Introduction
Red blood cells (RBCs) from a rhesus D (RhD)-positive fetus that get in contact with immune cells of an RhD-negative pregnant woman during birth can induce a pathogenic antibody (Ab) response against the RhD-positive RBCs, leading to fetal hemolytic disease in subsequent pregnancies with RhD-positive fetuses after transplacental passage. To prevent allo-immunization by RhD-positive fetal RBCs, the RhD-negative mother receives one prenatal and one postnatal injection of serum immunoglobulin G (IgG) containing polyclonal RhD-specific IgG Abs that is purified from healthy RhD-negative men immunized with RhD-positive RBCs. Such a passive anti-RhD IgG Ab treatment i) induces a rapid clearance of RhD-positive RBCs from the bloodstream of the mother and ii) inhibits the development of pathogenic anti-RhD Abs by the mother1.
However, the protective mechanism of passive anti-RhD treatment remains unclear. It is hypothesized that the clearance of RhD-positive RBCs is mediated through an Fcγ receptor (Fcγ R) IIIA-mediated Ab-dependent cellular cytotoxicity (ADCC) reaction and that rapid clearance prevents immunization1–4. To prevent immunization, it has also been suggested that polyclonal anti-RhD IgG Abs have to inhibit the activation of RhD-specific B cells through the co-ligation of the B cell receptor and an inhibitory receptor, such as the IgG inhibitory receptor FcγRIIB2,3. Furthermore, tolerance induction via antigen-presenting cells (APCs) has been posited to inhibit pro-inflammatory, RhD-specific T cell responses2,5,6. However, it is questionable whether FcγRIIIA crosslinking on APCs inhibits pro-inflammatory RhD-specific T cell responses but rather enforces pro-inflammatory T cell responses7. It is more likely that polyclonal anti-RhD IgG Abs target an inhibitory receptor (complex) on APCs to induce regulatory T cells and tolerance for inhibiting pro-inflammatory T cell and therewith also T cell-depemdent B cell responses.
Attempts to substitute this polyclonal anti-RhD IgG prophylaxis with RhD-specific monoclonal IgG Abs have failed because the monoclonal RhD-specific IgG Abs were relatively unstable due to intramolecular rearrangements or did not clear RhD-positive RBCs as rapidly as the available polyclonal anti-RhD IgG Abs in in vitro assays or clinical trials and/or did not sufficiently inhibit allo-immunization in clinical trials1,4,8.
It has become increasingly clear that the effector function of IgG Abs is highly regulated by the Abs’ Fc N-linked glycosylation pattern (Figure 1A). Non-fucosylated (afucosylated) IgG Abs have a higher affinity for activating FcγRs, such as FcγRIIIA, and thus induce a stronger ADCC reaction than do fucosylated IgG Abs9,10. This finding is currently translated, for example, into tumor-specific Ab therapy.
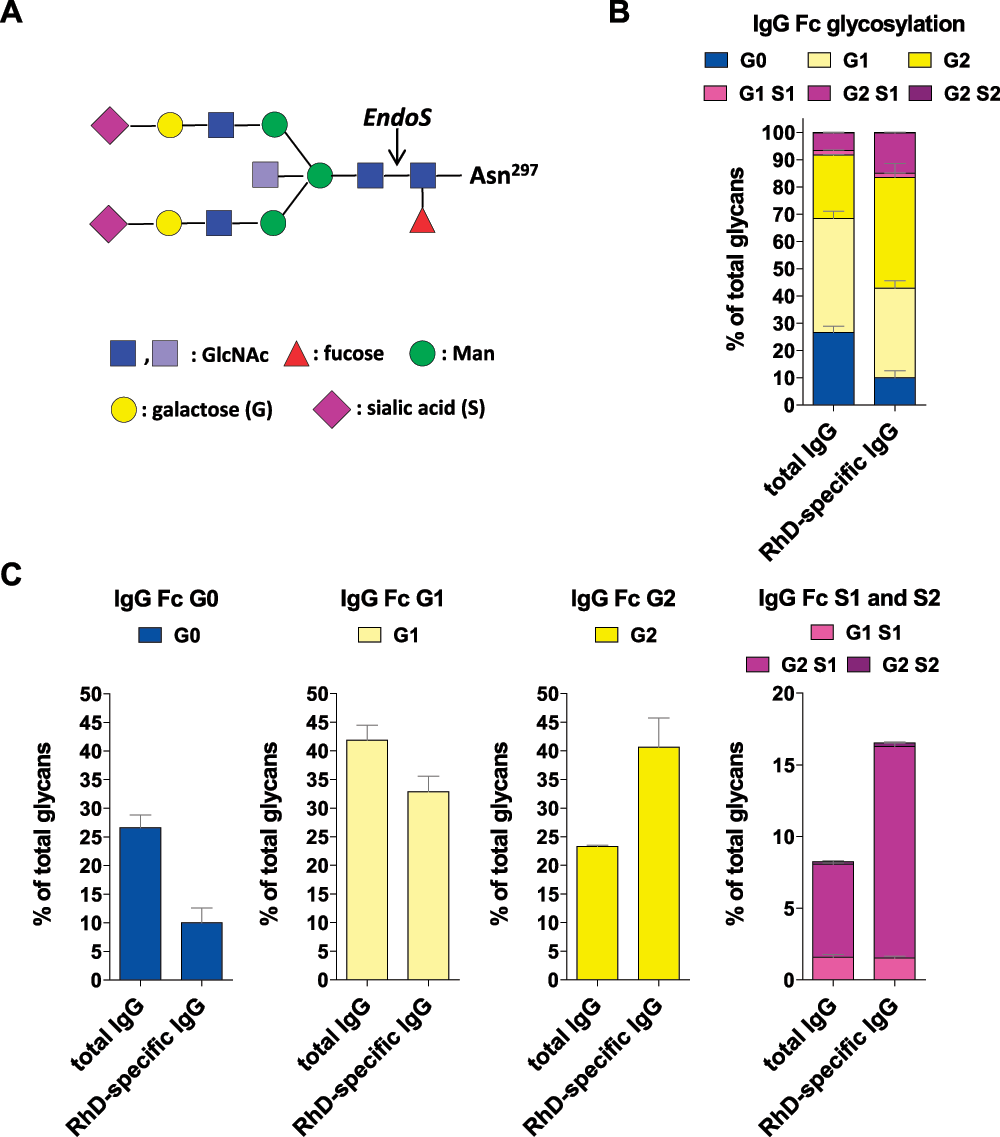
Figure 1. Polyclonal RhD-specific IgG Abs in Rhophylac® are sialylated.
(A) The biantennary IgG Fc glycan core structure, which is coupled to Asn 297, consists of two N-acetyl-glucosamines (GlcNAc; dark blue) and three mannoses (Man), which can be further decorated with fucose; bisecting GlcNAc (light blue) and terminal GlcNAc (dark blue), galactose (G) and sialic acid (S). (B and C) The purified total and RhD-specific IgG samples were hydrolyzed with EndoS and analyzed by MALDI-TOF MS. The cleavage site of EndoS is indicated by an arrow in (A). (B) The bar graph indicate the frequency of all glycan structures with 0, 1 or 2 galactose (G) residues and 0, 1 or 2 sialic acid (S) residues. The mean values with the standard error of the mean (SEM) from independent experiments are shown. (C) The bar graphs separately indicate the frequency of G0, G1, G2 and S1 and S2 glycan structures from the bar graph in (B).
Agalactosylated and asialylated (G0), autoantigen-specific serum IgG Abs correlate with pro-inflammatory immune responses and disease activity in patients with rheumatoid arthritis (RA)11–14. In contrast, pregnancy-induced and anti-tumor necrosis factor (TNF) therapy-induced remission in RA patients is associated with an increase in galactosylated and sialylated IgG Abs15,16. In this context, an anti-inflammatory role has been suggested for sialylated IgG Abs. Accordingly, the sialylated IgG subfraction of intravenous IgG (IVIG), which is purified from pooled human plasma from healthy donors and used to systemically treat autoimmunity in high doses (2 g/kg), exhibits anti-inflammatory activity17–20. We have recently shown that low doses of immune complexes (ICs) containing sialylated antigen-specific IgG Abs inhibit dendritic cell maturation and pro-inflammatory T and B cell immune responses in an antigen-specific manner21–23. ICs containing 15%, but not 5%, sialylated IgG Abs have further been sufficient to inhibit B cell activation in vitro23. Furthermore, it has recently been shown that ICs containing galactosylated, but not sialylated, IgG Abs are already sufficient to inhibit neutrophil activation24.
Thus, G0 IgG Abs enhance, whereas galactosylated and sialylated IgG Abs suppress, pro-inflammatory immune responses. Accordingly, ICs containing galactosylated and sialylated IgG Abs inhibit rather than induce an ADCC reaction by at least reduced binding affinity of galactosylated and sialylated IgG Abs to FcγRIIIA17,25 but also likely by active suppression mechanisms via inhibitory receptors on immune cells.
However, based on the assumption that anti-RhD IgG Abs clear RhD-positive RBCs through an ADCC reaction, the different outcomes of monoclonal anti-RhD IgG Abs have been particularly attributed to different levels of fucose1,2,4,26–29. By contrast, the role of anti-RhD IgG galactosylation and sialylation has hardly been investigated.
Based on the idea that anti-tumor as well as anti-RhD IgG Abs should induce a strong ADCC response by recruiting FcγRIIIA-expressing immune cells, the French biotechnology company Laboratoire Francais du Fractionnement et des Biotechnologies (LFB; Les Ulis, France) has generated a monoclonal anti-CD20 IgG1 Ab (ublituximab; LFB-R603) and a human monoclonal RhD-specific IgG1 Ab (roledumab, LFB-R59330) with low Fc fucosylation, low Fc galactosylation and low Fc sialylation based on their patent31. In the meantime the company has performed clinical phase I32 and II (NCT00952575; completed 2011) studies on roledumab33. The phase II study was designed to demonstrate the ability of roledumab to effectively eliminate exogenously administered RhD-positive RBCs from the circulation of an RhD-negative individual, thereby preventing RhD allo-immunization. The results have not been published yet.
However, based on the findings described above regarding the effector functions of differentially glycosylated IgG Abs, it is questionable whether an anti-tumor IgG1 Ab and an anti-RhD IgG1 Ab should have the same Fc glycosylation. Whether low-galactosylated, low-sialylated RhD-specific IgG Abs can inhibit the induction of pathogenic immune reactions against RhD-positive fetal RBCs or rather enhance allo-immunization is also questionable. To identify the Fc galactosylation and sialylation of RhD-specific IgG Abs in a commercially available polyclonal anti-RhD IgG product, we purified RhD-specific IgG Abs from the approved product Rhophylac® (CSL Behring, King of Prussia, PA, USA) and analyzed the Abs’ Fc glycosylation.
Methods
Purification of RhD-specific IgG Abs from Rhophylac®
Total IgG from the commercial polyclonal anti-RhD IgG product Rhophylac® was purified using protein-G-sepharose (GE Healthcare, Fairfield, CT, USA). RhD-specific IgG Abs from the purified total IgG Abs of Rhophylac® were enriched using RhD-positive human erythrocytes. For this purpose, anonymous RhD-positive erythrocyte concentrates were obtained from the blood bank of the Charité - University Hospital Berlin. The erythrocyte concentrate was washed with 1 mM EDTA in PBS. Next, 20 ml of erythrocyte concentrate was diluted 1:1 with purified total IgG from Rhophylac® in PBS, which contained approximately 600 µg of RhD-specific IgG Abs as indicated by the company, and was incubated for 2h at 4°C. The erythrocytes were then washed five times with PBS. Subsequently, RhD-specific IgG Abs were eluted with 0.15 M glycine pH 3.0; neutralized with 1 M Tris/HCl pH 9.0 and dialyzed against PBS. Two independent RhD-specific IgG purifications (A and B) were done. Enrichment of the RhD-specific IgG Abs was verified by fluorescence-activated cell sorting (FACS) analysis. IgG Fc glycosylation was characterized through MALDI-TOF mass spectrometry (MS).
FACS analysis
Enrichment of the purified polyclonal RhD-specific IgG Abs was verified by FACS analysis (FACS Calibur with CellQuest Pro software, version 6.0 (BD Biosciences, Franklin Lakes, NJ, USA)). RhD-positive human RBCs were stained with 260 μg/ml of total IgG from Rhophylac® (blue), 2 μg/ml purified RhD-specific IgGs (black) or 2 μg/ml of total IgG from Rhophylac® (red) and an anti-human IgG APC-coupled secondary Ab (#550931; BD Biosciences) in cold PBS containing 0.5% bovine serum albumin (Sigma-Aldrich; St. Louis, MO, USA). The stained cells were gated on the main erythrocyte population in a FSC/SSC blot to exclude fragments and aggregates and analyzed in an anti-human IgG (APC) histogram. The data were analyzed with FlowJo 7.2.5 from Tree Star, Inc. Ashland, Oregon, USA. An overlay of different histograms, containing the analysis of the RhD-specific IgG Abs from purification A, is shown in Supplementary figure 1. The FCS files can be found in the data files below.
Glycan analysis through MALDI-TOF MS
The purified total and RhD-specific IgG samples were hydrolyzed with recombinantly expressed endoglycosidase S (EndoS) from Streptococcus pyogenes, an enzyme that hydrolyzes N-glycans only from the Fc portion of the IgG Abs, to prevent the analysis of glycans from pontential contaminating RBC proteins34. The resulting N-glycans were purified through solid-phase extraction using reversed-phase C18 and graphitized carbon columns (Alltech, Deerfield, IL, USA), permethylated and further investigated by MALDI-TOF MS21. The spectra were recorded on an Ultraflex III mass spectrometer (Bruker Corporation, Billerica, MA, USA) equipped with a Smartbeam laser. Calibration was performed on a glucose ladder, and 2,5-dihydroxybenzoic acid (DHB) was used as the matrix. Spectra were recorded in reflector positive ionization mode and mass spectra from 3,000 laser shots were accumulated. The crude glycan analysis data of the two independent RhD-specific IgG Ab purifications A and B can be found in the data files below.
Results and discussion
To assess the Fc galactosylation and sialylation of RhD-specific IgG Abs in a commercially available polyclonal anti-RhD IgG product, we purified RhD-specific IgG Abs from Rhophylac® and analyzed the Abs’ Fc glycosylation (Figure 1, Supplementary figure 1 and Supplementary figure 2 and the data files). We found that the purified polyclonal RhD-specific IgG Abs were even more galactosylated and sialylated than the total Rhophylac® IgG Abs (Figure 1, Supplementary figure 1 and Supplementary figure 2 and the data files), which are comparable to total IgG Abs in immunosuppressive IVIG. The injection of RhD-positive RBCs into RhD-negative men obviously induced no pathogenic immune response. Comparable results have recently been generated by analyzing allergen-specific human IgG Abs after successful allergen-specific immunotherapy for birch pollen allergy21. Together, these results further indicate that tolerance induction induces mainly galactosylated and sialylated IgG Abs that have the potential to inhibit pathogenic immune responses via ICs21–24.
These results strongly suggest that only galactosylated and sialylated, immunosuppressive monoclonal RhD-specific IgG Abs can be substituted for polyclonal anti-RhD IgG products to inhibit pathogenic allo-immunity in RhD-negative pregnant woman. Low fucosylated, low agalactosylated and low sialylated RhD-specific IgG Abs might not only enhance pro-inflammatory immune responses against RhD-positive fetal RBCs but also have the potential to attack RhD-positive fetal RBCs after transplacental passage.
Author contributions
AW contributed to the experimental design and carried out the research. MB helped with the glycan analysis. ME contributed to the experimental design and prepared the manuscript. All authors were involved in the revision of the draft manuscript and have agreed to the final content.
Competing interests
No competing interests were disclosed.
Grant information
AW and ME were supported by the German Research Foundation (DFG: EH221-5 and SFB/TR654 project C9). In addition ME was a fellow of the Claussen-Simon-Foundation, and supported by the Max Planck Institute for Infection Biology, Berlin, Germany and by additional DFG grants (RTG1727 associated project 16, IRTG1911 project A4 and the cluster of excellence 306 “Inflammation at Interfaces” project K-TP2). MB was supported by the German Ministry of Research and Education (03IP511) and the Sonnefeld Foundation.
Acknowledgements
We thank Mattias Collin for support with EndoS.
Supplementary figures
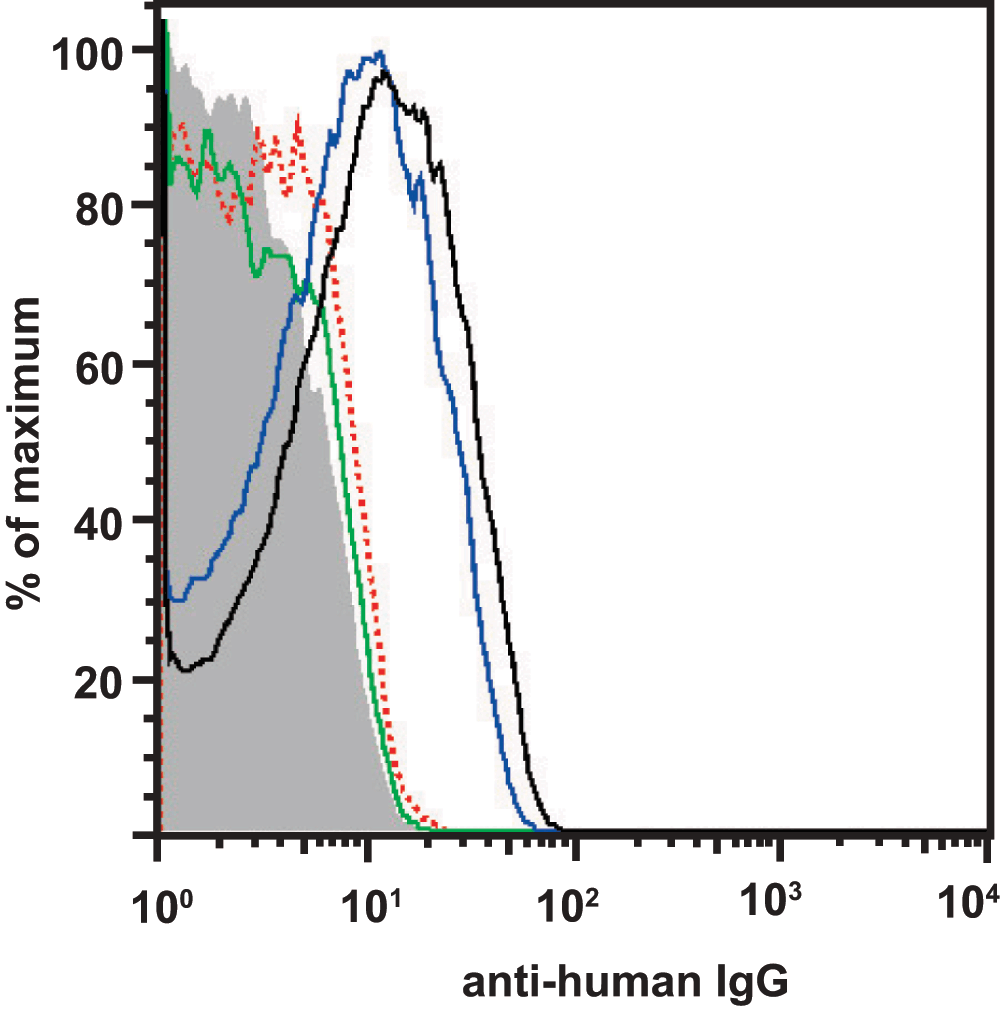
Supplementary figure 1. Verfication of the RhD-specific IgG purification.
Purification of RhD-specific IgG Abs was verified by FACS analysis. RhD-positive human RBCs were stained with 260 µg/ml of total IgG from Rhophylac® (blue), 2 µg/ml purified RhD-specific IgGs (black; purification A) or 2 µg/ml of total IgG from Rhophylac® (red) and an anti-human IgG APC-coupled secondary Ab and analyzed by FACS analysis. The gray curve represents the unstained RBCs and the green curve represents the RBCs stained only with the secondary antibody. An overlay of different histograms, containing the analysis of the RhD-specific IgG Abs from purification A, is shown.
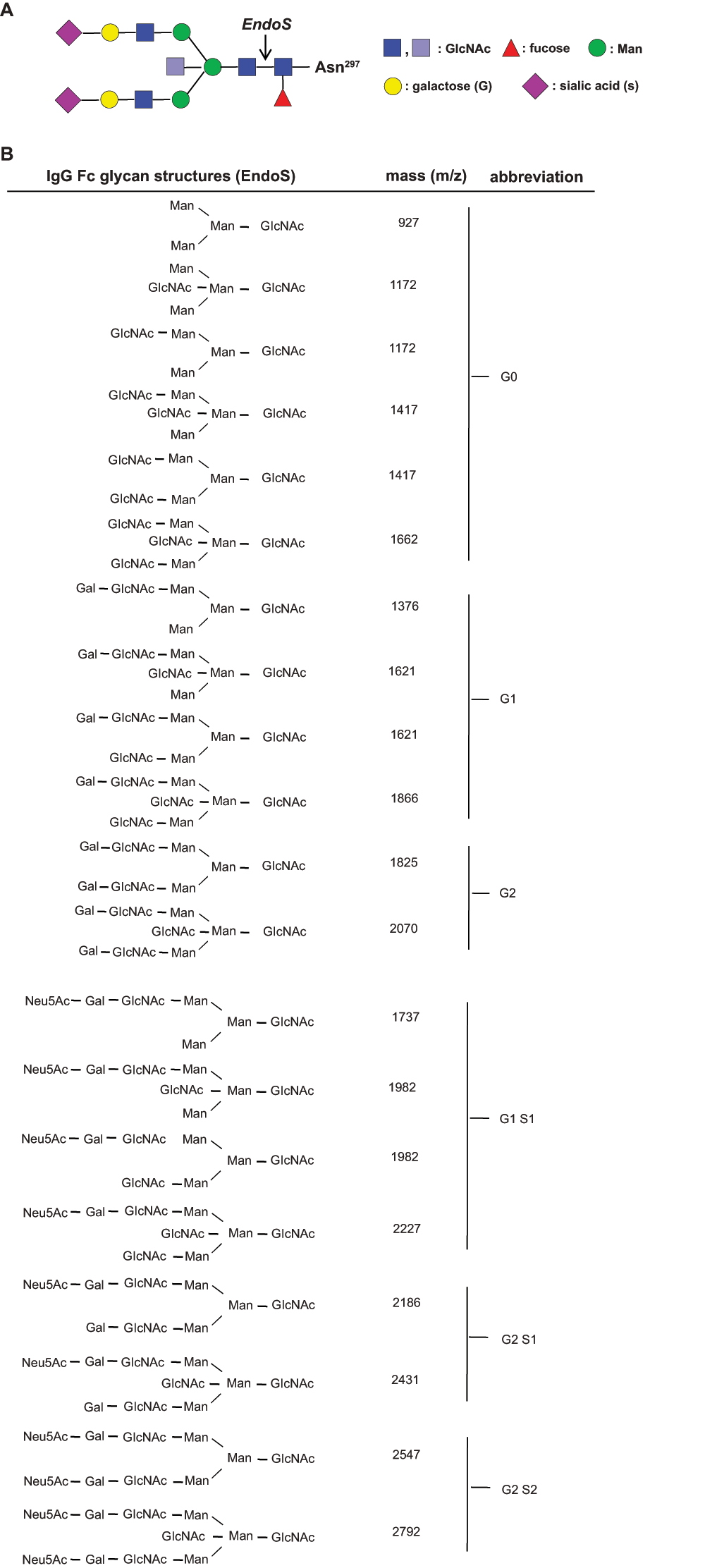
Supplementary figure 2. EndoS-released glycan structures.
(A) The biantennary IgG Fc glycan core structure, which is coupled to Asn 297 consists of two N-acetyl-glucosamines (GlcNAc; dark blue) and three mannoses (Man), which can be further decorated with fucose; bisecting GlcNAc (light blue) and terminal GlcNAc (dark blue), galactose (G, Gal) and sialic acid (N-acetylneuraminic acid (Neu5Ac), S, Sial). The cleavage site of EndoS is indicated by an arrow. (B) The molecular mass (m/z) of the possible Fc glycan structures (permethylated) released from Asn 297 upon EndoS treatment.
Faculty Opinions recommendedReferences
- 1.
Kumpel BM:
Lessons learnt from many years of experience using anti-D in humans for prevention of RhD immunization and haemolytic disease of the fetus and newborn.
Clin Exp Immunol.
2008; 154(1): 1–5. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 2.
Kumpel BM, Elson CJ:
Mechanism of anti-D-mediated immune suppression--a paradox awaiting resolution?
Trends Immunol.
2001; 22(1): 26–31. PubMed Abstract
| Publisher Full Text
- 3.
Kumpel BM:
In vivo studies of monoclonal anti-D and the mechanism of immune suppression.
Transfus Clin Biol.
2002; 9(1): 9–14. PubMed Abstract
| Publisher Full Text
- 4.
Kumpel BM:
Efficacy of RhD monoclonal antibodies in clinical trials as replacement therapy for prophylactic anti-D immunoglobulin: more questions than answers.
Vox Sang.
2007; 93(2): 99–111. PubMed Abstract
| Publisher Full Text
- 5.
Stott LM, Barker RN, Urbaniak SJ:
Identification of alloreactive T-cell epitopes on the Rhesus D protein.
Blood.
2000; 96(13): 4011–4019. PubMed Abstract
- 6.
Hall AM, Cairns LS, Altmann DM, et al.:
Immune responses and tolerance to the RhD blood group protein in HLA-transgenic mice.
Blood.
2005; 105(5): 2175–2179. PubMed Abstract
| Publisher Full Text
- 7.
Döbel T, Kunze A, Babatz J, et al.:
FcγRIII (CD16) equips immature 6-sulfo LacNAc-expressing dendritic cells (slanDCs) with a unique capacity to handle IgG-complexed antigens.
Blood.
2013; 121(18): 3609–3618. PubMed Abstract
| Publisher Full Text
- 8.
Kumpel BM, Goodrick MJ, Pamphilon DH, et al.:
Human Rh D monoclonal antibodies (BRAD-3 and BRAD-5) cause accelerated clearance of Rh D+ red blood cells and suppression of Rh D immunization in Rh D- volunteers.
Blood.
1995; 86(5): 1701–1709. PubMed Abstract
- 9.
Shinkawa T, Nakamura K, Yamane N, et al.:
The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity.
J Biol Chem.
2003; 278(5): 3466–3473. PubMed Abstract
| Publisher Full Text
- 10.
Nimmerjahn F, Ravetch JV:
Divergent immunoglobulin g subclass activity through selective Fc receptor binding.
Science.
2005; 310(5753): 1510–1512. PubMed Abstract
| Publisher Full Text
- 11.
Ercan A, Cui J, Chatterton DE, et al.:
Aberrant IgG galactosylation precedes disease onset, correlates with disease activity, and is prevalent in autoantibodies in rheumatoid arthritis.
Arthritis Rheum.
2010; 62(8): 2239–2248. PubMed Abstract
| Publisher Full Text
- 12.
Rademacher TW, Williams P, Dwek RA:
Agalactosyl glycoforms of IgG autoantibodies are pathogenic.
Proc Natl Acad Sci U S A.
1994; 91(13): 6123–6127. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 13.
Parekh RB, Dwek RA, Sutton BJ, et al.:
Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG.
Nature.
1985; 316(6027): 452–457. PubMed Abstract
| Publisher Full Text
- 14.
Scherer HU, van der Woude D, Ioan-Facsinay A, et al.:
Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid.
Arthritis Rheum.
2010; 62(6): 1620–1629. PubMed Abstract
| Publisher Full Text
- 15.
Rook GA, Steele J, Brealey R, et al.:
Changes in IgG glycoform levels are associated with remission of arthritis during pregnancy.
J Autoimmun.
1991; 4(5): 779–794. PubMed Abstract
| Publisher Full Text
- 16.
Van Beneden K, Coppieters K, Laroy W, et al.:
Reversible changes in serum immunoglobulin galactosylation during the immune response and treatment of inflammatory autoimmune arthritis.
Ann Rheum Dis.
2009; 68(8): 1360–1365. PubMed Abstract
| Publisher Full Text
- 17.
Kaneko Y, Nimmerjahn F, Ravetch JV:
Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation.
Science.
2006; 313(5787): 670–673. PubMed Abstract
| Publisher Full Text
- 18.
Arnold JN, Wormald MR, Sim RB, et al.:
The impact of glycosylation on the biological function and structure of human immunoglobulins.
Annu Rev Immunol.
2007; 25: 21–50. PubMed Abstract
| Publisher Full Text
- 19.
Nimmerjahn F, Ravetch JV:
Anti-inflammatory actions of intravenous immunoglobulin.
Annu Rev Immunol.
2008; 26: 513–533. PubMed Abstract
| Publisher Full Text
- 20.
Anthony RM, Kobayashi T, Wermeling F, et al.:
Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway.
Nature.
2011; 475(7354): 110–113. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 21.
Oefner CM, Winkler A, Hess C, et al.:
Tolerance induction with T cell-dependent protein antigens induces regulatory sialylated IgGs.
J Allergy Clin Immunol.
2012; 129(6): 1647–1655. PubMed Abstract
| Publisher Full Text
- 22.
Collin M, Ehlers M:
The carbohydrate switch between pathogenic and immunosuppressive antigen-specific antibodies.
Exp Dermatol.
2013; 22(8): 511–4. PubMed Abstract
| Publisher Full Text
- 23.
Hess C, Winkler A, Lorenz AK, et al.:
T cell-independent B cell activation induces immunosuppressive sialylated IgG antibodies.
J Clinical Invest.
accepted.
- 24.
Karsten CM, Pandey MK, Figge J, et al.:
Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of Fc gamma RIIB and dectin-1.
Nat Med.
2012; 18(9): 1401–1406. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 25.
Ackerman ME, Crispin M, Yu X, et al.:
Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity.
J Clin Invest.
2013; 123(5): 2183–2192. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 26.
Kumpel BM, Rademacher TW, Rook GA, et al.:
Galactosylation of human IgG monoclonal anti-D produced by EBV-transformed B-lymphoblastoid cell lines is dependent on culture method and affects Fc receptor-mediated functional activity.
Hum Antibodies Hybridomas.
1994; 5(3–4): 143–151. PubMed Abstract
- 27.
Kumpel BM, Wang Y, Griffiths HL, et al.:
The biological activity of human monoclonal IgG anti-D is reduced by beta-galactosidase treatment.
Hum Antibodies Hybridomas.
1995; 6(3): 82–88. PubMed Abstract
- 28.
Sibéril S, de Romeuf C, Bihoreau N, et al.:
Selection of a human anti-RhD monoclonal antibody for therapeutic use: impact of IgG glycosylation on activating and inhibitory Fc gamma R functions.
Clin Immunol.
2006; 118(2–3): 170–179. PubMed Abstract
| Publisher Full Text
- 29.
Beliard R, Waegemans T, Notelet D, et al.:
A human anti-D monoclonal antibody selected for enhanced FcgammaRIII engagement clears RhD+ autologous red cells in human volunteers as efficiently as polyclonal anti-D antibodies.
Br J Haematol.
2008; 141(1): 109–119. PubMed Abstract
| Publisher Full Text
- 30.
PCT/FR2010/050376 (international publication number: WO/2010/100383) Anti-rhesus d monoclonal antibody. Reference Source
- 31.
US 7931895 B2 patent “Monoclonal antibodies with enhanced ADCC function”; issued 2011. Reference Source
- 32.
Yver A, Homery MC, Fuseau E, et al.:
Pharmacokinetics and safety of roledumab, a novel human recombinant monoclonal anti-RhD antibody with an optimized Fc for improved engagement of FCγRIII, in healthy volunteers.
Vox Sang.
2012; 103(3): 213–222. PubMed Abstract
| Publisher Full Text
- 33.
Beck A, Reichert JM:
Marketing approval of mogamulizumab: a triumph for glyco-engineering.
MAbs.
2012; 4(4): 419–425. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 34.
Collin M, Olsén A:
Effect of SpeB and EndoS from Streptococcus pyogenes on human immunoglobulins.
Infect Immun.
2001; 69(11): 7187–7189. PubMed Abstract
| Publisher Full Text
| Free Full Text



Comments on this article Comments (0)