models often fail to predict human immune toxicities, and inconsistencies in the type, dose, and route of trained immunity inducers reduce reproducibility. Ethical concerns also arise, particularly when testing in vulnerable populations such as the elderly or immunocompromised, where immune activation could pose serious risks.122
While the concept of trained immunity as a therapeutic target holds significant promise, several challenges must be addressed to fully harness its potential. A deeper understanding of the precise mechanisms underlying trained immunity and its regulation is essential for developing targeted interventions. Moreover, potential off-target effects and concerns regarding long-term safety require thorough evaluation.26 Despite these challenges, the capacity to modulate trained immunity presents exciting therapeutic opportunities across a wide range of diseases.123 In certain conditions, inducing trained immunity can be beneficial, for example, enhancing cancer therapies or reversing sepsis-induced immunoparalysis. Notably, the Bacillus Calmette–Guérin (BCG) vaccine, approved by the FDA for the treatment of bladder cancer, is also being investigated for its potential efficacy in other cancers such as lymphoma and melanoma. Additionally, β-glucan, a compound traditionally used in East Asia to boost immune responses in cancer patients, is currently undergoing clinical trials in the United States in combination with immune checkpoint inhibitors.124,125
Autoimmune disorders are distinguished by an excessive and prolonged immune response against the body’s own tissues, primarily mediated by B and T cells. This chronic inflammation is a major contributor to tissue damage and disease progression. Recent research suggests that reprogramming of the innate immune system, including trained immunity, might contribute to the development and exacerbation of autoimmune diseases.126 Trained immunity can be induced by both endogenous trigger and environmental factors, leading to an increased inflammatory response and contributing to the pathogenesis of autoimmune disorder.127 Approaches designed to manage the underlying mechanisms of trained immunity, such as changed metabolic processes and epigenetic modifications, could offer new therapeutic avenues for managing chronic inflammation associated with autoimmunity.128
Epigenetic Therapy in Autoimmune Diseases: One promising therapeutic approach to modulate trained immunity involves reversing the epigenetic modifications that occur during immune training. By using various inhibitors, it may be possible to restore normal epigenetic regulation and reduce the chronic inflammation seen in autoimmune diseases.129 For instance, agents targeting DNA methyltransferases (DNMTs), including azacytidine and decitabine, are already used in oncology and could have potential applications for inflammatory diseases. Additionally, small molecules targeting proteins involved in lysine methylation, marks associated with transcriptionally silenced chromatin, can further manipulate gene expression.130
Histone deacetylase (HDAC) inhibitors, which target the acetylation state of histones, have also shown promise in reducing inflammation.129 Compounds like sodium phenylbutyrate, vorinostat, and TSA have been investigated in various animal models for diseases such as arthritis, diabetes, sepsis, and asthma, where they reduce pro-inflammatory cytokine production.131 Specifically, TSA and MI192 have been shown to inhibit IL-6 secretion in LPS-activated PBMCs, and TSA, along with givinostat (ITF2357), interferes with the stability of IL-6 mRNA, thereby decreasing IL-6 secretion in synovial fibroblasts and macrophages.132 Other molecules, such as romidepsin (FK228) and MPT0G009, inhibit the proliferation of synovial fibroblasts133 and can block angiogenesis in synovial tissue.134
Biological Therapies: Several biologic therapies that are used to treat autoimmune diseases can also modulate trained immunity. For instance, TNF inhibitors, including etanercept and adalimumab, regulate histone modifications, such as acetylation and trimethylation, to suppress the expression of CCL2 in monocytes. These changes have been associated with reduced RA activity.135
To actively suppress trained immunity, targeting IL-1β, a key mediator of inflammation through the inflammasome pathway, may be effective. While IL-1Ra (anakinra) is considered a marginal treatment in rheumatoid arthritis (RA),136 both anakinra and the IL-1-blocking antibody canakinumab have been shown to suppress symptoms and maintain disease control in systemic autoinflammatory syndromes.137 In the case of cryopyrin-associated periodic syndrome, early diagnosis and treatment31 with an IL-1 blocker are fundamental to preventing long-term complications.138 Hyper-IgD syndrome, characterized by sterile inflammation and uncontrolled trained immunity, can also benefit from treatment with IL-1 blockers.139,140
Another avenue involves targeting granulocyte-macrophage colony-stimulating factor (GM-CSF), which stimulates inflammation and may contribute to the development of trained immunity. Monoclonal antibodies against GM-CSF (namilumab, MOR10) and its receptor (Mavrilimumab) are currently being tested in clinical trials for patients with psoriatic arthritis.89
Manipulation of Metabolic Pathways: The metabolic reprogramming that occurs in trained immunity, particularly the shift from oxidative phosphorylation to aerobic glycolysis, is a critical process in immune cell activation. Pharmacological modulators targeting glucose metabolism are being explored as potential treatments for diseases driven by trained immunity. In animal models of arthritis and systemic lupus erythematosus (SLE), inhibitors like 2-deoxy-D-glucose (2-DG) and 3-bromopyruvate (3-BP), which block glycolysis, have shown protective effects.141,142
Lipid metabolism, another key aspect of trained immunity, can be modulated at various stages. For instance, cytochalasin D can be used to block CD36 internalization,143 while methyl-β-cyclodextrin prevents the formation of cholesterol crystals. The enzyme HMG-CoA reductase, responsible for cholesterol synthesis, can be inhibited with drugs like fluvastatin.31 Furthermore, suppressing inflammasome activation through NLRP3 inhibitors like Z-VAD-FMK,31 MCC950, and β-hydroxybutyrate144 has shown promise in reducing IL-1β production and inflammatory responses. Additionally, omega-3 polyunsaturated fatty acids (PUFAs), which have anti-inflammatory properties, are being explored as potential therapies for autoimmune diseases like type 1 diabetes, SLE, and RA.145,146 The modulation of lipoxins, resolvins, and protectins with aspirin has also been proposed for treating SLE, given their role in resolving inflammation.147
Trained immunity is an operational adaptation of the innate immune system that enhances responses to subsequent exposures, although it can also result in inappropriate inflammatory reactions in conditions like autoimmunity. It is observed in various contexts, such as the systemic acquired response in plants, cross-protection in insect reinfection models, and in mammals lacking B and T cells. A deeper understanding of the mechanisms behind trained immunity and its regulation may give rise to the creation of a new category of vaccines and immune therapies or targeted interventions. Ongoing research in this area is expected to reveal new therapeutic targets, aid in vaccine development, and foster innovative strategies to address infectious diseases, cancer, and immune-mediated diseases. While the concept of trained immunity as a therapeutic target holds potential, several challenges remain, including identifying the primary stimulators and adjuvants of trained immunity, understanding the mechanisms involved in its establishment, and ensuring the safety and effectiveness of TIBVs in diverse populations. To address such gaps, the following recommendations are forwarded:
• Mechanistic Insights: More study is essential to uncover the detailed molecular mechanisms that govern the initiation and persistence of trained immunity. A comprehensive understanding of the epigenetic alterations, signaling cascades, and metabolic reprogramming involved will offer valuable clarity on how trained immunity is regulated and how it might be therapeutically targeted or modulated.
• Targeted Modulation: Developing strategies to selectively modulate the trained immune response holds significant promise. The ability to enhance or dampen trained immunity in specific contexts could have profound implications for infectious diseases, cancer immunotherapy, and autoimmune disorders. Investigating the precise triggers and regulators of trained immunity will aid in the creation of targeted interventions.
• Translation to Clinical Applications: Translating the knowledge gained from preclinical studies of trained immunity into clinical applications is a crucial future direction. Clinical trials are essential to evaluate the safety, efficacy, and long-term effects of interventions that modulate trained immunity. Understanding how trained immunity can be harnessed in the development of novel vaccines and immunotherapies will be instrumental in improving clinical outcomes.
• Interplay with Adaptive Immunity: Investigating the link between trained immunity and adaptive immunity is another important area of future research. Understanding how these two arms of the immune system interact and influence each other’s responses will provide a comprehensive understanding of immune memory and may lead to novel strategies for immune modulation.
• Identifying inducers: Further research should be conducted to identify compounds and molecules that can trigger trained immunity.
• Identifying adjuvants: Further investigation should be conducted to develop adjuvants that can specifically activate innate immune cells and induce trained immunity.
• Reprogramming vaccine platforms: Investigation is necessary to design vaccines conferred by trained immunity in order to modify existing vaccine platforms to specifically target innate immune cells.
We would like to thank the University of Gondar and Debre Birhan Agricultural Research Center for their provision of an internet connection and library services, which facilitated the timely completion of this work.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)