Introduction
The auditory oddball paradigm, in which a series of repeated standard stimuli are interrupted by occasional deviant stimuli, has been used extensively in cognitive psychology to study early stages of auditory processing in humans1. Typically a sufficiently rare deviant stimulus evokes a long-duration negative potential shift beginning 100–200 ms after the stimulus onset, referred to as mismatch negativity (MMN). MMN has been considered an electrophysiological correlate of a mismatch between the incoming stimulus and a sensory memory trace2. MMN is not dependent on a subject’s attention and can be evoked even during sleep or anesthesia1. Electrical and magnetic recordings in human subjects have localized the MMN generator to the auditory cortex3, although a frontal component has also been observed4. In addition, there are some speculations on subcortical generators, especially the hippocampus, but those cannot be indisputably verified in noninvasive recordings5,6.
Whereas the psychophysical details of conditions evoking MMN have been thoroughly analyzed, relative little is known about the neuropharmacology and molecular mechanisms of MMN. This is largely due to the paucity of experimental studies of MMN in standard laboratory animals. MMN-like responses have been reported in various animal species such as cats7, guinea pigs8, rabbits9, monkeys10 and rats11,12. However, there are also reports of negative findings in rats implying that the evoked potentials are modified by the preceding stimuli or adapted in a stimulus-specific manner not resembling MMN13,14.
Therefore, the aim of the present study was to address a number of unresolved issues related to MMN in the rat. First, anesthesia was reported to attenuate MMN in the cat7. Therefore, we wanted to test whether MMN can be evoked in freely moving rats14,15 rather than in the anesthetized preparation11,12. Second, we compared the event-related potentials (ERPs) recorded using cortical and hippocampal electrodes to reveal a possible hippocampal generator. Third, to distinguish between MMN and long-term adaptation to standard auditory stimuli as suggested by Lazar and Metherate13, we repeated the oddball stimulus set on two daily sessions and on consecutive days. Fourth, to shed light on the neuropharmacology of MMN, we manipulated the cholinergic input to the cortex and hippocampus by systemic administration of scopolamine. We report evidence for repetition-induced attenuation of the mid-latency auditory ERPs but no correspondence to the sustained negativity around 100–200 ms in response to the deviant sound that is referred to as MMN in humans.
Methods
Animals
Male Wistar rats (National Laboratory Animal Center, University of Eastern Finland, Finland, n=12, weight 412 ± 9 g) were reared in groups of 2–4 until 5 months of age and individually thereafter in a controlled environment (temperature +21°C, lights on from 7:00 h to 19:00 h, water and food available ad libitum) Animals were housed in stainless steel metal cages, floor 31 cm x 45 cm, height 18 cm as according to the guidelines of the Council of Europe ETS123. At the age of 5–6 months, the rats were chronically implanted with two recording electrodes made of 50 μm insulated stainless steel wire (California Fine Wire Company Co, Grover Beach, CA, USA) in the hippocampus at the following stereotactic coordinates: AP (from Bregma) - 3.8, L (from Bregma) +3.1, V (from brain surface) - 3.1 with a vertical separation of the tips of 0.6 mm. In addition, two cortical screw electrodes (Wurth Electronics, Finland) were fixed on the (left and right) parietal bones (L ± 2.0 mm and A -7.5 mm from Bregma). A frontal screw served as the ground and a common reference electrode. The hippocampal electrode closest to the pyramidal cell layer and the right parietal cortical electrode were selected for the final analysis of evoked potentials. The rats were anesthetized with a mixture of pentobarbital and chloral hydrate (40 mg/kg i.p. each), and, for post-operative analgesia, they received 5 mg/kg of carprofen (Rimadyl®, Vericore, Dundee, UK) intraperitoneally. The rats were housed in individual cages after the surgery. Recordings started after at least 2 weeks of recovery period. The rats were involved in an EEG study for three weeks before the current study on evoked potentials. All animal procedures were carried out in accordance with the guidelines of the European Community Council Directives 86/609/EEC and approved by the Animal Experiment Board of Finland.
Data acquisition
In total 10 rats were recorded for the study but due to poor signal in some channels, the number of records in the analysis varies from 6 to 9. During the recordings the rat was able to freely move in a brown paste-board cylinder (70 cm diameter, 50 cm height) that was highly familiar to the rat due to previous EEG recordings. Two conventional speakers were placed on the opposite sides outside the cylinder. Auditory stimuli were created through a computer sound card (Sound Blaster 16, Creative Technology Ltd, Singapore, Singapore) and included pure sinusoidal tones of 7, 9 or 11 kHz pitch (tone duration 150 ms, 70 dB, rise/fall time 5 ms). The signal was analog filtered for the 1–1000 Hz band, amplified (× 1000–5000), and digitized at 2 kHz per channel for further processing using a commercial software (Experimenter’s Workbench, DataWave Technologies, Longmont, CO, USA).
At the end of the experiment, the rats were euthanized by an overdose of anesthetic pentobarbital and chloral hydrate each 80 mg/kg i.p. and the sites of the electrode tips were marked by passing a 30 μA anodal current for 5 s through each hippocampal electrode. Subsequently, the brains were immersion-fixed overnight with 4% formalin (formalin was diluted from 37% formaldehyde solution (Sigma-Aldrich)) and sectioned at 50 μm with a vibratome (Leica VT1000s). The sites of the electrolytic lesions were verified in sections stained with cresyl violet Sigma-Aldrich) by using a light Olympus CX microscope Figure 1.
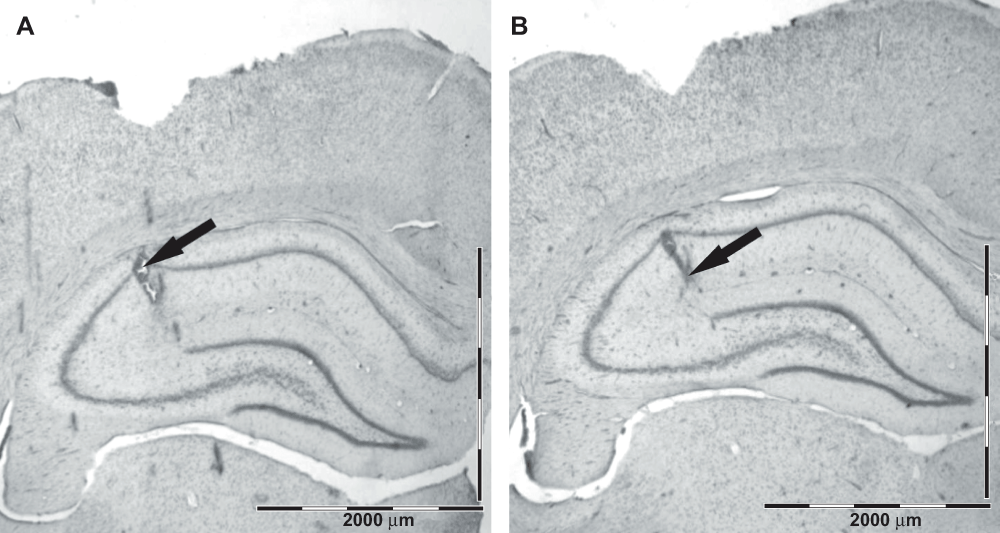
Figure 1. Histological verification of the electrode placement in the hippocampus.
The arrows point to the lesion marks corresponding to the two electrode tips, the upper one in the alveus/oriens and the deeper one in the fissure. Scale bar = 2000 μm.
Study design
The basic study protocol was a conventional mismatch (or oddball) paradigm consisting of one standard tone and one or two deviant tones. Under most conditions, the standard was 9 kHz and the deviants were 7 and 11 kHz tones. Both a low and a high deviant were used to exclude the contribution of tonotopy to auditory evoked potential (AEP) amplitudes. Every run consisted of 400 repetitions with a 1-s inter-stimulus interval. The three tones (7, 9 and 11 kHz) were presented in a pseudo-random order, so that the proportions of the standard, deviant 1 and deviant 2 tones were 85%, 7.5% and 7.5%, respectively.
Experiment 1 consisted of three consecutive days with the 9 kHz tone as the standard, and 7 and 11 kHz tones as the deviants. Similar recordings were performed during Experiment 2 (three weeks after Experiment 1) that also consisted of three consecutive runs. Day 1 replicated Day 1 of the Experiment 1, and was followed by a similar run on Day 2. In addition, Day 2 included a second run with the mismatch contingency reversed, so that 7 kHz became the standard and 9 kHz the deviant. Experiment 3 (one week later) included pharmacological manipulations and consisted only of two runs, one on Day 1 and the second on Day 4. In the first run the standard tone was 9 kHz and the deviants 7 and 11 kHz. In the second run the standard tone was 7 kHz and the deviant 9 kHz. Four rats received scopolamine (0.2 mg/kg, s.c.; Sigma-Aldrich) 20 min before the first run, and five rats before the second run. Saline was used as control treatment.
Data analysis
First, all signals were corrected for amplification. Waveform averaging and AEP peak detection were conducted by custom made routines in Visual Basic under Microsoft Excel® (version 2002).
The AEP in a typical rat had three middle-latency components, N40, P60 and P110 (N40 means a negative deflection at 40 ms). In addition, these components were followed by a broad negativity from 150 ms to 250 ms after the stimulus onset (Figure 2A, B). The amplitude of these components was calculated as a maximum deviation from the baseline. The baseline was calculated for each rat from the averaged response between 0 and 100 ms before stimulus onset. When calculating mismatch effect between standard and deviant AEP, we focused on the middle-latency components only (N40, P60 and P110).
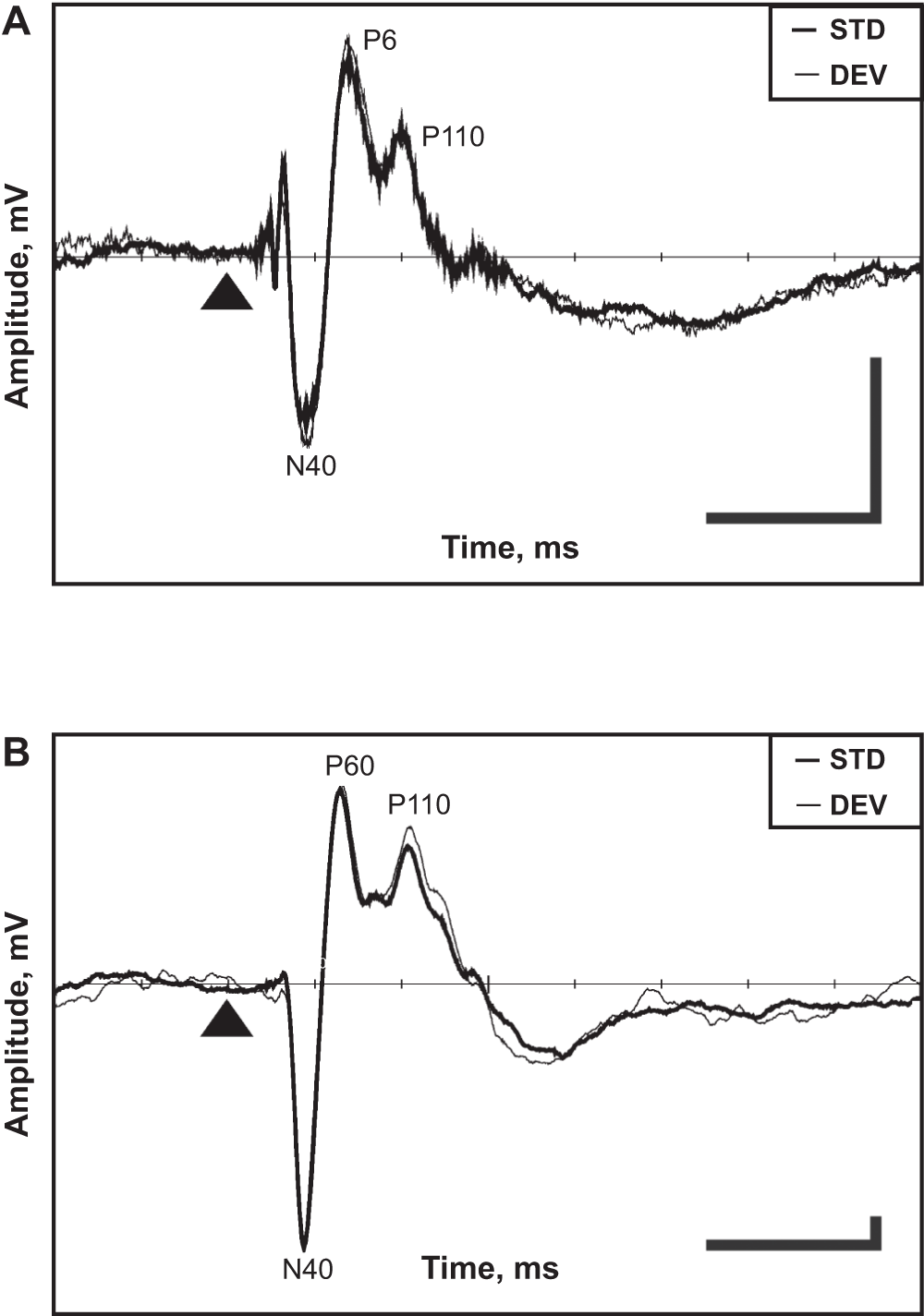
Figure 2. Representative examples of averaged AEPs obtained in the auditory oddball paradigm.
Cortical (A) and hippocampal (B) AEPs. The thin line denotes the response to the deviant tone and the thick line the response to the standard tone. The triangle marks the tone onset. The horizontal bar corresponds to 100 ms, the vertical bar to 0.04 mV (scale for the cortex is five times smaller than that for the hippocampus). Negativity is downward.
The statistical analysis was conducted by using SPSS for Windows 11.5 software. The standard and deviant responses were compared within-subjects using ANOVA with repeated measures with the run (1–3) or drug (placebo or scopolamine) as additional within-subject factors. The threshold for significance was set to p < 0.05.
Results
Electrode location
Histology verified the location of the hippocampal electrodes in the intended layers: the top electrode in the stratum pyramidale – stratum radiatum and the deeper one in the hippocampal fissure – outer molecular layer of the dentate gyrus. The typical location of the hippocampal electrodes is illustrated in Figure 1.
AEP components
Representative examples of an averaged cortical and hippocampal AEPs obtained in the auditory mismatch paradigm are shown in Figure 2. The components N40, P60 and P110 were identified for each rat and pooled for standards and deviants for all drug-free days. The exact latencies of these components are summarized in Table 1 and their mutual correlations in Table 2. The mutual Pearson correlation coefficients were high and significant for all components of the hippocampal response (if the absolute value of one component grows there is a high probability that other components will also grow). This suggests that physiological source(s) of AEP’s components is not completely independent. On the other hand, only the mutual correlations of the P60–P110 components in the cortical response reached a comparable significance level. Furthermore, neither cortical P60 nor P110 correlated with any hippocampal component, which suggests that the cortical and hippocampal responses are largely independent, with the exception of the early N40 component.
Table 1. Latencies for defined mid-latency components in [ms].
| CORTEX | n = 26 | | HIPPOCAMPUS | n = 20 |
|---|
| PEAK1_T | PEAK2_T | PEAK3_T | | PEAK1_T | PEAK2_T | PEAK3_T |
Mean
Sem | 44.42
0.68 | 70.82
1.04 | 98.26
0.93 | DEV | 44.11
0.39 | 66.72
1.27 | 103.37
0.49 |
Mean
Sem | 43.32
0.81 | 69.84
1.11 | 98.20
0.70 | STD | 43.50
0.34 | 65.96
1.33 | 103.35
0.40 |
Table 2. The correlation matrix for middle-latency components.
| | N40
CTX | P60
CTX | P110
CTX | N40
HIPP | P60
HIPP | P110
HIPP |
|---|
N40
CTX | Pearson Correlation
Sig. (2-tailed)
N | 1
.
94 | 0.04
0.667
94 | -0.15
0.141
94 | 0.26*
0.024
76 | 0.02
0.851
76 | -0.35**
0.002
76 |
P60
CTX | Pearson Correlation
Sig. (2-tailed)
N | | 1
.
94 | 0.41**
0.000
94 | 0.16
0.172
76 | 0.00
1.000
76 | 0.17
0.143
76 |
P110
CTX | Pearson Correlation
Sig. (2-tailed)
N | | | 1
.
94 | 0.32**
0.004
76 | -0.17
0.139
76 | -0.02
0.876
76 |
N40
HIPP | Pearson Correlation
Sig. (2-tailed)
N | | | | 1
.
76 | -0.58**
0.000
76 | -0.39**
0.001
76 |
P60
HIPP | Pearson Correlation
Sig. (2-tailed)
N | | | | | 1
.
76 | 0.54**
0.000
76 |
P110
HIPP | Pearson Correlation
Sig. (2-tailed)
N | | | | | | 1
.
76 |
Increased cortical response to the deviant tone
The overall analysis of all three days of Experiment 1 revealed larger cortical responses to the deviant tone compared to the standard tone (Figure 2A, and Figure 3). The difference was significant for N40 [F(1,7) = 7.7, p = 0.03] and P60 (p = 0.04) components and approached significance for P110 (p = 0.06). However, the shape of the average evoked response remained the same, and there was no evidence for the typical mismatch negativity as reported in human studies2. In contrast, the hippocampal response did not differentiate between the standard and the deviant tones (p ≥ 0.10 for all components). Together with the correlation table (Table 2) this finding speaks against the notion that the cortical response is a simple volume conducted signal from the hippocampus.
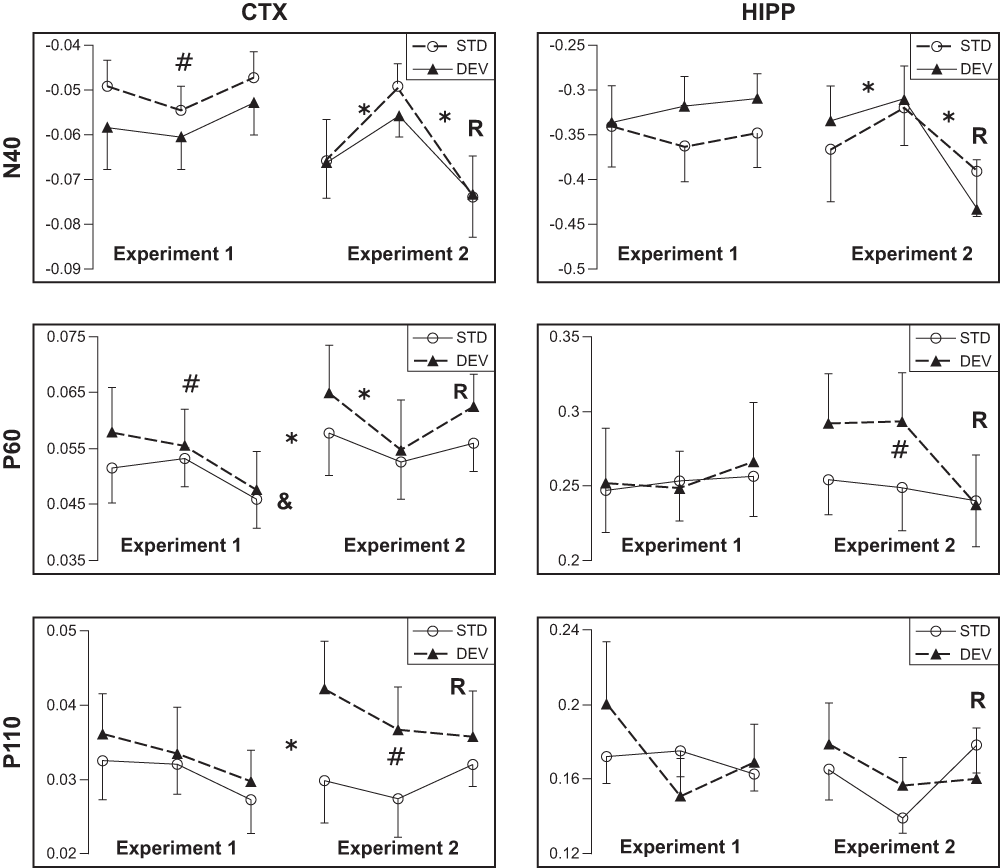
Figure 3. Effect of repetition on the AEP in response to the standard and the deviant tones.
Mean amplitudes of AEP components (N40, P60, P110) ± SEMs are given. In each chart the x-axis represent different runs of the test. Note the break between Run 3 of Experiment 1 and Run 1 of Experiment 2 to indicate the intervening days.
R under Run 3 of Experiment 2 indicates reversal of the mismatch contingency.
* significant difference between consecutive Runs (including between Run 3 of Experiment 1 and Run 1 of Experiment 2);
# significant difference between standard and deviant responses;
& significant repetition effect on component attenuation.
Repetition effect on the responses
The amplitude of cortical N40 response was relatively stable in Experiment 1, but the P60 component attenuated significantly between days [F(2,6) = 5.9, p = 0.04], and the P110 showed a similar, but non-significant trend [F(2,6) = 1.9, p = 0.24]. This trend could be observed for both standard and deviant tones (Figure 3). In contrast, none of the hippocampal components attenuated between days (all p values > 0.40).
The time dependency of AEP attenuation was further investigated in Experiment 2. First, we replicated the standard mismatch condition after 20 intervening days of rest. The cortical response to the standard tones reached the original (or higher) amplitude of Day 1 in Experiment 1 (Figure 3). The ANOVA for repeated measures revealed significant enhancement of cortical P60 [F(1,6) = 12.9, p = 0.01] and P110 (p = 0.03) components between Day 3 of Experiment 1 and Day 1 of Experiment 2. Interestingly, these were the same components that were also attenuated over three daily sessions in Experiment 1. Although a similar trend was observed in the N40 component in some animals, the difference did not reach significance at the group level (p > 0.15). The response enhancement after 20 intervening days could be observed to some extent for both standard and deviant stimulus (Figure 3). In contrast, hippocampal responses, which did not change significantly over the three days of Experiment 1, did not increase after the 20 intervening days of rest, either (all p > 0.35).
Next, we repeated the same mismatch condition on Day 2 of Experiment 2 to see whether this habituation of responses between days could be replicated. This time we saw an attenuation of cortical N40 [F(1,6) = 8.6, p = 0.03] and P60 [F(1,6) = 20.0, p = 0.004] components; and a similar, but not significant trend of P110 component [F(1,6) = 1.7, p = 0.24] (Figure 3). In addition, habituation of hippocampal N40 reached significance [F(1,5) = 12.9, p = 0.02]. Again habituation was similar for the standard and deviant responses. Furthermore, the difference between AEPs to the standard and deviant tones could be replicated. However, this time the most robust oddball effect was observed for cortical P110 [F(1,6) = 29.3, p = 0.002], while P60 showed only a trend (p = 0.07), and N40 no effect (p > 0.30). Unlike in Experiment 1, the hippocampal P60 component showed a clear oddball effect [F(1,5) = 15.2, p = 0.01].
Finally, we reversed the mismatch contingency on the second run of Day 2. The reversal resulted in a robust enhancement of both cortical [F(1,6) = 12.2, p = 0.01] and hippocampal N40 [F(1,5) = 28.7, p = 0.003] components, which increased even above the Day 1 (of Experiment 2) level (Figure 3). This change was observed for both the standard and deviant tones. No other cortical or hippocampal components were enhanced after the reversal (all p > 0.14), but the reversal removed the oddball effect for hippocampal P60 and cortical P110 components (Figure 3).
Scopolamine effect on the middle-latency components
Muscarinic receptors in the central nervous system (CNS) play an important role in the regulation of arousal, attention and synaptic plasticity16,17. To test the contribution of muscarinic receptors on the mismatch effect, we used the subtype nonspecific muscarinic antagonist, scopolamine18, in Experiment 3.
Scopolamine resulted in general attenuation of the cortical response, with significant effects in the N40 and P60 components (Figure 4; p = 0.03 and p = 0.04, respectively). In the hippocampal response, only the P60 component decreased significantly (p = 0.002). In Experiment 3, differences were no longer detected between the responses to the standard and deviant sounds for any of the cortical or hippocampal components. Furthermore, the effect of scopolamine did not differ for the standard vs. deviant response (for all sound × drug interactions p > 0.45).
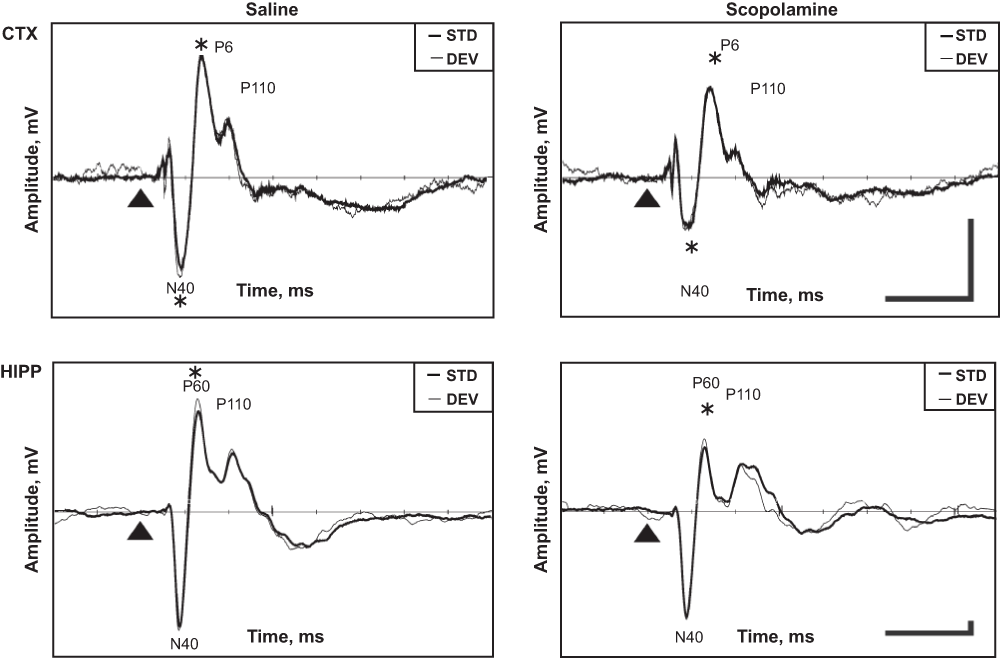
Figure 4. Scopolamine effect on cortical and hippocampus AEPs.
Representative example obtained from one rat in the auditory oddball paradigm. The thin line indicates the response to the deviant tone and the thick line the response to the standard tone. The triangle marks the tone onset. Horizontal bar corresponds to 100 ms, vertical bar to 0.04 mV (cortical scale is 5.7 times smaller than that for the hippocampus). Negativity is downward.
* significant difference between scopolamine and saline runs.
Discussion
The mismatch negativity (MMN) is well established phenomena in humans and widely studied within the field of cognitive neuroscience and psychology. However, MMN studies in laboratory animals are sparser and somehow controversial. We found evidence for repetition-induced attenuation of the mid-latency auditory ERPs but no correspondence to the sustained negativity around 100–200 ms in response to the deviant sound that is referred to as MMN in humans.
The rat auditory sensitivity as a function of stimulus frequency is very different from that of humans. The human auditory system is sensitive to frequencies from about 20 Hz to a maximum of around 20,000 Hz, although it is most sensitive between 2 and 5 kHz. In rats the auditory evoked potential increases in amplitude from 2 to 8 kHz reaching a plateau until 20 kHz19. Therefore having the deviant sounds higher than the standards can yield a false impression of MMN. This possibility was excluded in the present study by using a balanced number of higher and lower deviants and averaging their responses when comparing them to the standard. Nevertheless, the cortical ERPs in Experiment 1 had higher amplitudes in response to the deviant than the standard tones. Notably, the overall shape of the ERP did not change, and we found no evidence for a sustained shift – whether negative of positive – that would resemble the human MMN. Interestingly, no augmentation of the ERP to the deviant tone was observed in the hippocampus.
Whereas the number of high vs. low deviants was balanced in the present study, the standard and deviant responses differed in an important parameter, the repetition rate. The standard was presented at the proportion of 85%, while each deviant was presented only at 7.5%. One of the studies in anesthetized rats13 reported augmented responses to deviant sounds, which the authors interpreted in terms of repetition rate. In the present study, the cortical ERPs gradually decreased over three daily sessions (Experiment 1) and returned to the original levels after a three-week break between Experiments 1 and 2. The decrement of ERP from session to session was again replicated in Experiment 2. Notably, this decrement in ERP amplitude was roughly the same for the standard- and deviant-evoked responses. The most parsimonious way to interpret these findings is that both the response enhancement to deviant stimuli and general ERP decrement over time reflects gradual attenuation of auditory ERPs to stimulus repetition. This interpretation is also consistent with the disappearance of all differences between standard- vs. deviant-evoked responses after the standard and deviant stimuli were reversed. Namely, after the reversal the cumulative number of the former deviant stimuli soon approached that of the standard for that session. Thus our findings largely support the conclusion of Lazar and Metherate13 that the enhanced response to the deviant sound in an oddball paradigm can be attributed to differences in repetition rate.
Some of the present findings, however, cannot be explained by differences in repetition rate. First, after reversal of the task contingency, the N40 responses (for both the standard and the deviant tone) increased markedly in amplitude. A change in repetition rate could explain why the responses increased to the 9 kHz stimulus, the former standard that now became the deviant (proportion change from 85% to 15%, as only one deviant was used in this part of the experiment). However, this enhancement was also found for the 7 kHz stimulus that became much more frequent (7.5% vs. 85%). Moreover, the enhancement could be observed not only in the cortical channel that was sensitive to the repetition rate, but also in the hippocampus. A similar response to the reversal in the cortex and hippocampus may reflect general arousal or response enhancement in the thalamus or brainstem. A second finding that is at odds with the repetition rate hypothesis was the enhanced deviant-evoked hippocampal P60 and cortical P110 responses. It is possible that these changes after a three-week break in the experiment reflect a ‘declarative’ kind of memory recall as opposed to gradual response attenuation as a function of stimulus presentation. This finding warrants further studies.
Our conclusion that no auditory MMN exists in non-anesthetized rats contrasts with another studies conducted in anesthetized rats11,12,20. These studies found a sustained positive response over the auditory cortex to the deviating sound between 63 and 253 ms after the stimulus onset by electrocorticogram recording. In addition, the comparison between the responses to deviant alone vs. oddball deviant (i.e. deviant after the standard stimulus) revealed also a robust late positive response to the deviant alone between 220 and 350 ms after the stimulus onset. The shape of the latter response (Figure 2 in Ruusuvirta et al.20) is remarkably similar to a sinusoidal oscillation at 4 Hz, which is the theta frequency under urethane anesthesia. One interpretation of the study is that the deviant alone, as the most infrequent stimulus, induced a so-called theta reset and phase-locking of the theta rhythm to the auditory stimulus21, whereas the same deviant stimulus in the oddball context was less arousing and induced theta reset only occasionally, resulting in a flat positive response in the average. The sensitivity to theta reset may be much higher under urethane anesthesia than in urethane/xylazine combination anesthesia13 or in awake animals (present study), thus explaining the lack of these late components in these later studies. However, as for the P60 component all three studies seem to agree in that it is slightly larger for the deviant stimulus and dependent of the repetition rate (deviant alone is larger than the standard alone irrespective of stimulus pitch).
In light of previous studies and the present study it is highly unlikely that the awake rat has a similar auditory MMN response as reported for humans. Instead, stimulus repetition results in gradual attenuation of the mid-latency responses, which resembles attenuation of the N1-component of human auditory ERP22. One possible neuropharmacological mechanism underlying this repetition-related attenuation of auditory ERP is reduced cholinergic tone, as administration of scopolamine in the present study reduced the amplitudes of standard- and deviant evoked mid-latency responses. Thus the rat provides a model to study neuropharmacological regulation of the human N1-component, but other animal models need to be employed for the modeling of human MMN.
Author contributions
K.G. and H.T. designed the experiments. K.G. analyzed the data. K.G., H.T. and A.L. wrote the paper. A.L. carried out experiments. R.M. manufactured the electrodes, implanted them and conducted the brain histology.
Competing interests
The authors have no competing interests to declare.
Grant information
The authors declare that no grants were involved in supporting this work.
Acknowledgements
We thank Dr. M. Penttonen for constructive comments on the manuscript.
Faculty Opinions recommendedReferences
- 1.
Naatanen R, Alho K:
Mismatch negativity--a unique measure of sensory processing in audition.
Int J Neurosci.
1995; 80(1–4): 317–337. PubMed Abstract
| Publisher Full Text
- 2.
Naatanen M, Tervaniemi E, Sussman P, et al.:
"Primitive intelligence" in the auditory cortex.
Trends Neurosci.
2001; 24(5): 283–288. PubMed Abstract
| Publisher Full Text
- 3.
Alho K:
Cerebral generators of mismatch negativity (MMN) and its magnetic counterpart (MMNm) elicited by sound changes.
Ear Hear.
1995; 16(1): 38–51. PubMed Abstract
| Publisher Full Text
- 4.
Giard MH, Perrin F, Pernier J, et al.:
Brain generators implicated in the processing of auditory stimulus deviance: A topographic event-related potential study.
Psychophysiology.
1990; 27(6): 627–640. PubMed Abstract
| Publisher Full Text
- 5.
Kumaran D, Maguire EA:
Match mismatch processes underlie human hippocampal responses to associative novelty.
J Neurosci.
2007; 27(32): 8517–24. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 6.
Duncan K, Ketz N, Inati SJ, et al.:
Evidence for area CA1 as a match/mismatch detector: a high-resolution fMRI study of the human hippocampus.
Hippocampus.
2012; 22(3): 389–98. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 7.
Csepe V, Karmos G, Molnar M:
Evoked potential correlates of stimulus deviance during wakefulness and sleep in cat--animal model of mismatch negativity.
Electroencephalogr Clin Neurophysiol.
1987; 66(6): 571–578. PubMed Abstract
| Publisher Full Text
- 8.
Kraus N, McGee T, Littman T, et al.:
Nonprimary auditory thalamic representation of acoustic change.
J Neurophysiol.
1994; 72(3): 1270–1277. PubMed Abstract
- 9.
Ruusuvirta T, Korhonen T, Penttonen M, et al.:
Hippocampal evoked potentials to pitch deviances in an auditory oddball situation in the rabbit: No human mismatch-like dependence on standard stimuli.
Neurosci Lett.
1995; 185(2): 123–126. PubMed Abstract
| Publisher Full Text
- 10.
Javitt DC, Schroeder CE, Steinschneider M, et al.:
Demonstration of mismatch negativity in the monkey.
Electroencephalogr Clin Neurophysiol.
1992; 83(1): 87–90. PubMed Abstract
| Publisher Full Text
- 11.
Astikainen P, Stefanics G, Nokia M, et al.:
Memory-based mismatch response to frequency changes in rats.
PLoS One.
2011; 6(9): e24208. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 12.
Ruusuvirta T, Lipponen A, Pellinen E, et al.:
Auditory cortical and hippocampal-system mismatch responses to duration deviants in urethane-anesthetized rats.
PLoS One.
2013; 8(1): e54624. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 13.
Lazar R, Metherate R:
Spectral interactions, but no mismatch negativity, in auditory cortex of anesthetized rat.
Hear Res.
2003; 181(1–2): 51–56. PubMed Abstract
| Publisher Full Text
- 14.
von der Behrens W, Bäuerle P, Kössl M, et al.:
Correlating stimulus-specific adaptation of cortical neurons and local field potentials in the awake rat.
J Neurosci.
2009; 29(44): 13837–13849. PubMed Abstract
| Publisher Full Text
- 15.
Umbricht D, Vyssotki D, Latanov A, et al.:
Deviance-related electrophysiological activity in mice: Is there mismatch negativity in mice?
Clin Neurophysiol.
2005; 116(2): 353–363. PubMed Abstract
| Publisher Full Text
- 16.
Sarter M, Bruno JP:Cognitive functions of cortical acetylcholine: toward a unifying hypothesis.
Brain Res Brain Res Rev.
1997; 23(1–2): 28–46. PubMed Abstract
| Publisher Full Text
- 17.
Perry E, Walker M, Grace J, et al.:
Acetylcholine in mind: a neurotransmitter correlate of consciousness?
Trends Neurosci.
1999; 22(6): 273–80. PubMed Abstract
| Publisher Full Text
- 18.
Katzung BG, Masters SB, Trevor AJ, et al.:
Basic & clinical pharmacology (McGraw-Hill Medical; McGraw-Hill distributor. New York; London, ed. 11th,. 2009). Reference Source
- 19.
Knight RT, Brailowsky S, Scabini D, et al.:
Surface auditory evoked potentials in the unrestrained rat: Component definition.
Electroencephalogr Clin Neurophysiol.
1985; 61(5): 430–439. PubMed Abstract
| Publisher Full Text
- 20.
Ruusuvirta T, Penttonen M, Korhonen T:
Auditory cortical event-related potentials to pitch deviances in rats.
Neurosci Lett.
1998; 248(1): 45–48. PubMed Abstract
| Publisher Full Text
- 21.
Vinogradova OS:
Expression, control, and probable functional significance of the neuronal theta-rhythm.
Prog Neurobiol.
1995; 45(6): 523–583. PubMed Abstract
| Publisher Full Text
- 22.
Lu ZL, Williamson SJ, Kaufman L:
Behavioral lifetime of human auditory sensory memory predicted by physiological measures.
Science.
1992; 258(5088): 1668–1670. PubMed Abstract
| Publisher Full Text




Comments on this article Comments (0)