Keywords
Mast cell degranulation, Thermal sensitivity, TNF-alpha
Mast cell degranulation, Thermal sensitivity, TNF-alpha
In response to feedback from our reviewers, we have updated our research report. We have clarified in the discussion that the effects of TNF-α on nociceptors can be both direct and indirect i.e. mediated through other inflammatory molecules such as COX-2. We have also clarified that while we see the lack of neutrophil blockade in the presence of TNF-α neutralizing antibodies in a relatively small experimental group of animals, we verify neutrophil infiltration through independent assays of myeloperoxidase enzyme activity and histological analysis of hind paw tissue sections.
See the authors' detailed response to the review by Peter Drummond
See the authors' detailed response to the review by Rosa Torres
See the authors' detailed response to the review by Adrian Piliponsky
Pain is one of the cardinal components of inflammation and tissue-resident immune cells are important players in the regulation of protective nociceptive responses1. Compound 48/80 (c48/80)-provoked thermal hyperalgesic responses in the hind paw tissue of male ND4 Swiss mice are mediated by the degranulation of tissue-resident mast cells2. The increased sensitivity to thermal stimulus is dependent, in part, on neutrophil influx into the affected tissue, and on histamine signaling; the response is completely abrogated in mast cell-deficient mice, and is substantially reduced by blocking neutrophil influx or treatment with histamine receptor antagonists2. The importance of neutrophil influx in nociceptive cascades has been shown in different rodent models3–5 and the important regulatory roles of inflammatory cytokines including IL-1β, TNF-α, and IL-6 in pain signaling have also been demonstrated6,7. Here we show that c48/80-induced early thermal sensitivity in ND4 Swiss mice is markedly reduced by pre-treatment with a neutralizing antibody against TNF-α. Anti-TNF-α antibody administration does not significantly affect levels of mast cell degranulation or neutrophil influx into the affected tissue. We also found no increase in protein and mRNA levels of TNF-α in the tissue within the first 3 hours following c48/80 administration suggesting that this blockade targets pre-formed TNF-α including that stored in the granules of tissue-resident mast cells. Our data suggest that pre-formed TNF-α may act rapidly on nociceptors that are known to reside in close proximity to mast cells8 and modulate nociceptor sensitization thresholds9 and function10. TNF-α blockade therefore compromises the early, protective nociceptive withdrawal responses that normally act to protect tissues from further exposure to acute, injurious environmental stimuli11.
Three-six month old male ND4 Swiss mice (Harlan Laboratories, Indianapolis, IN) were housed in Macalester College’s research animal facility with a 12-hour light/dark cycle and free access to food and water. A total of 73 mice were used for the experiments shown here (21 controls; 52 experimental) to ensure that appropriate statistical analysis could be performed on data acquired from these experiments. Macalester College’s Institutional Animal Care and Use Committee approved all experimental procedures (Protocol B08Su1).
All drugs were administered using 0.9% saline (VWR, Radnor, PA) vehicle or phosphate buffered saline (EMD Millipore, Billerica, MA). Mice received bilateral intra-plantar (i.pl.) treatments with c48/80 (1.5μg/paw; 10μl; (Sigma-Aldrich, St. Louis, MO)) or saline alone as previously described2. Either 200μg/kg of anti-TNF-α neutralizing antibody (R&D Systems, Minneapolis, MN, Polyclonal Goat IgG, Catalog #: AB-410-NA) or 200μl vehicle was injected intravenously 30 minutes prior to c48/80 injection in a protocol adapted from Rocha et al.12. Rocha et al. administered anti-TNF-α antibodies i.v. 5 mins prior to carrageenan treatment in a mouse model of mechanical hyperalgesia12.
To assess thermal sensitivity, single mice treated with i.pl. c48/80 or vehicle were placed in a Plexiglas cylinder on a hotplate analgesia meter (Harvard Laboratories, Edenbridge, KY) maintained at 51.0 ± 0.5°C and removed when prolonged retraction, flipping/licking of the hind paw, or jumping with both hind paws off the hotplate were observed, but no later than 40 seconds, as previously described2. Two baseline hotplate latencies were taken 24 and 48 hours before the experiment. Mice with >10 second differences between baselines or <15 second averages were excluded from the experiment. Nociceptive thermal sensitivity was quantified by subtracting the mean baseline thermal latency from the experimental thermal latency at each time point for each mouse.
Change in hind paw width measured using digital calipers (±0.1mm; VWR, Radnor, PA) was calculated as an average of the left and right paw widths. Baseline paw widths for each mouse were taken pre-treatment and subtracted from post-treatment paw widths to calculate tissue edema as previously described2.
Footpads were extracted from hind paws of mice euthanized by CO2 inhalation, weighed, frozen at -80°C in 5.6μl/mg tissue weight of 50mM K2HPO4 buffer (pH 6.0) (Sigma-Aldrich, St. Louis, MO) containing 0.05% hexadecyltrimethylammonium bromide (HTAB) (Sigma-Aldrich, St. Louis, MO), thawed, homogenized in 5x the storage volume of HTAB buffer, sonicated with a 550 Sonic Dismembrator (Fisher Scientific, Waltham, MA), freeze-thawed, and centrifuged (AllegraX-15R; Beckman Coulter, Inc., Pasadena, CA) as previously described2,13. Absorbance was recorded using a BioTek PowerWave XS plate reader (BioTek, Winooski, VT) at 450nm after a 20-minute incubation in 50mM phosphate buffer (pH 6.0) with 0.025% hydrogen peroxide (Sigma Aldrich, St. Louis, MO) and 0.167mg/ml o-dianisidine-dihydrochloride (Sigma Aldrich, St. Louis, MO) at room temperature in the dark. Myeloperoxidase (MPO) levels were normalized to tissue weight and presented as OD/g of wet tissue.
For protein and gene expression studies, hind paws were excised from mice euthanized by CO2 inhalation, flash-frozen in liquid nitrogen and stored at -80°C.
For protein studies, flash-frozen paws were homogenized in Cell Lysis Buffer (Cell Signaling Technology, Beverly, MA) supplemented with protease inhibitor (Cocktail Set IV; EMD Biosciences, Billerica, MA) using a Tissue-Tearor (BioSpec; Model 985370). Homogenates were incubated on ice for 20 minutes, centrifuged at 2000 rpm for 10 minutes at 4°C, and lysate supernatants stored at -80°C. We quantified TNF-α cytokine levels by ELISA according to the manufacturer’s instructions (eBioscience, San Diego, CA).
To measure cytokine gene expression, total RNA was extracted from flash-frozen plantar tissue (Total RNA Mini Kit, Midwest Scientific, St. Louis, MO), quantified with Nanodrop ND-1000 Spectrophotometer (Thermo Scientific, Wilmington, DE), and reverse-transcribed in a 2720 Thermal Cycler (Life Technologies, Grand Island, NY) using the Superscript III First-Strand Synthesis System (Life Technologies, Grand Island, NY) with 100ng of RNA per reaction. Relative transcript abundance was determined by quantitative comparative reverse transcriptase PCR (qRT-PCR)14 using TaqMan Gene Expression Assay Primer/Probe Sets and TaqMan MasterMix (Life Technologies, Grand Island, NY) in a Bio-Rad iCycler (Bio-Rad, Hercules, CA) using β-2-microglobulin (β2m; Mm00437762_m1, Life Technologies, Grand Island, NY) and TNF-α (Mm00443260_g1, Life Technologies, Grand Island, NY) primer sets. Fold expression was normalized to β-2-microglobulin levels and calculated as previously described14,15.
Excised hind paws (from mice euthanized by CO2 inhalation) were fixed in 10% buffered formalin (VWR, Radnor, PA) for 24 hours, transferred to 70% ethanol (Sigma-Aldrich, St. Louis, MO), decalcified for 1–2 weeks in 15% EDTA, hydrated, and embedded in paraffin. 4μm sagittal sections were stained with toluidine blue (Tb) at pH 2.3 for 3 minutes (Sigma-Aldrich, St. Louis, MO) or hematoxylin and eosin (H&E) (Sigma-Aldrich, St. Louis, MO) for the detection of mast cells or neutrophils, respectively, at 400x magnification with ≥3 biological replicates per treatment. Tb-stained mast cells were counted in 10 fields/section and degranulation was scored based on the number of granules observed outside the boundary of the cell: intact (0), mild (1–10), moderate (10–20), and extensive (20+) as previously described2. The same investigator performed all counts and was blinded to treatment groups. Neutrophils were imaged between the heel and the toes on the plantar side of the hind paw section using an Olympus CX21LED light microscope and camera (Olympus Corporation of the Americas, Center Valley, PA).
Data were processed using Microsoft Excel (Redmond, WA) and graphed with PRISM 5.0 (GraphPad, San Diego, CA). All statistical analyses were performed using JMP 9.0 (SAS, Cary, NC). All data are presented as the mean ±SEM. Data were analyzed using one-way ANOVA, and the Tukey-Kramer HSD post hoc test, at each time point. Statistical significance was defined as p<0.05. Thermal sensitivity and hind paw edema measurements included 15–18 mice per treatment group. Other analyses used 3–9 mice per treatment group. All data represent 2–3 independent experiments.
Male ND4 Swiss mice bilaterally injected with 1500ng of c48/80 in the hind paws showed hyperalgesic withdrawal responses ~10 seconds sooner than their baseline withdrawal 30 minutes after treatment; these responses were resolved by 2.5 hours (Figure 1A). Pre-treatment with an intravenous injection of 200μg/kg TNF-α neutralizing antibody 30 minutes prior to c48/80 injection significantly abrogated these responses at 30 minutes and 1.5 hours after intra-plantar treatment with c48/80; pre-treated mice showed hyperalgesic withdrawal responses <5 seconds sooner than their baseline responses 30 minutes after treatment (Figure 1A). Withdrawal responses of control and anti-TNF-α treated groups were significantly different at 1.5 hours after treatment and indistinguishable at 2.5 hours when the c48/80-provoked hyperalgesic responses were resolved. Anti-TNF-α pretreatment also significantly reduced hind paw edema compared to mice without pre-treatment at 30 minutes but not at 1.5 and 2.5 hours (Figure 1B). Saline-treated control mice had no increase in thermal sensitivity and showed no tissue swelling (Figure 1A–B). Overall, thermal sensitivity was reduced in a more sustained manner compared to tissue edema.
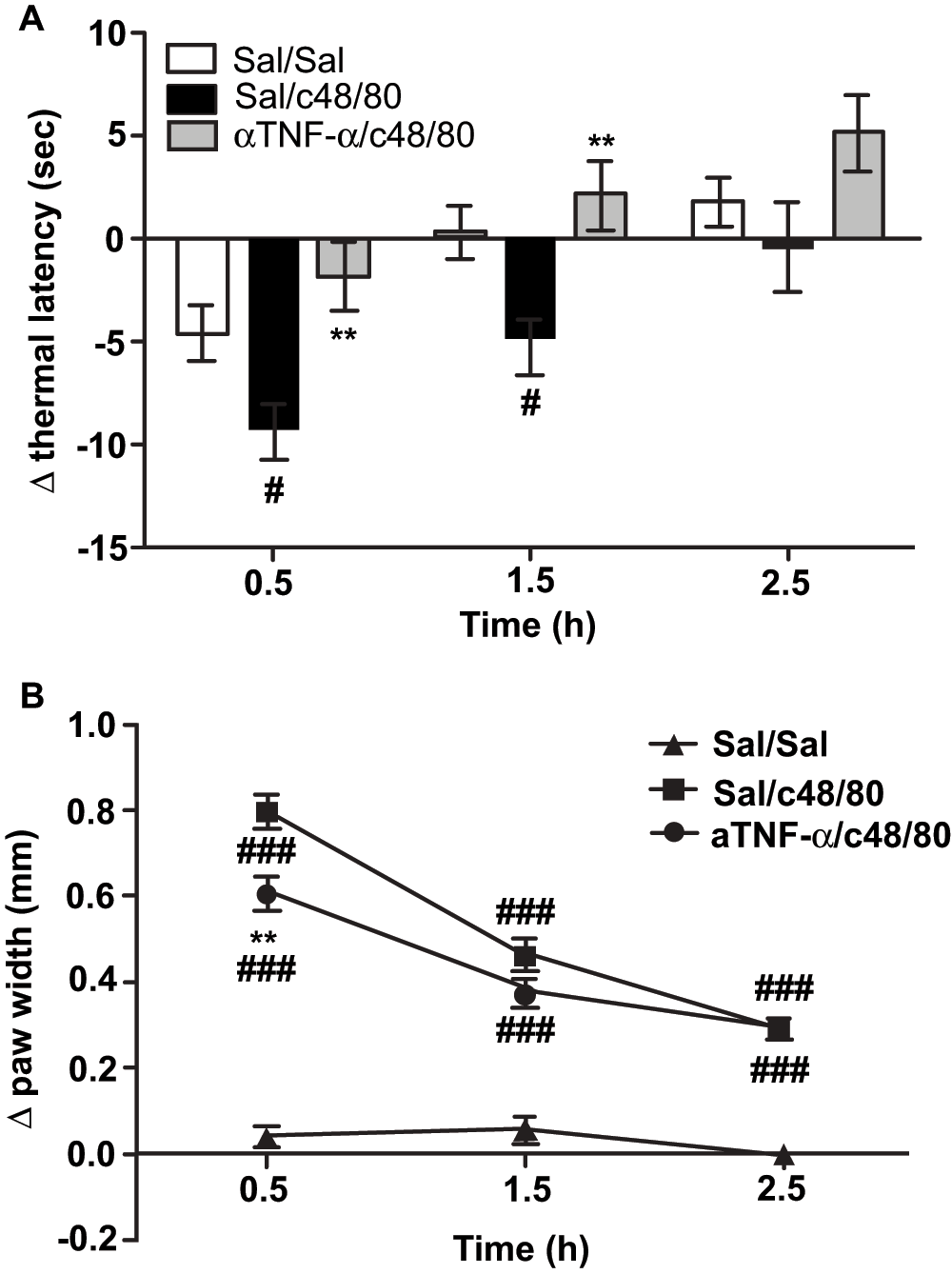
Mice were pre-treated with 200μg/kg anti-TNF-α neutralizing antibody or vehicle (200μl, i.v.) 30 minutes before bilateral intra-plantar c48/80 or 0.9% saline injections (1.5μg/paw; 10μl). The bars represent the mean change in thermal paw withdrawal latency (A) and the change in paw width (B) from baseline and error bars represent ± SEM. Anti-TNF-α administration abrogated thermal hyperalgesia at 0.5 and 1.5h after c48/80 injection (A) and reduced paw edema significantly at 0.5h (B). Significances are compared to Sal/Sal (# = p<0.05; ## = p<0.001) and Sal/c48/80 (* = p<0.05; *** = p<0.001). n = 12 in Sal/Sal; n = 18 for Sal/c48/80; n = 19 for anti-TNF/c48/80 treatment groups; data are pooled from 2 independent experiments.
To confirm that the antibody pre-treatment did not have an effect on the extent of mast cell degranulation caused by c48/80 that would have consequently affected mast cell-mediated hyperalgesia, we evaluated the levels of degranulation in toluidine blue-stained 4μm paraffin sections as previously described2 and confirmed that the levels of mild, moderate and extensive degranulation were not altered in anti-TNF-α treated mice (Figure 2).

Mice were pre-treated with 200μg/kg anti-TNF-α neutralizing antibody or vehicle (200μl, i.v.) 30 minutes before bilateral intra-plantar c48/80 injection (1.5μg/paw; 10μl). Mice were euthanized 2.5h after c48/80 administration; their hind paws were harvested, preserved in 10% buffered formalin and ethanol, and 4μm paraffin sections stained with toluidine blue for mast cell visualization. Mast cells were counted at 400x and their degranulation status was assessed as described previously2. Bars represent mean percentages of mast cells examined that are assigned to intact or mild, moderate and extensive degranulation status respectively and the error bars represent ±SEM. n = 3 mice per treatment group; data are representative of 2 experiments.
Taken together, pre-treatment with anti-TNF-α neutralizing antibody did not change the extent of local mast cell degranulation in the mouse hind paw tissue but markedly reduced resulting thermal sensitivity and tissue swelling provoked by injection of the basic mast cell secretagogue c48/80.
Mast cells contain pre-formed TNF-α in their granules16 that is released early upon degranulation16. We analyzed TNF-α protein and mRNA levels in c48/80-treated and untreated hind paw tissue to determine whether there was new synthesis of TNF-α immediately following treatment with the basic mast cell secretagogue. We found that neither protein nor mRNA levels of the cytokine detectably increased in the tissue within 3 hours following intra-plantar c48/80 injection (Figure 3A–B). Thus, it is most likely that the anti-TNF-α neutralizing antibody treatment targets pre-formed, rather than newly synthesized, TNF-α molecules and this blockade contributes to the resulting decrease in thermal sensitivity in the hind paw tissue.
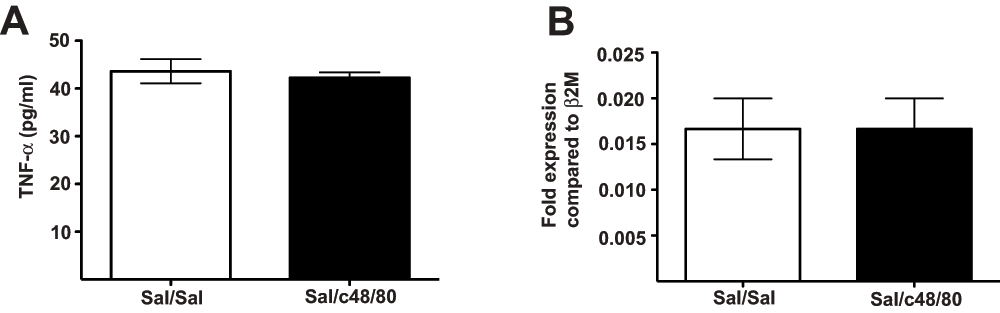
Mice were pretreated with 200μg/kg anti-TNF-α neutralizing antibody or vehicle (200μg, i.v.) 30 minutes before bilateral intra-plantar c48/80 injection (1.5μg/paw; 10μl). 2.5h after c48/80 treatment, their hind paws were excised and preserved for TNF-α protein quantification by ELISA (A). Another set of mice was euthanized at 3h after c48/80 treatment; their footpads were excised for TNF-α mRNA abundance quantification by qRT-PCR (B). Bars represent average pg/ml TNF-α protein (A) and fold-expression of TNF-α transcripts compared to β2m (B) and the error bars represent ±SEM. n = 6 (Sal/Sal); 9 (Sal/c48/80) for ELISA assays and n = 3 mice per treatment group for qRT-PCR; data are representative of 3 (ELISA) and 2 (qPCR) experiments.
Neutrophil influx into the affected tissue contributes to c48/80-provoked hind paw thermal sensitivity2 and TNF-α is a known neutrophil attractant3,17. We have previously shown that the blockade of neutrophil influx can abrogate mast cell-dependent thermal sensitivity in the mouse hind paw2. Therefore, we investigated whether pre-treatment with the TNF-α neutralizing antibody had an effect on neutrophil influx. We found that levels of myeloperoxidase (an enzyme indicating the presence of activated neutrophils) in the hind paw tissue of mice injected bilaterally with 1500ng of c48/80 were not significantly reduced with anti-TNF-α pre-treatment (Figure 4A). We further confirmed this by examining the presence of neutrophils in 4μm paraffin embedded hind paw tissue sections stained with H&E and found that infiltrating neutrophils were present in the hind paws of mice that received TNF-α neutralizing antibody (Figure 4B). Neutrophil numbers in the hind paw tissue of mice following c48/80 injection with and without anti-TNF-α pre-treatment were approximately a total of ~150 neutrophils in 10 randomly chosen visualized sections per paw (see Data File). Therefore, pre-treatment with anti-TNF-α neutralizing antibody had little to no inhibitory effect on the levels of tissue infiltrating neutrophils and myeloperoxidase enzyme activity in the hind paw tissue of c48/80-treated mice.

Mice that received c48/80 intra-plantar injections following anti-TNF-α pretreatment did not have significantly different levels of myeloperoxidase enzyme activity compared to mice that received vehicle pretreatment (A). Hind paws were harvested from euthanized mice and assayed for MPO activity; bars represent average MPO activity as OD/g wet tissue and error bars represent ±SEM (A). Mice were euthanized 2.5h after c48/80 administration, their hind paws were harvested, preserved in 10% buffered formalin and ethanol, and 4μm paraffin sections stained with hematoxylin & eosin for neutrophil visualization at 1000x. Similar to c48/80-treated mice (B), mice pre-treated with anti-TNF-α neutralizing antibody showed clear evidence of neutrophil influx (indicated by black arrowheads) into the affected hind paw tissue (C). n = 3 mice per treatment group for MPO assay; data are representative of 2 experiments.
Pain is a complex physiological and pathological phenomenon with intricate underlying neuro-immune circuitry. Pain can be either protective or maladaptive1,11 and in the former instance, serves as an early warning system that prompts an organism to retreat from noxious environmental stimuli.
We have previously shown that mast cells are required for enhanced sensitivity to heat stimulus following c48/80 treatment in the hind paw tissue of mice and blockade of neutrophil influx or histamine signaling can abrogate these responses2. Here we show that pre-treatment with TNF-α neutralizing antibody just prior to c48/80 administration significantly reduces early thermal hyperalgesic responses. TNF-α neutralizing antibody treatment does not affect mast cell degranulation; levels of mild, modest and extensive degranulation following c48/80 treatment were similar to those previously described2. Mast cells contain pre-formed TNF-α in their cytoplasmic granules and this pro-inflammatory cytokine is one of the earliest mediators released by these cells upon activation16. We looked for evidence of changes in TNF-α protein and mRNA levels shortly after c48/80 treatment and found none, suggesting that the pre-formed TNF released by mast cells may be the main source of this cytokine in these experiments. As TNF-α is a known attractant of neutrophils17, we expected TNF-α blockade to result in a reduction of infiltrating neutrophils. We have previously shown that blockade of neutrophil influx can abrogate c48/80-provoked thermal sensitivity2. However, we found here that in the first 2.5 hours following c48/80 administration, TNF-α neutralizing antibody pre-treatment had little to no effect on recruitment of tissue neutrophils. The influx of neutrophils in the presence of TNF-α neutralizing antibodies is supported by several different kinds of evidence – myeloperoxidase activity in the tissue, visualization of neutrophils by H&E staining and neutrophil counts in H&E stained tissue sections. However, given our relatively small sample size for these assays, it is possible that a reduction in neutrophil influx can be seen with larger numbers of mice. Nevertheless, our observations are in keeping with earlier studies that show that mast cell mediators other than TNF-α can contribute to the induction of neutrophil influx18. In these experiments, pain was significantly abrogated as early as 30 minutes after c48/80 treatment despite the presence of neutrophils.
TNF-α is one of the early inflammatory cytokines with a known role in various nociceptive cascades6,7. Direct injection of TNF-α in the hind paw tissue produces thermal and mechanical hyperalgesia in rodents6,19; anti-TNF-α antibody administration shortly before challenge reduces the hyperalgesic responses to carrageenan12 in mice and blockade of TNF-α induced neutrophil influx impairs thermal carrageenan pain in rats3.
Our findings here add nuance to the prevailing model of mast cell and neutrophil modulation of peripheral nociception with evidence that TNF-α blockade can also abrogate very early thermal pain responses without a proportional reduction in neutrophil influx. Hind paw thermal pain provoked by c48/80 is markedly abrogated despite the presence of infiltrating neutrophils in the tissue. In a model of carrageenan-induced pain in rats, Cunha and colleagues have shown that levels of TNF-α in the tissue are not affected by the absence of infiltrating neutrophils i.e. these cells are not the primary sources of TNF-α3 but rather the release of TNF-α and other cytokines such as IL-1β in the tissue following an inflammatory insult serves to attract infiltrating neutrophils that continue to fuel the nociceptive cascade3. In preliminary experiments, we have found that pre-treatment with antibodies against IL-1β reduces neutrophil influx into the tissue but does not abrogate c48/80-provoked thermal pain at early time points (data not shown). Therefore, the rapid anti-hyperalgesic effect that we observe here may be due to the blockade of earlier effects of TNF-α on nociceptor sensitization mediated by cyclooxygenase (COX) and p38 mitogen-activated protein (MAP) kinase activation9,20 or effects of TNF-α on nociceptor ion channels10,21. Our observations here suggest that pre-formed TNF-α, e.g. from mast cell granules in the skin mast cells, is the likely target of the TNF-α neutralizing antibody that produces an early reduction of thermal sensitivity. This is underscored by the documented proximity of nerves and mast cells in the tissue8 and the known effects of mast cell-derived TNF-α on nerve physiology - elongation of cutaneous nerves in oxazolone contact hypersensitivity22, and susceptibility of sensory neurons to agents such as capsaicin, in a model of tracheal vascular hyper-permeability23. These known interactions further support the idea that nociceptive neurons could be early downstream targets of the first release of pre-formed TNF-α immediately after mast cell degranulation. In this study, we show that early, protective, nociceptive withdrawal responses to heat stimulus are compromised by the blockade of TNF-α signaling. It has been documented that individuals who use TNF-targeted biological therapeutics to manage chronic autoimmune and inflammatory conditions can experience a higher risk of bacterial and viral infections24. Our findings here suggest that the use of TNF-α blockade therapies may also compromise the protective, nociceptive responses of such individuals leaving them more vulnerable to tissue damage from injurious environmental stimuli.
LP contributed to study design, conducted hyperalgesia, edema and histology experiments and analyzed the data. TM contributed to study design and data analyses, conducted protein analyses and co-wrote the manuscript. EB and LP conducted enzyme activity assays and data analyses. JA and AS performed histological analysis and imaging. AA performed gene expression studies. DC designed the study, supervised experiments, analyzed data and wrote the manuscript.
DC is supported by Macalester College and NIH Academic Research Enhancement Award (Grant R15 NS067536-01A).
The Comparative Pathology Shared Resource at the University of Minnesota provided technical support. In the Chatterjea laboratory, we thank Grace Linder and Emily Ewan for assistance with protein and mRNA quantification studies, Kidist Zewdie and Elena Tonc for logistical assistance, and all other lab members for their support.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (update) 27 Sep 13 |
read | read | read |
|
Version 1 21 Aug 13 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)