Keywords
T helper cell; global microarray analysis; allosteric inhibitor; TFactS; CD28; CD3; Akt; NF-kB
T helper cell; global microarray analysis; allosteric inhibitor; TFactS; CD28; CD3; Akt; NF-kB
Mapping global changes in gene expression has proven extremely useful in revealing previously unappreciated connections between groups of expressed genes and biological events, such as development and tumorigenesis. We have previously studied the role of the Akt kinase in T cells, and showed that a subset of NF-κB-dependent genes required Akt for optimal upregulation during T cell activation1. However, the gene expression program controlled by Akt signaling in activated T cells has not been elucidated. Although studies of individual transcription factors and their target genes have elucidated several aspects of Akt signaling2,3, understanding the overall program of transcriptional regulation controlled by the Akt pathway requires global expression analysis. We used global expression profiling to identify genes whose induction is dependent on Akt. A study from the Cantrell lab4 concluded that the chief role of Akt in CD8+ cytotoxic T cells is to control the transcriptional programs that direct effector versus memory cell fate. However, Akt may not have the same role in all T cell subpopulations. For example, constitutively active Akt can stimulate cell growth and survival of CD4+ T cells but not CD8+ T cells5,6.
In the current study, we tested the effects of a selective, allosteric inhibitor of Akt 1 and 2 (Akti1/2)7–10 on activated T cells and further explored potential mechanism of action of Akt, by performing network analysis of gene expression data and validating the expression changes of selected genes by real-time qPCR analysis. Our findings demonstrate that Akt inhibition by Akti1/2 significantly affects ribosomal protein expression and the cytokine-cytokine receptor interaction. Asthma and the antigen receptor signaling pathways were most affected by Akti1/2 in activated T cells. Moreover, Akt inhibition can decrease the enrichment of NF-κB- and Myc-targeted genes. These effects may contribute to the functions of dysregulated Akt activation in tumorigenesis, as well as in normal T cell activation and development6.
The importance of Akt for T cell activation and transformation led us to explore the underlying pathways and mechanisms (or target genes and downstream cellular pathways) by a genome-wide gene expression profiling approach. Therefore the aims of the present study were to (1) identify, at the genome-wide level, the genes that are differentially expressed in activated T helper cells, with or without Akt inhibition and (2) conduct bioinformatics analyses to identify the pathways and possible mechanisms involved.
Biotin-anti-mCD28 (37.51) and biotin-anti-mCD3ε (145-2C11) were obtained from BD-Biosciences (San Jose, CA). Streptavidin was obtained from Invitrogen (Carlsbad, CA) and Akti1/2 was from EMD Biosciences (San Diego, CA). rhIL-2 was obtained through the NIH AIDS Research and Reference Reagent program, Division of AIDS, NIAID, NIH (Cat.#136 from Hoffman-LaRoche, Inc.).
The D10 T cell line, a fast-growing variant of the D10.G41 murine Th2 T cell clone11–13 was maintained in RPMI 1640 media (Mediatech, Manassas, VA), supplemented with 10% heat-inactivated bovine growth serum (BGS; Hyclone, Logan, UT), 0.1 mM nonessential amino acids (Lonza, Walkersville, MD), 2 mM l-glutamine, 50 µM 2-ME, 100 U/ml penicillin, 100 µg/ml streptomycin (Mediatech, Manassas, VA) and 25 IU/ml rhIL-2.
D10 T cells were left untreated or pretreated with 10 µM Akti1/2 for 1 h and then stimulated with biotinylated anti-mCD3/CD28 and streptavidin for 0, 2, 6 and 12 hrs. RNA extraction was performed using a commercially available kit (RNeasy, Qiagen, Frederick, MD) according to the manufacturers’ recommendations. RNA quality was confirmed based on a RNA integrity number >8 by use of the Agilent 2100 bioanalyzer (Agilent Technologies, Palo Alto, CA). The microarray analysis was performed by Genomics and Proteomics Core Laboratories (GPCL) of the University of Pittsburgh, USA. An Illumina mouse RefSeq8 chip was used. Microarray data have been deposited in the GEO database and are accessible through the GEO series accession number GSE45221.
To compare the molecular characteristics between different time points, Automated Efficiency Analysis was first performed using 7 transformation methods, 9 normalization methods and 5 tests for differentially expressed genes14. A global normalization method and the J5_Quantile95_None method were applied on each time point. The differentially expressed genes were identified using caGEDA with a reasonable threshold of J5 for each time point15. To survey the spectrum of biological functions within genes, which were differentially expressed between different groups, functional classification of the genes were performed using Pathway Express (a pathway level Impact Analysis as described by Draghici et al., 200716). Pathway Express was designed to provide both statistical and biological significance in the indication of which pathways may be affected by the observed changes in gene expression. The results are summarized as Impact scores and p-values. Pathway-Express orders the affected pathways in the decreasing order of their expected importance for the given condition.
Quantitative real-time PCR was performed using the ABI StepOnePlus Real-time PCR system (Applied Biosystems, Foster City, CA) as described previously1. Amplification was performed on a cDNA amount equivalent to 25 ng total RNA with 1×SYBR green universal PCR Master mix (Applied Biosystems) containing deoxyribonucleotide triphosphates, MgCl2, AmpliTaq Gold DNA polymerase, and forward and reverse primers. Specific primers for each gene were purchased from SABiosciences (Qiagen, Frederick, MD). Experimental samples and no-template controls were all run in duplicate. The PCR cycling parameters were: 95°C for 10 min, and 40 cycles of 94°C for 15s, 60°C for 1 min. The amount of cDNA in each sample was calculated by the comparative threshold (Ct) method and expressed as 2exp(Ct) using 18S RNA as an internal control. Statistical significance was determined using the Student’s T test. All statistical tests were performed using GraphPad Prism (GraphPad Prism, San Diego, CA).
TFactS was used to predict the activities of transcription factors in our microarray data17. The lists of up- and down-regulated genes were compared to a list of curated target gene signatures. The nominal p-value (Pval) represents the risk of a false positive for a single test. Since the list of query genes is systematically compared to each target gene signature, a multi-testing condition is required. The e-value (Eval) represents the expected number of false positives for a given nominal value. It is computed using the formula: Eval=Pval*T, where T is the number of tests.
We previously demonstrated that Akt activity was rapidly inhibited in T cells by addition of the allosteric inhibitor Akti1/2, which inhibited phosphorylation of Akt within one minute, an effect that can last as long as twelve hours1. In the present study, microarray analysis was performed at three different time points after CD3/CD28 stimulation to characterize the effects of Akt inhibition on the T cell gene activation program in helper T cells. Thus, D10 T cells (a murine Th2 T cell line) were pre-incubated with Akti1/2 or solvent, then stimulated for 2–12 hours. After stimulation, mRNA was isolated, which was converted into labeled cDNA for hybridization to Illumina chips for microarray analysis (Figure 1A). We first determined which genes were differentially modulated after T cell receptor (TCR) stimulation using caGEDA with reasonably chosen thresholds for different time points (2 h, 6 h and 12 h). Further validation and downstream analysis were then performed to confirm some of the differentially expressed genes and to extract functional information from the dataset (Figure 1B).
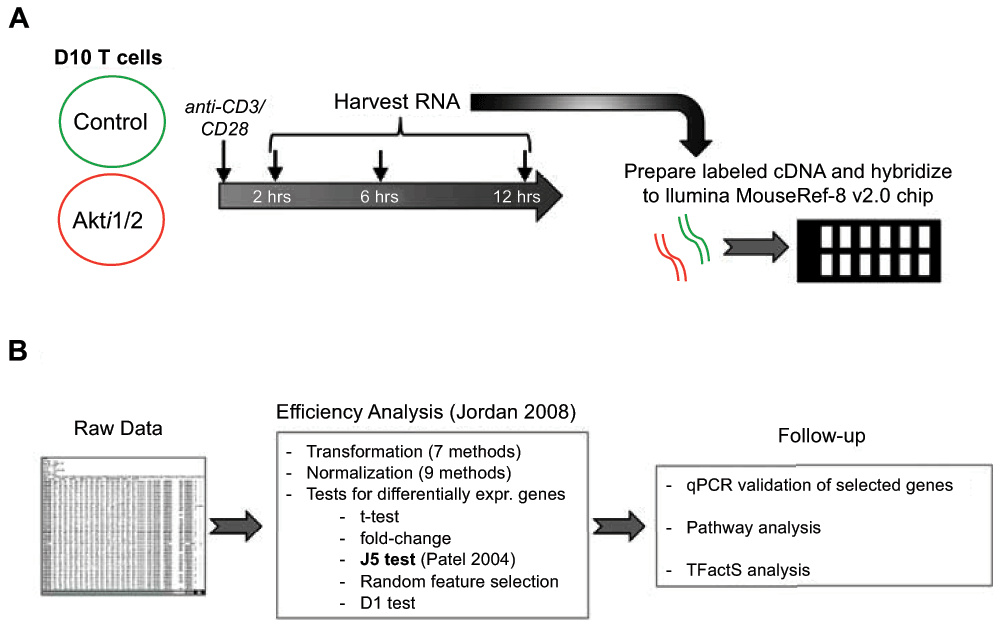
Flowchart of experimental outline (A) and data analysis (B), as described further in the text.
We identified differentially expressed gene sets that were dependent on Akt among the three different time point groups. We compared the gene expression patterns of cells plus or minus addition of Akti1/2. First, we generated two gene lists for each time point. Gene list one represents the genes that were differentially expressed between the unstimulated and CD3/CD28 stimulated group in the absence of Akti1/2. Gene list two represents the genes that were differentially expressed between the unstimulated and CD3/CD28 group in the presence of Akti1/2. When comparing the two gene lists, three different patterns were observed:
1. Genes significantly modulated by CD3/CD28 alone but not modulated in the presence of Akti1/2 (genes in this category showed Akt–dependent expression after T cell activation; first sheet in Data File 1–Data File 3 and Supplementary Figure 1)
2. Genes significantly modulated by CD3/CD28 alone but less strikingly modulated in the presence of Akti1/2 (genes in this category showed some dependence on Akt; middle sheet in Data File 1–Data File 3 and Supplementary Figure 2) and
3. Genes not modulated by CD3/CD28 alone but significantly modulated in the presence of Akti1/2 (genes in this category displayed Akti1/2-specific expression; last sheet in Data File 1–Data File 3 and Supplementary Figure 3).
By examining multiple time points after stimulation, we were able to obtain a kinetic picture of gene expression ± Akt inhibition. The 6 h Akti1/2 (+) and Akti1/2 (-) comparison showed the highest number of differentially expressed genes, and there were fewer differentially expressed genes after two or twelve hours of TCR/CD28 stimulation. Among these, only the genes that expressed the most consistent differences (either increased or decreased expression) were selected for further analysis. Genes with no known function were excluded.
Our previous work identified several NF-κB target genes that were dependent on Akt after TCR stimulation in T helper cells, including those encoding the cytokines TNF-α, GM-CSF, and IL-10, among others1. Analysis of the microarray data confirmed the dependency of these genes on Akt activation, which inspired confidence in our results. Moreover, expression of the mRNAs encoding many secreted proteins was also decreased by Akt inhibition, including IL-13, IL-5, IL-3 and IL-4 (Figure 2). The protein products of these genes (except IL-3) were examined in our previous paper1, which confirmed similar decreases after Akt inhibition. Moreover, we found that expression of Ltb (encoding lymphotoxin β), Areg (encoding amphiregulin) and genes encoding the chemokines CCL1, CCL3 and CCL4 were also affected by Akt inhibition (Figure 2).
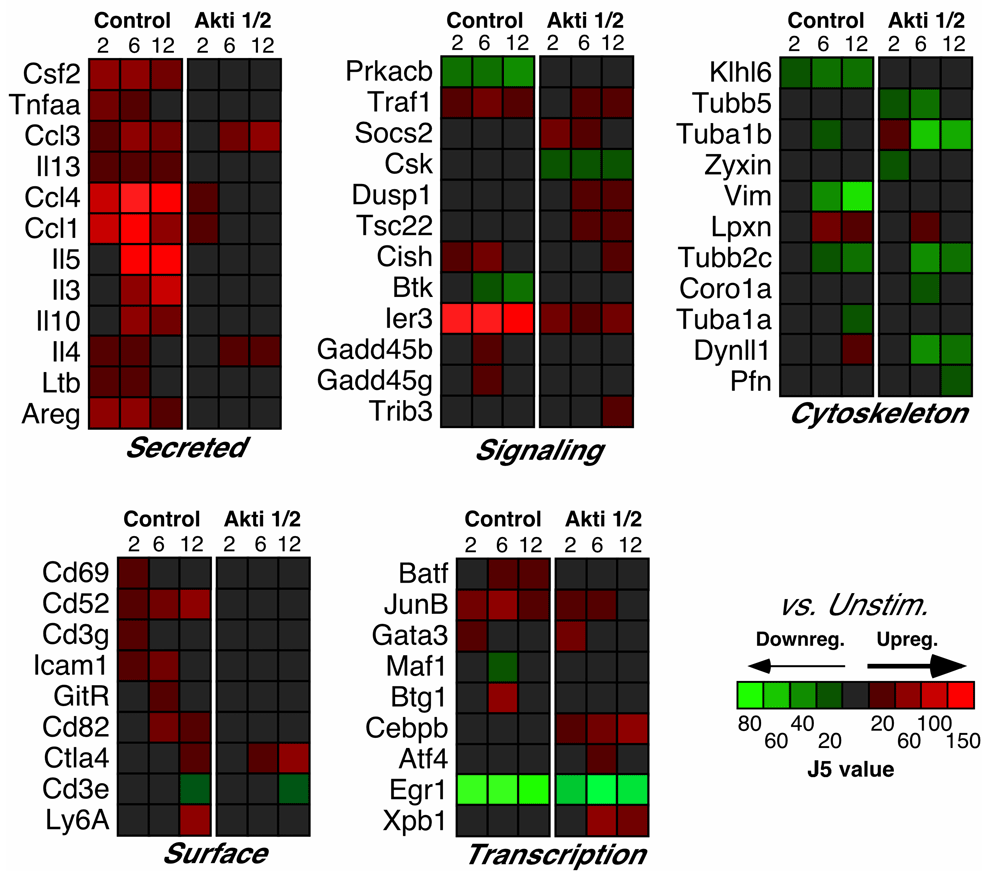
Relative levels of expression are represented by the J5 score.
Of note, several cell-surface proteins, including CD69, CD52 and CD82 were among the Akt-dependent genes after T cell activation (Figure 2). The CD82 molecule is a type III integral membrane protein with four transmembrane domains and is part of the tetra-span-transmembrane (TST) family, which also includes CD9, CD37, CD53, CD63 and CD81/TAPA-118. Interestingly, proteins of this family are involved in cell activation. For example, engagement of CD82 can deliver a co-stimulatory signal, similar to CD28, for full T cell activation, leading to strong IL-2 production19. CD52 is a small glycopeptide molecule and tethered to the outer surface of the plasma membrane by a glycosylphosphatidylinositol (GPI)-anchor20. CD52 crosslinking can also provide a co-stimulatory signal that causes the activation of normal human T lymphocytes21 and induction of CD4+ regulatory cells22. Since Akt was reported to mimic CD28 co-stimulation in a T cell line by synergizing with TCR-induced signals to increase transcription of the IL-2 promoter23, activation of these other co-stimulatory molecules might also function through this pathway.
Interestingly, inhibition of Akt resulted in impaired up-regulation of the genes encoding many ribosomal proteins (Figure 3). These included: Rps6 (a major substrate of ribosomal protein kinases24); Rps8, Rps9 (reported to be activated in various tumors, such as colon cancers25); Rps10, Rps15, Rps24 (mutations in these gene result in Diamond-Blackfan anemia)26; Rpl7a (which interacts with a subclass of nuclear receptors and inhibits their ability to activate transcription)27; Rplp1 (important for elongation during protein synthesis28). All these proteins belong to either small or large subunits of ribosomes; changes in their expression may contribute to an increase in protein synthesis to accommodate numerous cellular processes involved in T cell activation, in addition to the possible connections to cancer. Importantly, the expression of 18S ribosomal genes, which was used for our qPCR housekeeping gene, did not differ between our experimental groups.
In order to validate the microarray data and obtain more quantitative data on specific genes, real-time PCR analysis was performed at different time points after CD3/CD28 stimulation ± Akti1/2. Akt is known to directly phosphorylate FOXO3a. Once phosphorylated, FOXO3a is excluded from the nucleus and becomes transcriptionally inactive29. Klf6 is one of the FOXO-targeted genes. Real time PCR analysis confirmed that in the Akti1/2 group, there was an up-regulation of Klf6 as expected (Figure 4). Ier3 (also named IEX-1, immediate early response gene X-1), Il13, Ccl1 and Ccl4 were selected for real-time PCR validation because their expression was greatly enhanced after CD3/CD28 stimulation and decreased in the presence of the Akt inhibitor. Of note, the gene expression of Ccl1 and Il3 was rapidly up-regulated by CD3/CD28 stimulation, while the up-regulation of Ier3 and Ccl4 expression by CD3/CD28 stimulation was delayed. Egr1 was also selected as a control gene, which showed no CD3/CD28 dependent increase in gene expression. Thus, Akt inhibition could affect different genes that showed diverse kinetics after CD3/CD28 stimulation. For all genes tested, the expression changes assessed by real-time PCR confirmed the microarray results, giving us confidence in the overall quality of the dataset.
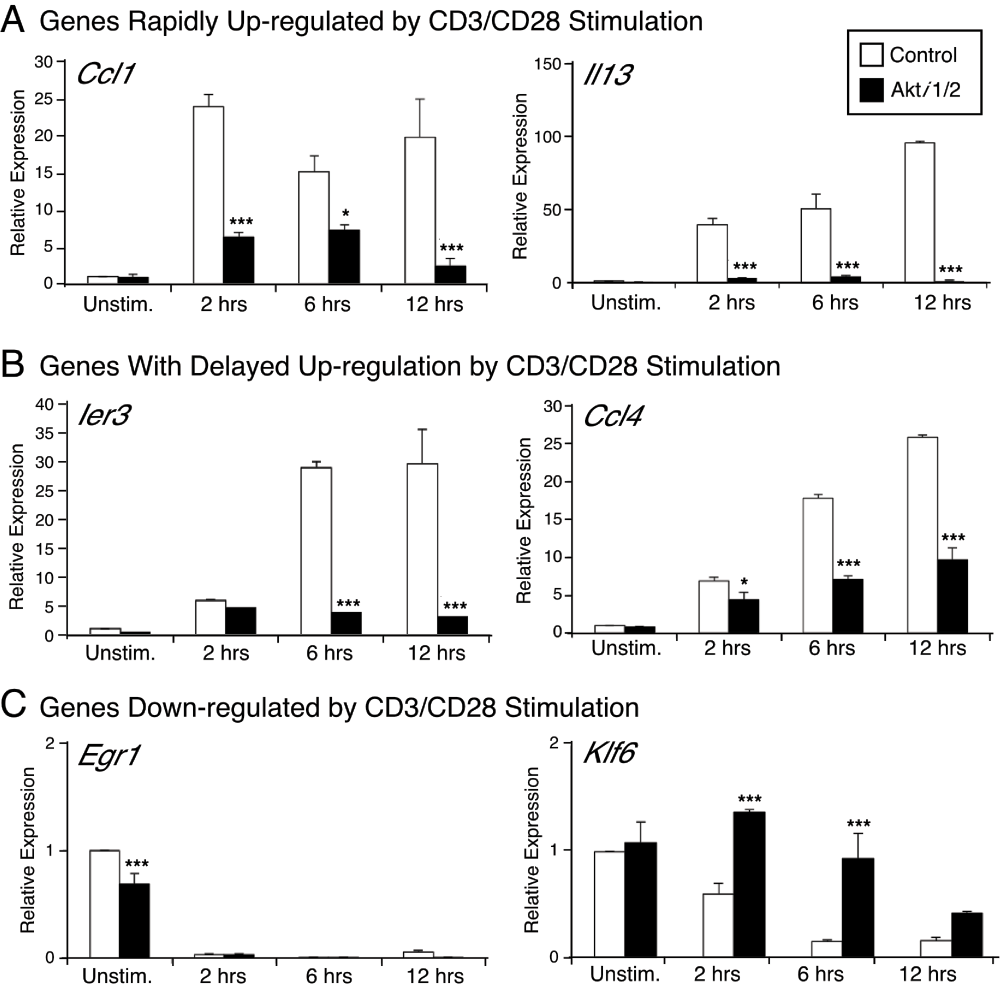
qPCR was used to validate the gene expression of Ier3, Il13, Klf6, Egr1, Ccl1 and Ccl4. The RNA samples were the same as those used for the microarray. Results are presented as relative mRNA expression, compared to the unstimulated control sample, normalized to 18S RNA expression. *** p<0.001, * p<0.05 compared with the control group.
From our previously published array data, we found that at two, six and twelve hours after CD3/CD28 stimulation. Akti1/2 elicited a wide range of effects on expression of numerous genes, including both over- and under-expressed genes. In the present study, genes that showed changes in expression after two, six and twelve hours of stimulation in the presence of Akti1/2 were largely not overlapping and cannot be combined for subsequent analysis. There were 54, 71 and 58 genes dependent on Akt at the two, six and twelve hour time points, respectively, after CD3/CD28 stimulation (Data File 1–Data File 2 and Supplementary Figure 1).
To determine whether our gene expression signature was enriched in specific subsets of genes with known biological functions, bioinformatic functional classification analysis of the genes that were differentially expressed was carried out as described in the Methods. The functional classifications of gene sets are illustrated in Figure 5. We found that genes involved in ribosome, cytokine-cytokine receptor interaction, antigen receptor signaling pathway, hematopoietic cell lineage and asthma were significantly enriched among genes affected by Akt inhibition in the presence of CD3/CD28 stimulation.
Co-expressed genes are often regulated by common transcription factors. Therefore, we separately analyzed the genes that showed altered expression at 2, 6 and 12 h time points after CD3/CD28 stimulation using TFactS, an algorithm that used a catalog of well-characterized transcription factor targets to predict the activity of transcription factors based on microarray data17. Enrichment of transcription factors was found at all the time points tested (Figure 6). These included NF-κB family members (RelA, Rel, NF-κB1), Myc, Jun and CREB. At the 2 h time point, Myc, NF-κB family members (NF-κB1, RelA and Rel) and Egr1 were significantly enriched. RelA, NF-κB1 and Rel, along with Myc and Egr1 were also significantly regulated after 6 h of CD3/CD28 stimulation. Moreover, other transcription factors, including Sp1, CEBPB, Ets1, Jun and Nfam1, were also enriched at the 6 h time point. At 12 h after stimulation, however, fewer transcription factors were regulated, with Myc and NF-κB1 still significantly regulated. In summary, the most significantly enriched transcription factor binding sites in the regulatory regions of Akt-dependent genes after CD3 and CD28 stimulation were those of NF-κB family members and Myc.
Akt activation impacts the expression of genes responsible for cell proliferation and survival30,31. The majority of previous global gene expression studies have investigated transcriptional programs under the combined control of both PI3K and Akt32,33. Despite much attention in recent years to the role of Akt-regulated metabolism in T cells34, a recent study provided clear evidence that Akt indeed contributes to changes in gene expression after activation of cytotoxic T cells4. We have also been interested for some time in achieving a greater level of clarity in separating the effects of PI3K and Akt during helper T cell activation35. Our recent study identified a subset of NF-κB-dependent genes that require Akt for optimal upregulation during helper T cell activation by using a targeted gene profiling approach1. In the present study, we performed a broader microarray analysis to characterize the global changes in gene expression resulting from inhibition of Akt in activated CD4+ helper T cells. Analysis of the affected genes revealed pathways that are central to the effects of Akt on helper T cell activation. Finally, analysis of the enrichment in transcription factor binding sites in our target genes further confirmed NF-κB as a regulator of these genes in response to TCR stimulation.
The generation and maintenance of memory T cells is central to the development of protective immunity, as characterized by a rapid and vigorous response after a secondary encounter with a given pathogen or antigen36,37. Recent studies have suggested that proper regulation of Akt activity is essential for the development of memory T cells. Riou et al. found that Akt plays a critical role in the phosphorylation of FOXO3 in CD4+ central memory T cells (TCM), thereby promoting TCM survival38. Abrogating the Akt survival pathway led to a greater degree of apoptosis in TCM as compared with effector memory T cells (TEM), confirming that TCM are more dependent on these pathways for their survival. Sustained and strong activation of Akt was also shown in CD8+ cytotoxic T cells (CTL) to coordinate the TCR and IL-2-induced transcriptional programs that control expression of key cytolytic effector molecules, adhesion molecules, and cytokine and chemokine receptors that distinguish effector from memory and naïve T cells4. It has been suggested39 that Akt simultaneously induces and represses expression of key genes, leading to the development of effector CTL, with the FOXO transcription factors being at the center of this process. Thus, Kim et al. found that Akt appeared to function as a cellular rheostat, controlling distinct facets of the program that governed differentiation of Ag-activated CD8+ T cells into effector cells or memory CD8+ T cells39. Myristoylated Akt transgenic mice were found to accumulate memory phenotype CD4+ T cells and to develop both tumors and autoimmunity40, effects that could, in principle, be due in part to non-metabolic outcomes of Akt activation, such as NF-κB activation41.
Interestingly, the expression of Klf6, a FOXO-target gene, was increased in activated T helper cells after Akti1/2 treatment in our study. Although Klf6 did not appear in the differentially expressed gene list at any of the time points, real-time PCR showed that its expression was increased after Akt inhibition. It is known that FOXO transcription factors control regulatory T cell development and function42. Moreover, early Treg-cell-lineage commitment is regulated by Akt and FOXO family transcription factors43,44. Further studies are needed to clarify whether Akt has a similar effect on CD4+ memory cells. FOXO and NF-κB are both known targets for Akt45,46. The enrichment of multiple NF-κB family members in Akt-dependent genes confirmed our previous study emphasizing the important role of NF-κB in Akt-dependent biological processes1. FOXO-target genes were not enriched in the Akt dependent gene list after T helper cell activation, although their expression (such as that of Klf6) was increased after Akt inhibition. The Akt-dependent transcription factors that are induced by TCR stimulation in T helper cells might be different from those induced in CTL.
Of note, Myc target genes were also found enriched in the subset of Akt-dependent genes after T cell activation. It is well known that Myc is associated with cell activation. However, it is now thought that Myc is not an on-off specifier of a particular transcriptional program(s) but is a universal amplifier of gene expression, increasing output at all active promoters47. Relevant for our findings, N-Myc was reported to function as a regulator of cell growth by stimulating expression of genes functioning in ribosome biogenesis and protein synthesis48. Akt was also reported to cooperate with c-Myc to increase ribosome biogenesis and cell growth, which includes the synthesis of rRNA and ribosomal proteins, processing of 45S rRNA, and assembly of functional ribosomal subunits40. In our present study, we showed that Akt inhibition decreased the expression of many ribosomal proteins, including components of both the small (40S) and large (60S) ribosomal subunits. Putting together these disparate observations, it may be that in activated T helper cells, one of the mechanisms through which Akt broadly regulates ribosomal subunit transcription is by activating Myc.
Akt is also known to enhance ribosomal protein production through the mammalian target of rapamycin (mTOR) pathway. However, it was reported that direct inhibition of Akt only weakly suppressed the phosphorylation of S6 and S6K1 in CTLs4 and Akti1/2 could only weakly suppress the CD3/CD28 stimulation dependent phosphorylation of S6 in Jurkat T cells and primary T cells (unpublished data from our lab). Akt could signal through mTORC1-dependent and independent mechanisms to promote rDNA transcription in mammalian cells40. Dissecting out the mTOR-independent mechanism that Akt utilizes to regulate ribosomal biogenesis is crucial to understand the therapeutic response to Akt inhibitors in cancer.
The project was conceived by LPK. Experiments were carried out by JC. Data were analyzed by JC and LPK, in consultation with the University of Pittsburgh Genomics and Proteomics Core Laboratory (GPCL). The paper was written by JC and LPK.
We thank James Lyons-Weiler and Haiwen Shi of the University of Pittsburgh Genomics and Proteomics Core Laboratory (GPCL) for assistance with data analysis.
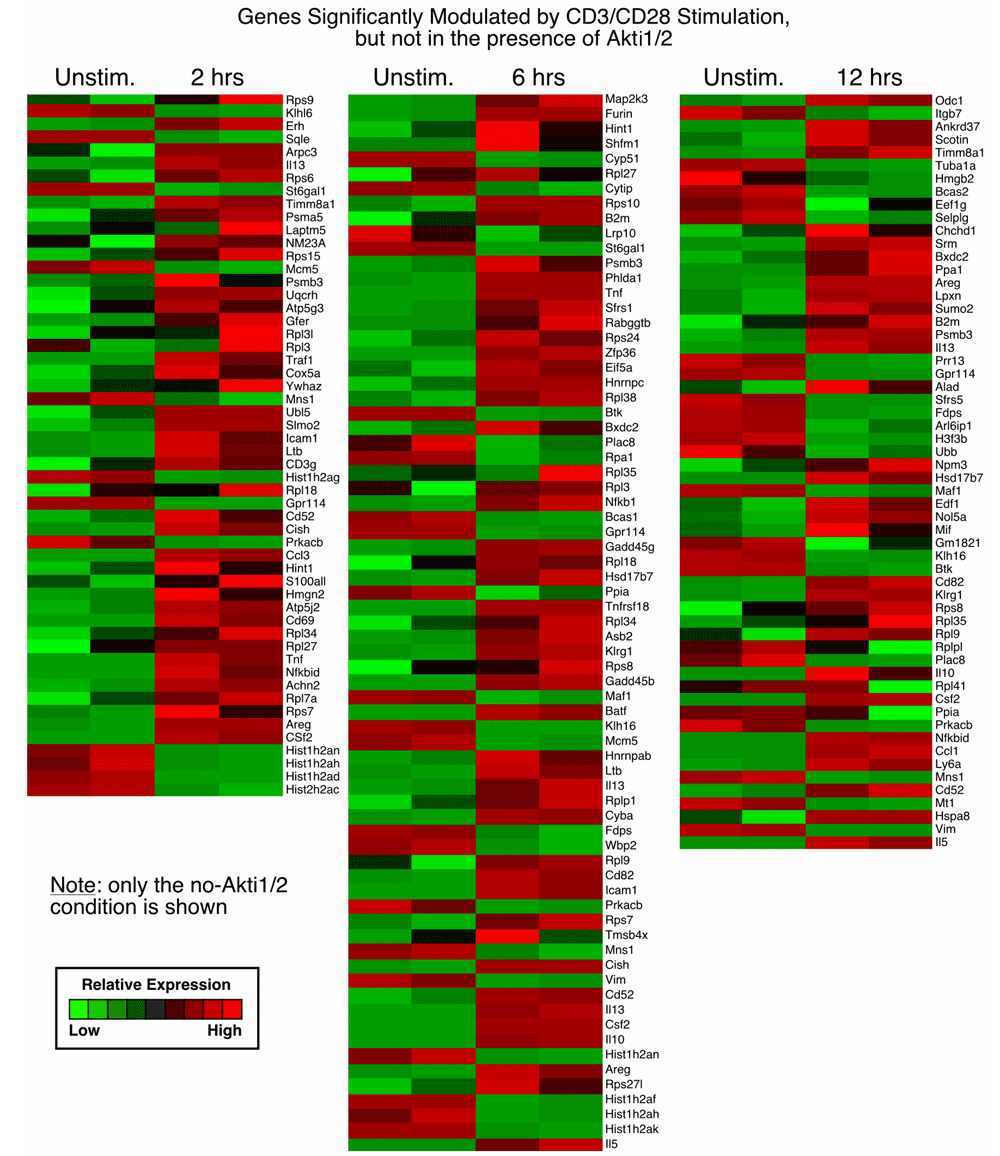
Each row represents a gene and each column shows one of the duplicates from two different groups (from left to right 0 h vs. 2 h, 0 h vs. 6 h, 0 h vs. 12 h). The red and green colors reflect high and low expression levels, respectively.
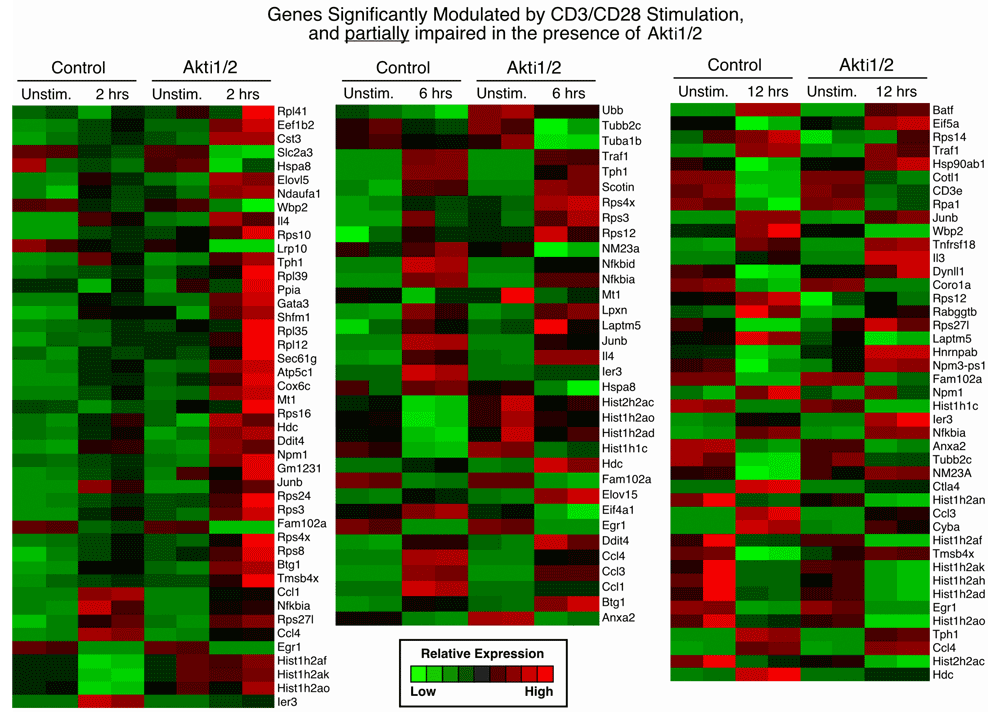
Each row represents a gene and each column shows one of the duplicates from two different groups (from left to right 0 h vs. 2 h, 0 h vs. 6 h, 0 h vs. 12 h). The red and green colors reflect high and low expression levels, respectively.
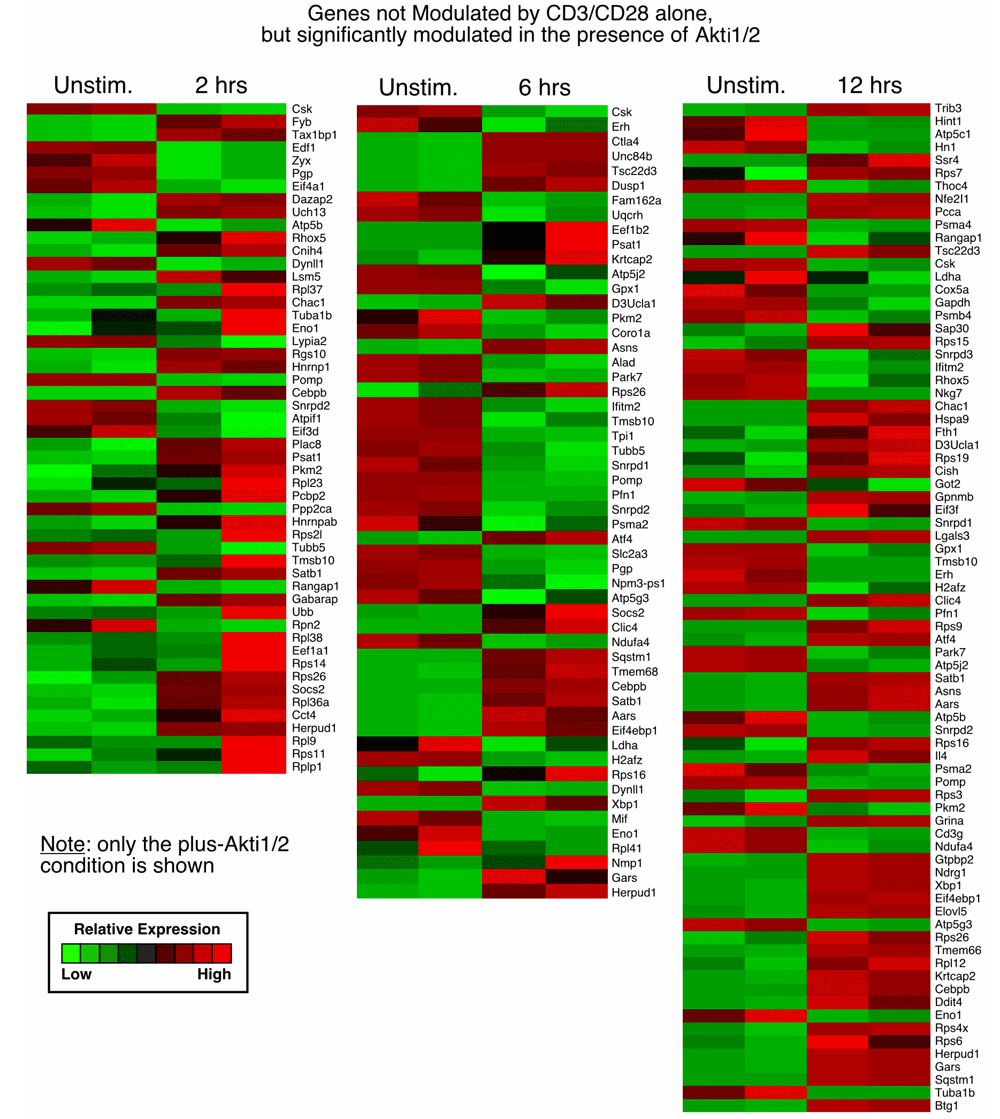
Each row represents a gene and each column shows one of the duplicates from two different groups (from left to right 0 h vs. 2 h, 0 h vs. 6 h, 0 h vs.12 h). The red and green colors reflect high and low expression levels, respectively.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (update) 15 May 13 |
read | read | read |
|
Version 1 15 Apr 13 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)