Keywords
apoptosis, FAM129B, Wnt/β-catenin, siRNA, melanoma, tumor
apoptosis, FAM129B, Wnt/β-catenin, siRNA, melanoma, tumor
The incidence of melanoma continues to rise across the U.S. at a rate faster than any other cancer2. Malignant melanoma has a poor prognosis with a 5-year survival rate of only 15%3. The recently approved therapeutic, vemurafenib, extends median patient survival by 7 months4–6. This major advance raises expectations that even greater rates of survival might be attainable with combination therapies.
Activation of the Wnt/β-catenin pathway decreases tumor growth and cooperates with ERK/MAPK pathway inhibitors to promote apoptosis in melanoma1,7–13. Analysis of melanoma tumor samples show a positive correlation between nuclear β-catenin staining and decreased tumor depth, increased patient survival and increased time to metastasis14–17. Moreover, treatment with WNT3A-containing conditioned media or stable overexpression of WNT3A in mouse B16 or human A375 melanoma cells reduces cell number in vitro10–12. Allografts of mouse B16 or mouse xenografts of human A375 cells overexpressing WNT3A decrease tumor size compared to control17,18. Recently, we found that activation of Wnt/β-catenin signaling concurrent with the inhibition of the ERK/MAPK pathway synergistically elevates apoptosis in a subset of BRAF- and NRAS-mutant cultured human melanoma cells18,19. Given the interaction between Wnt/β-catenin signaling and pathways known to be critical for melanoma pathogenesis, the identification of Wnt/β-catenin regulators might prove to be informative in developing novel approaches to treat this disease.
In the present study, we identify novel regulators of Wnt/β-catenin signaling in melanoma by performing a large-scale small-interfering RNA (siRNA) screen of a Wnt/β-catenin responsive reporter in human HT1080 fibrosarcoma cells, and by identifying siRNA targets that are also regulated by ERK/MAPK signaling and that have been previously associated with melanoma. By integrating these three approaches, we identified FAM129B as a potential regulator of Wnt/β-catenin signaling. FAM129B is a 746 amino acid protein that contains an amino-terminal pleckstrin homology (PH) domain and a differentially phosphorylated carboxy-terminal region20. FAM129B is known to inhibit TNFα-dependent apoptosis in HeLa cells21. FAM129B is expressed in melanoma and promotes tumor cell invasion into collagen matrices in an ERK/MAPK phosphorylation-dependent manner20. In the present study we demonstrate that FAM129B promotes Wnt/β-catenin signal transduction in melanoma and that reducing levels of FAM129B with siRNA reduces the ability of WNT3A to increase apoptosis in melanoma cells.
In order to identify novel regulators of Wnt/β-catenin signaling, we performed a siRNA screen. We used HT1080 cells stably transduced with a luciferase reporter of β-catenin-mediated transcription (BAR)22. We screened 28,044 pools of siRNAs. 19,490 gene products were targeted by one or more siRNA pool. Cells were transfected with siRNAs and treated with WNT3A-conditioned media to activate the reporter. BAR activity was normalized to the activity of Renilla luciferase driven by the constitutive TK promoter to control for total cell number. siRNAs targeting positive control proteins such as the known Wnt/β-catenin inhibitor, AXIN2, modulated BAR activity by at least 2.0 fold with a p-value less than 0.01 (Figure 1b). Using this as a criterion, we found that 10,215 siRNA pools regulated BAR activity. Of the 19,490 gene products targeted by one or more siRNA in our screen, we identified 5189 gene products for which every given siRNA significantly regulated BAR activity (Data File 1).
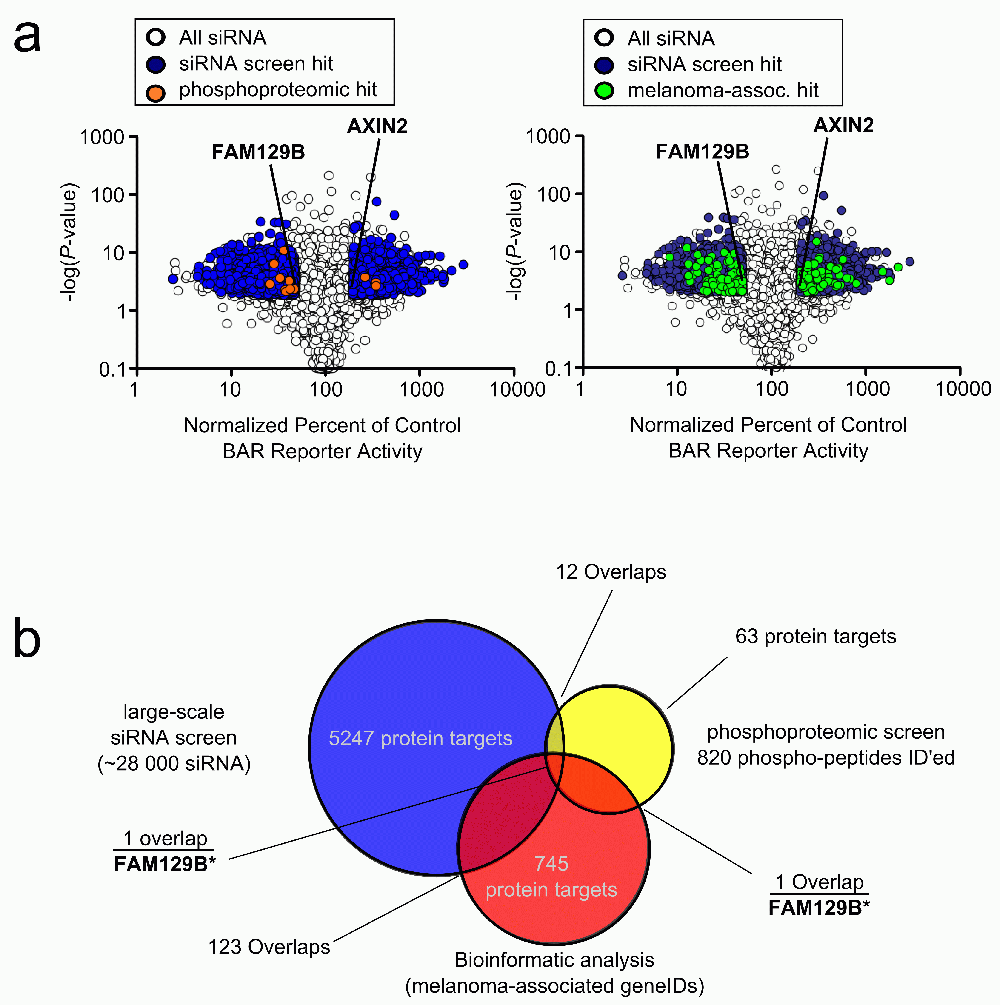
(a) Volcano plots depicting siRNA screen hits overlaid with phosphoproteomic data or bioinformatic data (left panel). Median effect of each siRNA treatment as a percent of control siRNAs were plotted against the p-value of that treatment. If, for a given gene, all siRNAs targeting that gene showed a twofold change in normalized reporter activity and a p-value <0.01, that gene was classified as a hit. This screen identified 5,189 gene products as hits, which are depicted in dark grey. Overlapping phosphoproteomic data from Old WM et al. (2009)15 are depicted in light grey. The known regulator of Wnt/β-catenin signaling, AXIN2, is indicated, as is FAM129B (right panel). Data plot is the same as the left panel with melanoma-associated genes plotted in light grey instead. (b) Venn diagram depicting overlaps between phosphoproteomic dataset, siRNA screen and melanoma-associated genes. 12 protein targets overlap between the phosphoproteomic hits and the siRNA screen, 1 protein target overlaps between the phosphoproteomic hits and melanoma-associated protein targets, and 123 proteins overlap between the siRNA screen hits and melanoma associated protein targets. Only FAM129B overlaps with all three datasets.
To refine the results of our large-scale siRNA screen, we performed an integrative analysis of our siRNA screen regulators by cross-referencing these regulators with a list of genes previously identified in melanoma, and a list of gene products phosphorylated downstream of MEK and ERK in melanoma. First, we identified 12 proteins in common between the siRNA and phosphoproteomic screens (Figure 1a and Data File 3a). Next, we generated a list of melanoma-associated genes using a custom biopython script (Data File 2 and Query Script). We identified 745 melanoma-associated genes by querying the NCBI gene database. Of these, one gene (FAM129B) encoded a protein that was differentially phosphorylated following MEK inhibition (Figure 1 and Data File 3a) and 123 were gene targets of siRNA pools that regulated Wnt/β-catenin signaling (Figure 1b and Data File 3b). Finally, we discovered FAM129B as the only melanoma-associated gene that both modulated Wnt/β-catenin signaling and was phosphorylated following MEK activation, (Figure 1b and Data File 3a).
The siRNA screen suggested that FAM129B is a regulator of Wnt/β-catenin signaling. In order to confirm this possibility, we designed three independent siRNAs targeting FAM129B. First, we confirmed that all three siRNAs inhibit expression of FAM129B protein in HT1080 fibrosarcoma, A2058 melanoma and A375 melanoma cells (Figure 2a). Next, we asked whether the siRNAs inhibited the ability of WNT3A to activate BAR. Indeed, we found that each FAM129B siRNA reduced the ability of WNT3A to activate BAR in all three cell lines (Figure 2b). We also tested whether FAM129B siRNAs reduce the ability of WNT3A to elevate expression of the endogenous β-catenin target gene, AXIN2. Similar to inhibition of BAR, FAM129B siRNAs significantly reduced levels of AXIN2 transcript relative to control siRNA (Figure 2c). From these data, we conclude that FAM129B knockdown inhibits the ability of WNT3A to promote β-catenin mediated transcriptional activation.
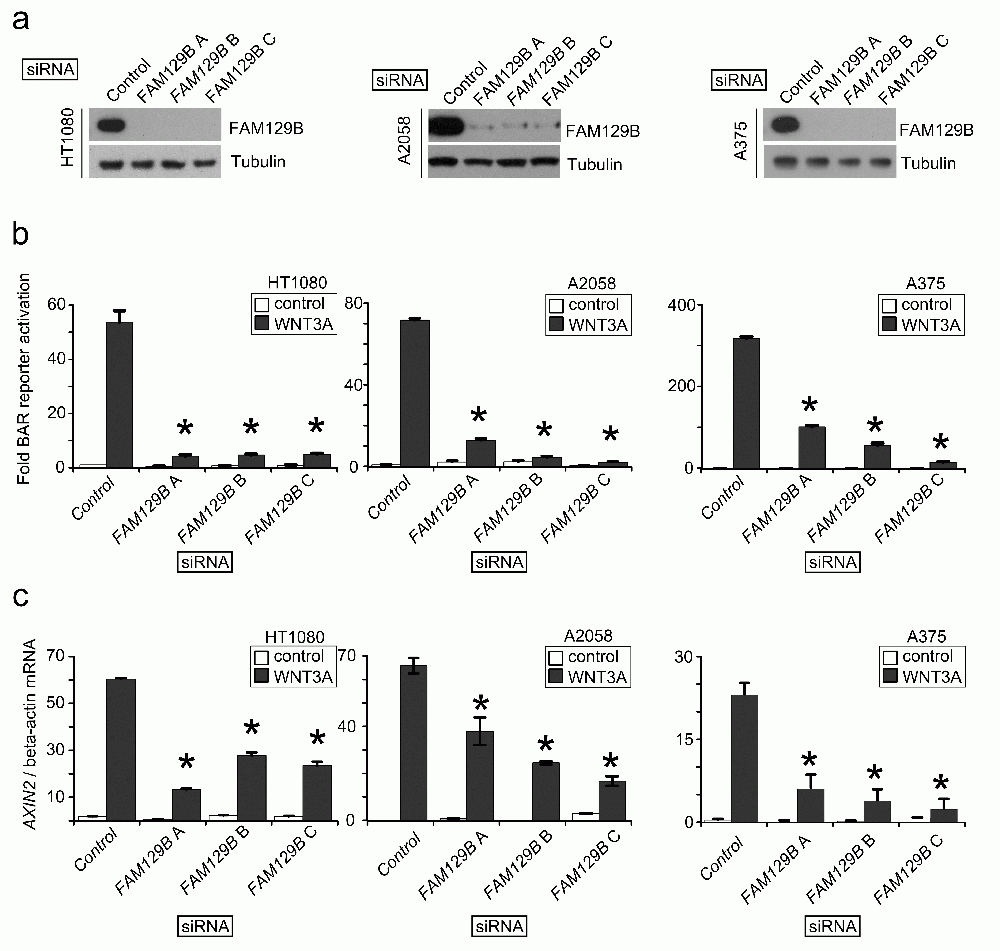
(a) Immunoblots show three independent siRNAs reduce steady-state levels of FAM129B following 72 hr treatment with 20 nM siRNA. The beta-tubulin immunoblot serves as a control. Three independent siRNAs targeting FAM129B inhibit FAM129B expression in HT1080 (left), A2058 (middle), and A375 cells (right). (b) FAM129B siRNA inhibit WNT3A-dependent luciferase reporter activity (BAR reporter) normalized to constitutively expressed Renilla luciferase in HT1080 (left), A2058 (middle), and A375 cells (right). (c) FAM129B siRNA inhibit Wnt-dependent AXIN2 expression in HT1080 (left), A2058 (middle), and A375 cells (right) relative to beta-actin mRNA expression by qPCR. Columns and error bars represent mean and SEM, respectively. Data are representative of at least three separate biological replicates. *p<0.05 by unpaired, two-tailed T-test.
While FAM129B modulates Wnt/β-catenin signaling in the above assays, these experiments do not rule out the formal possibility that reducing levels of FAM129B might affect other signaling pathways. We therefore generated A375 melanoma cell lines stably transduced with a luciferase-based reporter to the TNFα pathway. We then transfected cells with FAM129B siRNAs and stimulated the reporters with cognate ligands. While FAM129B siRNAs inhibit activation of the BAR reporter by WNT3A across a wide range of doses (Figure 3a), FAM129B siRNA has only negligible effects on TNFα-dependent NFκB reporter activity (Figure 3b). While this result does not allow the conclusion that FAM129B functions solely as a modulator of β-catenin signaling, this result does suggest that FAM129B is not required for activation of all pathways.
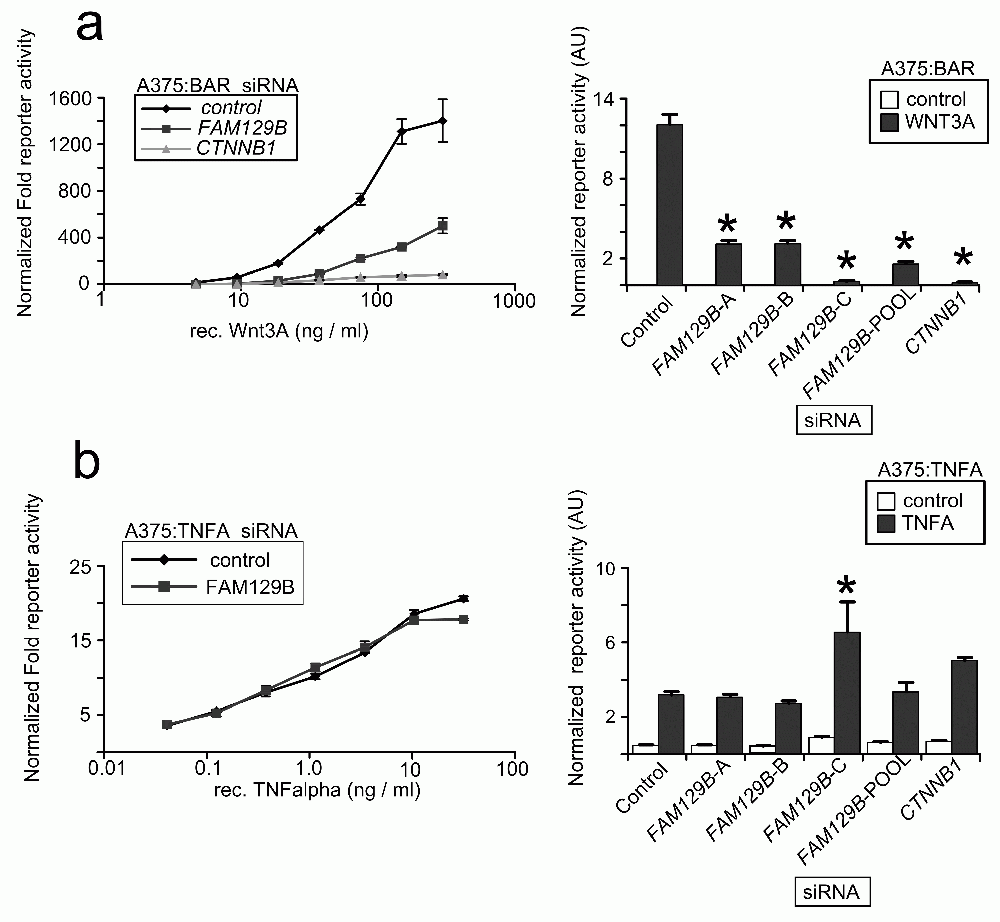
(a, left panel) pooled FAM129B siRNAs inhibit Wnt-dependent BAR reporter activity over a wide range of doses. Increasing doses of WNT3A d increases activation of the BAR reporter (normalized to constitutive Renilla luciferase in control treated cells). WNT3A does not activate the reporter in the presence of FAM129B or CTNNB1 siRNAs. (Right panel) A375 cells were treated with siRNAs as indicated and treated with an EC50 dose of WNT3A (50 ng/ml). All FAM129B siRNA and positive control CTNNB1 siRNA inhibit Wnt-dependent BAR reporter activity. (b) The same experiment was carried out as in (a, left panel) in A375 lines TNFα/NFκB reporter. Data in the left panel indicate dose-dependent activation of the NFκB reporter by TNFα. However, FAM129B siRNAs do not inhibit the activation of the TNFα/NFκB reporter. (Right panel) FAM129BsiRNA do not regulate activity of the NFκB reporter activated by 1.5 ng TNFα/ml in A375 cells. High dose TNFα (10 ng/ml) does differentially activate the reporter. Data represent 3 separate biological replicates. *p<0.05 by unpaired, two-tailed T-test.
The combined treatment with WNT3A protein and compounds that inhibit ERK/MAPK signaling synergizes to induce robust apoptosis in cultured melanoma cells18,19. If FAM129B is required for Wnt/β-catenin signaling, then FAM129B loss of function should inhibit this synergy. We monitored apoptosis in A375 melanoma cells by western blot for cleaved caspase-3 and immunofluorescence staining for TUNEL (terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling). As previously reported18,19, A375 cells treated with control siRNA and the combination of WNT3A and PLX4720 exhibit robust levels of cleaved caspase-3 (Figure 4a). siRNA mediated knockdown of FAM129B decreases the levels of cleaved caspase-3 in response to WNT3A siRNA (Figure 4a–4c). Moreover, when measuring WNT and PLX4720-dependent apoptosis by TUNEL staining, we found that siRNA mediated FAM129B knockdown reduced the number of TUNEL positive cells as compared to control siRNAs. Collectively, these results show that FAM129B is required for the synergy between Wnt3A and PLX4720 to induce melanoma apoptosis.
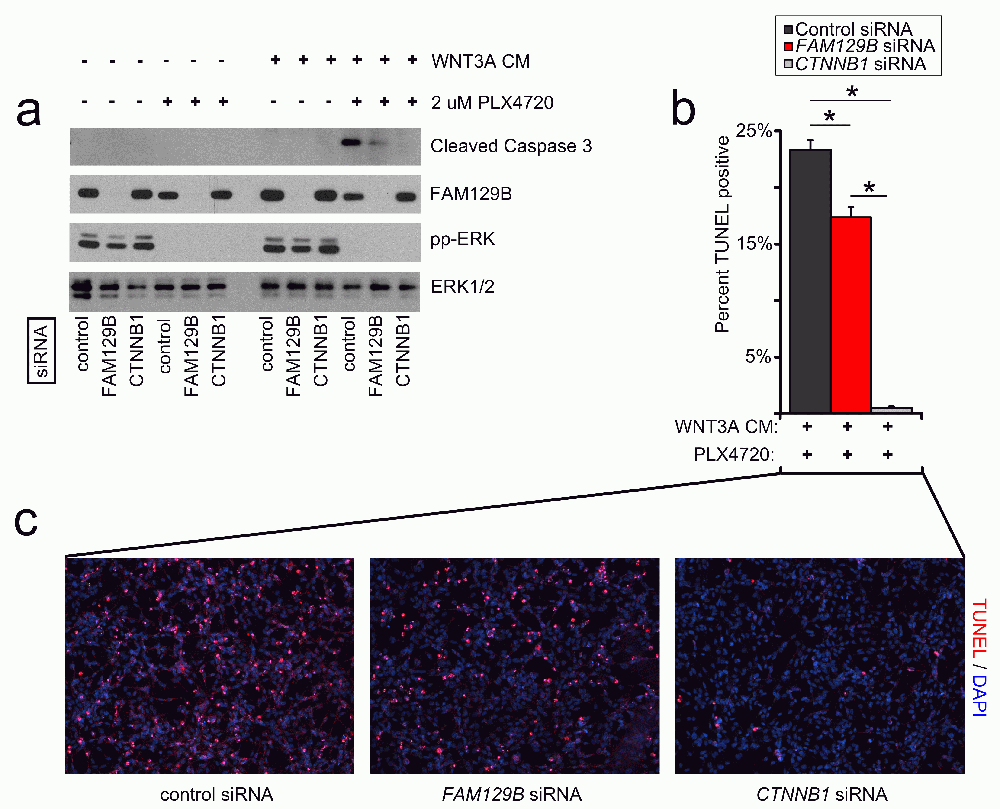
(a) FAM129B siRNA inhibits Wnt-dependent apoptosis as monitored by cleaved caspase-3 immunoblot. A375 cells were treated with pooled control, pooled FAM129B siRNA, or CTNNB1 siRNA as indicated for 48 hr. Cells were subsequently treated with DMSO or 2 µM PLX4720, and L-conditioned or WNT3A-conditioned media for 24 hr as indicated. Knockdown of FAM129B was monitored by FAM129B immunoblot, inhibition of ERK/MAPK signaling by phospho-ERK immunoblot, and total ERK was used as normalization. Relative levels of cleaved caspase-3 were quantitated by normalizing cleaved caspase-3 pixel density to ERK1/2 for each condition relative to the maximum cleaved caspase-3 level. Data are representative of at least 3 biological replicates. FAM129B siRNA inhibit cleaved caspase-3 levels to between 16 and 41% of maximum. (b) FAM129B siRNA inhibits Wnt-dependent apoptosis as quantified by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) immunofluorescence (IF). A375 melanoma cells were treated as above, fixed and stained using TUNEL. Percent TUNEL positive cells calculated as a percent of DAPI positive cells. (c) Representative immunofluorescence of A375 cells treated with the indicated conditions. TUNEL staining is depicted in red and DAPI staining is depicted in blue. Columns and error bars represent the mean and SEM of three separate biological replicates. *p<0.05 by student’s T-test.
We combined phosphoprotoemics and siRNA screening to identify novel regulators of Wnt/β-catenin signaling in human melanoma. We focused on FAM129B, a previously identified protein that has not formerly been linked to Wnt/β-catenin signaling. Using independent siRNAs, we confirmed that FAM129B is required for Wnt3A to activate a β-catenin dependent reporter and reduces the ability of Wnt3A to enhance the expression of the β-catenin target gene AXIN2. We demonstrated that loss of function of FAM129B inhibits the apoptosis of melanoma cells induced by the combined treatment with WNT3A and PLX4720.
FAM129B siRNAs suppress apoptosis in melanoma cells treated with WNT3A and PLX4720. This result was surprising given that the transfection of FAM129B siRNA in HeLa cells promotes increased apoptosis in response to TNFα and cyclohexamide21. The discrepancy between the ability of FAM129B siRNAs to suppress Wnt-dependent apoptosis in melanoma and the ability of these siRNA to promote TNFα-mediated apoptosis in HeLa remains unresolved, although it does suggest that FAM129B may function in a manner that is dependent on cellular context. Alternatively, the differences in apoptotic response with FAM129B loss of function may merely reflect the regulation of Wnt/β-catenin signaling in these two cell types. Uncovering the underlying roles of FAM129B in the cell may well illuminate how FAM129B exerts these opposing effects on apoptosis in response to different stimuli. Future studies should probe the role, if any, of TNFα/NFκB in melanoma apoptosis and the cross-talk between Wnt/β-catenin and TNFα/NFκB signaling in cell lines, such as HeLa, that respond to TNFα by apoptosis.
Detailed information on the β-catenin activated reporter plasmid (pBARLS) has been previously described23,24. Briefly, the reporters are generated from lentiviral plasmids that contain 12 TCF/LEF binding sites (5´-AGATCAAAGG-3´) or Nuclear Factor Kappa B (5´-GGGAATTTCC-3´) signaling pathways separated by distinct 5-base pair linkers upstream of a minimal promoter and the firefly luciferase open reading frame. The reporters also contain a separate PGK (phosphoglycerate kinase) promoter that constitutively drives the expression of a puromycin resistance gene for mammalian cell selection. These reporters were generated by Travis L. Biechele in the lab of Randall T Moon as previously published23,24.
Human A375 and A2058 cells were a generous gift from Cassian Yee (Fred Hutchinson Cancer Research Institute, Seattle, WA). HT1080 cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA). Stable reporter lines were generated as previously described22. Cell lines were cultured in a Thermo Forma steri-cult humidified incubator (#3310, Thermo Scientific, Rockford, IL) at 37°C and 5% CO2. All cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, #11965–084 Invitrogen, St. Louis, MO) containing 10% fetal bovine serum and 1% Penicillin/Streptomycin (Invitrogen, Grand Island, NY), except A375 cells, which were grown in DMEM containing 5% FBS and 1% P/S.
Control (LCM) and WNT3A-conditioned media (WNT3A CM) used to activate the Wnt/β-catenin signaling pathway were prepared as previously described25. To monitor reporter activity and transcript activity, cells were treated with 10% WNT3A CM or LCM overnight before proceeding to subsequent assays. To monitor effects on apoptosis, cells were treated with 1% LCM or WNT3A and DMSO (Sigma St. Louis, MO, product 472301) or 2 uM PLX4720 (Symansis, Timaru New Zealand SY-PLX4720).
The large-scale siRNA screen was performed as previously described26, with minor modifications. Briefly, HT1080 cells stably transduced with BAR firefly luciferase and Renilla luciferase lentivirus were reverse-transfected in 1536-well plates, with a final concentration of pooled siRNA at 25 nM. 48 hours after reverse transfection, cells were treated with WNT3A-conditioned media. Following overnight incubation, β-catenin dependent transcription was measured by assaying firefly luciferase activity and normalized by monitoring constitutively expressed Renilla luciferase activity as described in the Promega Dual glo luciferase assay system technical manual (Promega, Madison Wi). All siRNAs were designed with a proprietary algorithm27.
Approximately 200,000 A375, A2058, or HT1080 cells (as estimated by hemocytometer counts) were reverse transfected at a final dose of 20 nM siRNA in 6-well format using 5 µl RNAi max/well (Invitrogen, Grand Island, NY). Medium GC universal stealth control siRNA was used as a negative control (Cat. No. 12935–112, Invitrogen, Grand Island, NY). Invitrogen’s stealth siRNA targeting FAM129B were designed using the BLOCK-iT RNAi designer and are described below. The sequence for "FAM129B A" is UCACGGACAUGAACCUGAACGUCAU. The sequence for "FAM129B B" is ACUGAGGUGCGAGAUGUCUUCUUCA. The sequence for "FAM129B C" is CAGCAGCGAUUUGAUGUGUCCAGCA. As a positive control for inhibition of Wnt/β-catenin signal transduction by siRNA, we used silencer select siRNA targeting CTNNB1 with the sequence GGUGGUGGUUAAUAAGGCUTT (Invitrogen, Grand Island, NY).
24 hr after siRNA transfection, cells were plated in 96-well plates at a density of 20,000 cells/well. Twenty-four hours after plating, cells were treated with the indicated conditions, and luciferase activity was measured 15 hours later with a Dual-Luciferase Reporter Assay kit (Promega, Madison, Wi) and an Envision multilabel plate reader (PerkinElmer, Waltham, MA) according to the manufacturer’s suggestions.
24 hr after siRNA transfection, cells were split into a 12-well cluster plate at approximately 50% confluency. 24 hr later, cells were treated with WNT3A- or L-conditioned media. After overnight treatment, RNA was isolated using Trizol reagent according to the manufacturer’s instructions (Invitrogen). 1 µg of RNA was reverse transcribed using Fermentas’ RevertAid M-MuLV Reverse Transcriptase (Fermentas, Glen Burnie, MD). QPCR was performed on a Lightcycler 480 (Roche, Indianapolis, IN) using Lightcycler 480 DNA SYBR Green 1 master mix (04707516001 Roche, Indianapolis, IN). The following primers were used for qPCR: "AXIN2 F" CTCCCCACCTTGAATGAAGA and "AXIN2 R" TGGCTGGTGCAAAGACATAG; and, "ACTB F" AGAGCAAGAGAGGCATCCTC and "ACTB R" CTCAAACATGATCTGGGTCA.
To test for siRNA knockdown, replicate cell lysates from low throughput reporter assays were pooled and treated with 10× RIPA lysis buffer (500 mM Tris, pH 7.5, 1.5 M NaCl, 10 mM EDTA, 10% Igepal CA-630, 1% SDS, and 2% sodium deoxycholate all purchased from Sigma, St. Louis, MO). For monitoring cleaved caspase-3, 90% confluent 12-well plates were treated for 24-hr with the indicated conditions described in the "cell lines and cell culture" section. Media were collected and cells were rinsed once (gently) with PBS. Cells were lysed on-plate in 100 µl 1× RIPA buffer containing protease and phosphatase inhibitors (Complete EDTA-free and PhoStop by Roche, Indianapolis, IN). Cells were disrupted by scraping of a 1000 µl pipette tip against the plate. Apoptotic cells present in the media and PBS wash were centrifuged at 300 g, rinsed once with PBS, and lysed with the RIPA buffer collected from the plate lysis. Cell lysates were cleared by centrifugation at 20,000 g at 4°C for 10 minutes. Protein lysates were separated by SDS-PAGE using NuPAGE 4%–12% Bis-Tris gels (NP0336BOX, Invitrogen, Grand Island, NY) in MES buffer, and transferred onto a nitrocellulose membrane (162–0115, Bio-Rad, Hercules, CA) using IDEA scientific GENIE transfer apparatuses (Idea Scientific, Minneapolis, MN). Blots were probed using polyclonal rabbit anti FAM129B (#HPA023261 Sigma, St. Louis, MO), monoclonal mouse anti Tubulin (#T7816 Sigma, St. Louis, MO), monoclonal mouse anti β-catenin (C2206 Sigma, St. Louis, MO), polyclonal Rabbit anti cleaved-caspase-3 (#9661 cell signaling), Rabbit anti ERK1/2 (#9102 cell signaling, Danvers, MA), Rabbit anti phospho ERK1/2 (#9211 cell signaling, Danvers, MA).
Glass coverslips were coated with poly-l-lysine in a 24-well dish, rinsed with PBS, and dried. Following reverse transfection as described above, cells were seeded at a density to achieve 90 to 100% confluency at harvest. Twenty-four hours after seeding, cells were treated with the indicated conditions and incubated for 24 hours with the indicated conditions as described above in the "cell lines and cell culture" section. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed using an in situ cell death detection kit (Roche, Indianapolis, IN). Briefly, the medium was gently aspirated, to keep apoptotic bodies on the slide, and cells were fixed in 4% paraformaldehyde for 1 hour at room temperature. Cells were gently rinsed twice with PBS and permeabilized with 0.1% Triton X-100 (Sigma, St. Louis, MO) in 0.1% sodium citrate (Sigma, St. Louis, MO) for 2 min on ice. Cells were rinsed twice with PBS and 40 ml of TUNEL reaction mixture was added directly on top of the slide; cells were incubated for 1 hour at 37°C in a humidified incubator. Slips were rinsed three times with PBS and mounted on Superfrost Plus glass slides with ProLong Gold anti-fade mounting medium containing 4´,6-diamidino-2-phenylindole (DAPI) (Invitrogen, Grand Island, NY). Images were obtained on a Nikon TiE inverted wide-field high-resolution microscope. DAPI and TUNEL positive nuclei were quantified blinded for 5 fields per slide using NIS elements (Nikon Instruments Inc, Melville, NY).
WC, RTM, AC and JB conceived the study. MF, SM, and NC automated the large-scale screen. MBM, MAC, BR, and WTA designed and carried out the large scale screen. WC carried out the research in melanoma cells and identified melanoma-associated genes. WC prepared the first draft of the manuscript. AC, JB, and RTM contributed to the experimental design and preparation of the manuscript. All authors were involved in the revision of the draft manuscript and have agreed to the final content.
WC was supported by a Pharmaceutical Sciences Training grant (5T32GM007750). AJC is funded by the NIH/National Cancer Institute (NCI) (K08CA128565).
We thank Cassian Yee for contributing the A375 and A2058 cells. Thanks to Travis Biechele for providing the beta-catenin reporter constructs. MC and WA were employees of Rosetta/Merck. JB is an associate of the Howard Hughes Medical Institute. RTM is an investigator of the Howard Hughes Medical Institute. We are indebted to these funding agencies for their continued support of our work. The contents of this manuscript are the sole responsibility of the authors and do not necessarily represent the official views of the NIAMS, NCI, NIH, or the Howard Hughes Medical Institute.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (update) 10 Oct 13 |
read | read |
|
Version 1 31 May 13 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)