Keywords
Early heart development; heart jogging; Gene Ontology; Orthology
This article is included in the University College London collection.
Early heart development; heart jogging; Gene Ontology; Orthology
The methods subsection - generation of the list of zebrafish jogging genes section: edited to make it clearer how the list of zebrafish jogging genes was generated and the limitation of the approach taken to create a list of ‘heart jogging genes’.
In Discussion- paragraph 4: edited to create more positive statements about the role of the 'jogging ortholog' genes, in human heart development.
The methods subsection - generation of the list of zebrafish jogging genes section: edited to make it clearer how the list of zebrafish jogging genes was generated and the limitation of the approach taken to create a list of ‘heart jogging genes’.
In Discussion- paragraph 4: edited to create more positive statements about the role of the 'jogging ortholog' genes, in human heart development.
See the authors' detailed response to the review by Jeroen Bakkers
See the authors' detailed response to the review by Vincent VanBuren
An understanding of heart development is important for the treatment of both congenital and acquired heart disease. The majority of heart development studies use model organisms for ethical and practical reasons. Transparent fish embryos, as well chick embryos, enable the developing heart to be studied in real time1, and the mouse continues to be a key model organism used to investigate mammalian heart development2. Although there is substantial evolutionary conservation in the development of left-right axis asymmetry, there is divergence between species3. The earliest events in mammalian heart development are of great interest, but are poorly understood relative to externally developing organs, due to practical constraints.
For the majority of developing vertebrate embryos left-right asymmetry is controlled by a ciliated region; the left-right organizer node in the mouse and human, and the Kuppfer’s vesicle in the zebrafish4,5. In the zebrafish, laterality cues from the Kuppfer’s vesicle determine asymmetry in the developing heart, and consequently the direction of heart jogging and heart looping. At 24 hours post-fertilization (hpf) the symmetrical zebrafish heart tube is displaced relative to the dorsal midline, with a leftward ‘jog’. At 36hpf the heart tube then loops to the right to create the asymmetric heart5,6. Cilia within the Kuppfer’s vesicle are known to be instrumental in establishing left-right asymmetry and consequently play a significant role in determining the direction of heart jogging7 and heart looping8. However, a failure of heart jogging does not necessarily imply that there will be a failure in heart looping, and vice versa. In addition, asymmetric cell migration has been implicated as a key factor in the process of heart jogging9–13. Several of the genes involved in zebrafish heart jogging have been identified from mutation, morpholino and functional complementation studies6,10,13–26.
We sought to determine whether the use of Gene Ontology (GO) annotation could offer mechanistic clues to early mammalian heart development. GO is a controlled vocabulary that is used to describe gene product function27. GO describes three aspects of a gene product’s biology: the biological process that the gene product is involved in, the specific molecular function of the gene product and the cellular component that the gene product is located in. GO terms are associated in a directed acyclic graph (DAG), and thus have defined relationships to each other.
The process of heart looping has been described in a variety of higher eukaryotes2,28,29, and the occurrence of dextral-looping, the early phase of heart looping, appears to be conserved from zebrafish to chicken to humans. In addition, many congenital heart abnormalities, such as dextrocardia and isomerisms are thought to be due to abnormal heart looping2,30 and ciliary dysfunction has been associated with 50% of patients with congenital heart disease and heterotaxy31. However, the process of heart jogging has only been described in zebrafish6. Biben and Harvey describe a leftward shift of the developing mouse caudal heart prior to looping, which may be analogous to heart jogging in zebrafish28, but to our knowledge this has not been investigated further, and heart jogging is not considered to occur in mammals. Consequently, when the ontology describing heart development was expanded32, limitations were included to prevent the association of the GO term ‘heart jogging’ to mammalian gene products33. However, an absence of evidence is not evidence of absence, hence it remains a possibility that heart jogging also occurs in mammalian systems.
Although there has been substantial progress in heart development research1,3,4,29, there are clearly gaps in our understanding of early heart development, particularly in the mammal. Functional enrichment analysis of genes known to be involved in zebrafish heart jogging, and also of the human and mouse orthologs of these zebrafish heart jogging genes, identifies many conserved biological processes, functions and cellular locations across these three species. The results of these analyses support the role of cilia in symmetry breaking and the importance of cell signalling in early heart development.
A list of 30 zebrafish genes that affect heart jogging was compiled using a variety of approaches. Twelve zebrafish proteins were identified as they were already annotated to the ‘heart jogging’ GO terms, the remaining 18 proteins were then identified using the ZFIN (http://zfin.org/) Site Search, with the search phrase 'heart jogging', and filtering using the 'Expression/Phenotypes' category. This search retrieves figures from papers that have ‘heart jogging’ in the figure legend, and thus are likely to be describing specific zebrafish genes (and proteins) involved in this process. Many of these genes had not yet been curated with GO terms. Each of the papers identified in this way were reviewed; of the 23 zebrafish genes identified in these papers five (Bmpr1aa, Tbx1, unm_hu119, unm_hu202, unm_hu304) were eliminated, as none of these papers provided experimental evidence for the involvement of these genes in heart jogging. This left 30 zebrafish proteins with strong evidence for a role in the heart jogging process (Table 1). The experimental evidence describing the association of each gene to the process of heart jogging was manually reviewed, to ensure consistent criteria were applied.
The evidence for these 30 zebrafish proteins having a role in heart jogging comes from mutant, morpholino or functional complementation studies, as described in the associated publications.
|
Zebrafish gene symbol (protein ID) |
Human gene symbol (protein ID) |
Mouse gene symbol (protein ID) |
|---|---|---|
| acvr1l6 (Q9DGI6) | ACVRL1 (P37023) | Acvrl1 (Q61288) |
| apc57 (F1QN37) | APC (P25054) | Apc (Q61315) |
| bmp46,13 (O57574) | BMP4 (P12644) | Bmp4 (P21275) |
| bmp7a6 (Q9PTF9) | BMP7 (P18075) | Bmp7 (P23359) |
| vbmpr2a20 (Q288P3) | BMPR2 (Q13873) | Bmpr2 (O35607) |
| bmpr2b20 (Q288P2) | ||
| camk2a14 (Q32PV2) | CAMK2A (Q9UQM7) | Camk2a (P11798) |
| camk2b214 (E7F012) | CAMK2B (Q13554) | Camk2b (P28652) |
| camk2g114 (Q4V9P8) | CAMK2G (Q13555) | Camk2g (Q923T9) |
| ccdc1036 (Q6DGB6) | CCDC103 (Q8IW40) | Ccdc103 (Q9D9P2) |
| ccdc406 (Q56A40) | CCDC40 (Q4G0X9) | Ccdc40 (Q8BI79) |
| cobl23 (I1X3U9) | COBL (O75128) | Cobl (Q5NBX1) |
| dand515 (Q76C29) | DAND5 (Q8N907) | Dand5 (Q76LW6) |
| dnaaf16,10 (Q7ZV84) | DNAAF1 (Q8NEP3) | Dnaaf1 (Q9D2H9) |
| dub22 (Q0P484) | RCSD1 (Q6JBY9) | Rcsd1 (Q3UZA1) |
| fgfr218 (Q8JG38) | FGFR2 (P21802) | Fgfr2 (P21803) |
| foxh16,58 (Q9I9E1) | FOXH1 (O75593) | Foxh1 (O88621) |
| foxj1a25 (Q08CI2) | FOXJ1 (Q92949) | Foxj1 (Q61660) |
| foxj1b25 (F1R8Z9) | ||
| fzd222 (Q90YL7) | FZD2 (Q14332) | Fzd2 (Q9JIP6) |
| gsk3b17 (Q9IBD2) | GSK3B (P49841) | Gsk3b (Q9WV60) |
| has213 (Q9DG41) | HAS2 (Q92819) | Has2 (P70312) |
| lrrc66 (B3DH20) | LRRC6 (Q86X45) | Lrrc6 (O88978) |
| nipbla21 (F5HSE3) | NIPBL (Q6KC79) | Nipbl (Q6KCD5) |
| Niplblb21 (F1QBY1) | ||
| nkd124 (Q2TJA6) | NKD1 (Q969G9) | Nkd1 (Q99MH6) |
| nphp326 (P0CI65) | NPHP3 (Q7Z494) | Nphp3 (Q7TNH6) |
| pkd26 (Q6IVV8) | PKD2 (Q13563) | Pkd2 (O35245) |
| ptpn11a16 (Q7ZW17) | PTPN11 (Q06124) | Ptpn11 (P35235) |
| southpaw10,19 (Q7ZZT5) | no mammalian orthologs | |
The HUGO Gene Nomenclature Committee Comparison of Orthology Predictions (HCOP) search tool (http://www.genenames.org/cgi-bin/hcop.pl) was used to identify the closest possible human and mouse ortholog for each of the 30 zebrafish genes. HCOP displays predictions from 11 homology prediction tools, including EnsemblCompara, Homologene and Inparanoid34. For all but one gene, southpaw, HCOP returned human or mouse homologs for the zebrafish genes. The lack of a close mammalian ortholog of southpaw was confirmed with a UCSC BLAT analysis against the human and mouse genomes35. BLAST analysis36 showed that the closest possible human and mouse homolog for the zebrafish southpaw gene was Nodal (33% identity). Indeed, both southpaw and nodal are specifically expressed in the left lateral plate mesoderm5,37 and knockdown of murine Nodal in this region leads to a disruption of cardiac asymmetry, as does injection of southpaw morpholinos, suggesting a functional orthology between southpaw and Nodal5,37. However a reciprocal HCOP search showed that the zebrafish genes nodal-related 1 and 2 are the closest orthologs of human NODAL. Hence we have not included a human or mouse ortholog for zebrafish southpaw (Table 1). Three pairs of zebrafish paralogs (bmpr2a/bmpr2b; foxj1a/foxj1b; nipbla/nipblb) have a single corresponding ortholog in human and mouse. Therefore, there are 26 human and 26 mouse orthologs to the 30 zebrafish genes identified as relevant to zebrafish heart jogging (Table 1).
The human ‘jogging ortholog’ genes were fully manually annotated, by an experienced GO curator38. Individual PubMed queries were run for each gene using the approved human gene symbol and filtering on ‘human’. To achieve full annotation, all of the relevant publications (a total of 232) containing unique functional data for each gene were annotated, regardless of the specific biology described in each paper. This approach enabled consistent annotation of all experimental data relating to each gene, thus ensuring an unbiased overview of any common processes associated with these genes. In addition, the GO term ‘heart looping’ was associated with a ‘jogging ortholog’ human gene if dextrocardia or situs inversus totalis phenotypes had been associated with a mutation in the gene, in order to follow the generally agreed view that leftward heart looping will have resulted in these phenotypes2.
The Mouse Genome Informatics functional enrichment tool VLAD (VisuaL Annotation Display; http://proto.informatics.jax.org/prototypes/vlad-1.0.3/) was used to look for overrepresentation of GO terms in each gene list relative to the whole genome of the organism. The annotation datasets used for the analysis were zfin (4th March 2013), goa_human (5th March 2013) and mgi (7th March 2013) for the zebrafish, human and mouse analyses respectively, and the ontology dataset used was dated 10th March 2013. The query gene lists (as UniProt IDs) were pasted into the ‘Query Set’ field, the ‘Universe Set’ field was left blank (to specify all genes in species specific annotation file) and the ‘Display Settings’ options selected were ‘pruning threshold’:3 and ‘collapsing threshold’:6. No evidence codes were excluded from the analyses. For this analysis the total number of genes (universe set size) having annotations in the biological process ontology were 14,577, 30,441 and 24,813 for zebrafish, human and mouse respectively. In line with common practice, when using functional analysis tools, enriched GO terms with 1 or 2 associated query genes were excluded from the final results table.
A list of 103 mouse genes likely to play a role in early heart development was created by combining gene lists derived from three sources: The Mouse Genome Informatics Mammalian Phenotype Ontology browser http://www.informatics.jax.org/searches/MP_form.shtml39, the QuickGO browser http://www.ebi.ac.uk/QuickGO/40 and the ‘jogging ortholog’ gene list described above (see Mousegenelist.csv in Data File). The Mammalian Phenotype Ontology browser was queried for genotypes annotated with the terms ‘abnormal direction of heart looping’, ‘situs inversus totalis’, ‘dextrocardia’ and ‘mesocardia’, creating a list of 180 genotypes with an associated gene. Due to the multiple phenotypes associated with each of these genotypes only 58 genes were identified through this approach, and of these only 5 overlap with the 26 ‘jogging ortholog’ genes. Thirty-five genes were identified by filtering on the GO term ‘determination of heart left/right asymmetry’ and its child terms, the evidence code IMP (Inferred by Mutant Phenotype), and the mouse taxon. Of these only two are also present in the ‘jogging ortholog’ gene lists and 11 are present in the phenotype gene list. Twenty-six mouse ‘jogging ortholog’ genes were added to this combined gene list, and any duplicated genes were removed.
Thirty zebrafish genes were annotated to the GO term ‘heart jogging’ or one of its child terms based on experimental data from the literature (Table 1). Human and mouse orthologs of these genes were identified, as described in the Methods section, resulting in a list of 26 mammalian ‘jogging orthologs’.
The human ‘jogging ortholog’ genes were then fully annotated with GO terms based on published experimental data. All manual annotations to the human, mouse and zebrafish genes can be visualized with the QuickGO Gene Ontology browser http://tinyurl.com/humanortholog, http://tinyurl.com/mouseortholog and http://tinyurl.com/zebrafishgenes.
The zebrafish heart jogging gene list and the human and mouse ‘jogging ortholog’ gene lists were analysed using the VLAD enrichment tool. This identified 155 biological process GO terms that were significantly enriched in the zebrafish (see Human_data.csv in Data File), 431 in the human (see Human_data.csv in Data File) and 402 in the mouse (see Mouse_data.csv in Data File) gene lists. The enriched GO terms from all three species were grouped into five biological areas: Development, Patterning, Cellular Process, Signalling and Movement. The relative enrichment of key GO terms from each area was compared across all three species (see Biological_process_summary.csv in Data File; summarized in Table 2).
The enriched GO terms were grouped into specific ontology areas, with a selection of more specific child term (preceded with a dash) also included. The full list of grouped GO terms can be found in Table S4, which also shows the genes annotated to each term from each of the three species. k: the number of genes in each gene list annotated to the GO term; M: the number of genes in the species proteome annotated to the GO term.
Enrichment of heart development terms. As expected there was a significant enrichment of developmental process terms in all three gene lists, including an enrichment of the GO term ‘heart development’. However, there was also enrichment of terms such as ‘renal system development’ and ‘nervous system development’, indicating the role of these proteins in regulating the development of a range of organ systems and tissues. These data analyses also show an enrichment of terms describing specific, but universal, cellular processes, such as signalling and regulation of transcription (Table 2). These terms represent essential aspects of development, but are grouped discretely due to their roles in many other biological processes.
‘Pattern specification’, described in GO as a ‘developmental process that results in the creation of defined areas or spaces within an organism to which cells respond and eventually are instructed to differentiate’ and several of its more specific child terms (such as ‘specification of symmetry’), were also enriched in all three gene lists. Within the symmetry ontology, the GO term ‘determination of heart left/right asymmetry’ is annotated to all 30 genes in the zebrafish jogging gene list, however, it is only associated with 8 and 4 jogging ortholog genes in the human and mouse respectively. Of the 97 zebrafish genes associated with ‘determination of heart left/right asymmetry’ 31% are also present in the zebrafish jogging gene list. In contrast, only 11% of the human and 8% of the mouse genes associated with this term are also ‘jogging orthologs’. These results confirm an overlap in the functional role of the zebrafish jogging genes and the human and mouse orthologs in the determination of heart left/right symmetry. However, this relatively low level of overlap may reflect the limitations of model organism and human research in this area, rather than a lack of functional conservation of these genes.
In addition, there were some differences in the developmental terms that were enriched between species. For example, the GO terms ‘vasculature development’ and ‘sensory organ development’ are enriched in both the human and mouse ‘jogging ortholog’ gene lists (Table 2), but neither of these processes are enriched in the zebrafish jogging ortholog genes. This difference may reflect the type of experiments zebrafish are used for, rather than reflecting a difference between zebrafish and mammals in the genes required for these developmental processes.
Enrichment of cilia terms. Terms in the cellular component organization or biogenesis ontology were enriched across all three gene lists (Table 2 and Biological_process_summary.csv in Data File). Specifically there was an enrichment of terms describing ‘cilium morphogenesis’ and ‘protein complex assembly’ (Figure 1). Within each of these, some more specific terms were enriched, for example the human and mouse ‘jogging ortholog’ gene lists were enriched for the term ‘axonemal dynein complex assembly’, whilst the zebrafish and human gene lists showed an enrichment of the term ‘cilium assembly’.
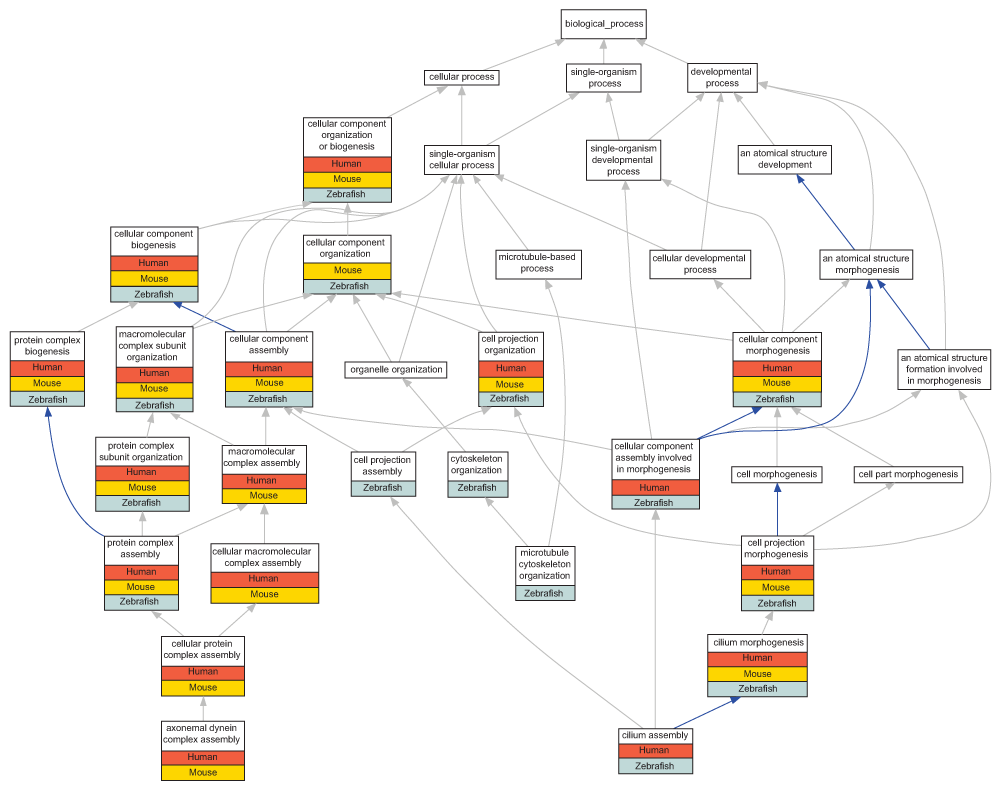
The GONUTs view of relationships between enriched terms from the cellular component organization or biogenesis ontology84. The grey arrows are used where a term has an ‘is a’ relationship to its parent term, the blue arrows indicate a ‘part of’ relationship. The bars below each GO term indicates which of these terms are enriched in the zebrafish ‘jogging’ gene list, and the human and mouse ‘jogging ortholog’ gene lists.
Terms such as ‘regulation of cell projection organization’ were also enriched in the human and mouse ‘jogging ortholog’ gene lists. ‘Regulation’ terms have a ‘regulates’ relationship with the relevant processes; for example the term ‘positive regulation of cell projection organization’ has a ‘positively_regulates’ relationship to the term ‘cell projection organization’. In GO an important benefit of building a DAG, rather than a flat-list of controlled vocabulary terms, is that relationships can be used to make inferences from one term to another. However, the VLAD enrichment tool does not automatically create a transitive relationship between ‘regulation’ terms and the processes or functions they regulate. Consequently genes annotated to a ‘regulation’ term will not be associated with the regulated process term (unless there is an independent annotation to the process term). It is also important to recognise that it can be difficult for a curator to choose between annotating to the biological process itself, or to the term describing the regulation of that biological process, based on the published experimental data. Therefore, to get a full picture of the genes involved in a process, including the genes that regulate the process, it is necessary to combine the genes annotated to GO terms describing both the ‘process’ and the ‘regulation of the process’. For example, 10 zebrafish, 12 human and 14 mouse genes within the ‘jogging ortholog’ gene lists are annotated to either ‘cell projection organization’ or ‘regulation of cell projection organization’ (or children of these terms). This represents 33%, 40% and 47% of these zebrafish, human and mouse ‘jogging’ gene lists respectively, indicating that the process of cell projection organization is an important function for this group of genes. In addition, many of the ‘jogging’ and ‘jogging ortholog’ genes annotated to ‘cell projection organization’ terms have also been annotated to the cellular component term ‘cell projection’ (7, 11, 13, genes in zebrafish, human and mouse, respectively, see Data File). The enrichment of the biological process term ‘cell projection organization’ and cellular component term ‘cell projection’ within these gene lists is consistent with the key role of cilia located in the node/Kuppfer’s vesicle to determine heart left/right asymmetry in all three species.
Enrichment of cell migration terms. Cell migration also plays a key role in the establishment of the heart cone, heart jogging and heart looping9,10 and enrichment of the GO term ‘cell migration’ is seen in the ‘jogging’ gene lists of all three species (Table 2 and Biological_process_summary.csv in Data File). Lenhart et al. (2013)9 identified FoxH1, spaw, Bmp4, Lefty2 and Has2 as essential to the asymmetric cell migration that leads to heart jogging. However, our literature review suggests that some genes may have functions in both cilia assembly, within the Kuppfer’s vesicle, and cell migration. For example, thymocytes from Foxj1 transgenic mice display defective migration41, whereas Foxj1-null mice are defective in ciliogenesis42. Similarly, in zebrafish, Fzd2 has been shown to play a role in cilium assembly22 as well as pancreatic insulin-cell migration43. Consequently, further investigations into the role of these genes in heart jogging cell migration may provide further insight into this process.
In order to investigate the contribution of individual genes in the multiple processes associated with early heart development we created human and mouse heart development gene lists and examined the associated GO biological processes terms. A list of 103 mouse genes with roles in early heart developmental processes was created by merging the three gene lists created using the Mouse Genome Informatics phenotype browser, the QuickGO browser as well as the ‘jogging ortholog’ gene list (Mousegenelist.csv in Data File).
GO captures a range of biological processes that a single gene is involved in. By comparing the overlap between the GO terms associated with specific gene lists it is possible to see what cellular mechanisms are likely to be contributing to the various heart developmental processes. Using the QuickGO browser, genes in the zebrafish ‘heart jogging’ gene list, which were associated with the GO terms ‘heart looping’, ‘signal transduction’, ‘cell migration’ and ‘cell projection organization’ (and all child terms, including ‘regulation’ terms), were downloaded, as well as the genes associated with these terms that were also present in the mouse ‘early heart development’ gene list (Mousegenelist.csv in Data File).
In the zebrafish ‘heart jogging’ gene list a similar proportion of the genes have the potential to play a role in cell projection organisation (10 genes), cell migration (8 genes) and signal transduction (13 genes) (Figure 2A). In the list of 103 mouse genes that are associated with early heart development, either by phenotype, annotation or homology to the zebrafish ‘heart jogging’ gene list, 82 have been annotated to the GO term heart looping. In contrast to the zebrafish ‘jogging’ gene list, signal transduction appears to play a major role in the mouse early heart development, with 27 genes associated with both signal transduction and heart looping, whereas only 18 and 9 genes, respectively, are associated with cell migration and cell projection organization (Figure 2B). These results fit well with what is known about these gene lists. The zebrafish ‘jogging’ gene list defines a group of genes whose functions are required very early in heart development, when the role of cilia in symmetry breaking initiates the heart jogging process. Whereas, in the mouse ‘early heart development’ gene list the genes included have roles in heart looping, which is developmentally later event than heart jogging. Therefore, although the initial events associated with breaking of left-right symmetry are represented within this gene list, the genes involved in the later process of ensuring the complex looping of the heart tube, through controlled signalling and cell migration, contribute to a large proportion of this list.
While annotating the 26 human ‘jogging ortholog’ genes we noticed that almost half of these genes have not been associated with a specific disease phenotype (Table 3). However, of the 26 genes examined, mutations in 14 had been associated with a disease phenotype, a fifth of which were ciliopathies. Dextrocardia or situs inversus totalis (reversal or mirroring of the major visceral organs) was associated with 6 of the human ‘jogging ortholog’ genes. Location of the heart on the right side (rather than the left) is generally agreed to be the result of left-handed, instead of right-handed looping of the heart tube in early embryogenesis2. The association of these ‘jogging ortholog’ genes with heart looping defects confirm that there is conserved functional homology between at least some of these orthologous zebrafish and human genes in the very early stages of heart development, which lead to the initial heart asymmetry. All four of ciliopathy-associated ‘jogging orthologs’ were also described as associated with situs inversus totalis, confirming the conserved role of these genes in the cilia within the symmetry determining left-right organizer.
The associated diseases are described in the listed publications.
|
Human gene symbol (protein ID) | Heart relevant phenotype | Other associated phenotypes |
|---|---|---|
| ACVRL1 (P37023) | - | Hereditary haemorrhagic telangiectasia type 2 (HHT2)59, HHT2 with pulmonary hypertension60 |
| APC (P25054) | - | Familial adenomatous polyposis coli-161 |
| BMP4 (P12644) | - | Microphthalmia, syndromic 662, orofacial cleft 1163 |
| BMP7 (P18075) | - | - |
| BMPR2 (Q13873) | - | Pulmonary hypertension64 |
| CAMK2A (Q9UQM7) | - | - |
| CAMK2B (Q13554) | - | - |
| CAMK2G (Q13555) | - | - |
| CCDC103 (Q8IW40) | Dextrocardia, situs inversus totalis65 | Ciliary dyskinesia, primary, 1765 |
| CCDC40 (Q4G0X9) | Situs inversus totalis66 | Ciliary dyskinesia, primary, 1567, Kartagener’s Syndrome66 |
| COBL (O75128) | - | - |
| DAND5 (Q8N907) | - | - |
| DNAAF1 (Q8NEP3) | Situs inversus totalis68,69 | Ciliary dyskinesia, primary, 1368,69 |
| FGFR2 (P21802) | - | Several craniosynostosis70,71, see OMIM for more information |
| FOXH1 (O75593) | Ventricular septal defect72, transposition of the great arteries54 | - |
| FOXJ1 (Q92949) | - | - |
| FZD2 (Q14332) | - | - |
| GSK3B (P49841) | - | - |
| HAS2 (Q92819) | - | - |
| LRRC6 (Q86X45) | Situs inversus totalis73 | Ciliary dyskinesia, primary, 19, Kartagener’s Syndrome73 |
| NIPBL (Q6KC79) | Cardiac septal defects (not confirmed as associated with NIPBL mutations)74 | Cornelia de Lange syndrome 174,75 |
| NKD1 (Q969G9) | - | Colorectal adenocarcinoma76 |
| NPHP3 (Q7Z494) | Situs inversus totalis77 | nephronophthisis type 378, Meckel syndrome type 777, renal-hepatic-pancreatic dysplasia77, 79 |
| PKD2 (Q13563) | Dextrocardia, situs inversus totalis53 | Polycystic kidney disease 253,80 |
| PTPN11 (Q06124) | atrioventricular canal defects81 | juvenile myelomonocytic leukemia82, LEOPARD syndrome81, Noonan syndrome81,83 |
| RCSD1 (Q6JBY9) | - | - |
We have used GO to annotate the key genes involved in zebrafish heart jogging and their human and mouse orthologs. Heart jogging is not a process that is thought to occur in mammals. However, these genes are conserved between species and play essential roles in many developmental processes. The information available about these genes in several diverse species can be used to shed light on the roles of these genes and possible mechanisms in heart jogging and other heart developmental processes. Our analyses are in agreement with the well described essential role of cilia in early development4,5,31, with a third of the zebrafish ‘heart jogging’ genes associated with the biological process ‘cell projection organization’ (Table 2).
However, it is also important to recognise that although there is considerable evidence for conserved mechanisms of heart development across vertebrates there are also areas of divergence44. For example, in the mouse, zebrafish and Xenopus the rotation of cilia is responsible for the early asymmetric gene expression pattern around the left-right organizer, whereas cilia do not play a role in symmetry breaking in the chicken or pig44.
The early phases of heart development are particularly difficult to study in mammals, however various approaches are enabling progress in this area2,29,45,46 and using phenotype, annotation and orthology data we have created a list of 103 genes with a putative role in early mouse heart developmental processes. Furthermore, the phenotypes associated with experimentally generated mutant mice provide further clues to the likely role of these genes in human heart development; the genes associated with situs inversus totalis phenotypes are most likely to have functional roles within the node. Conversely, genes not associated with situs inversus totalis but associated with an abnormal direction of heart looping, dextrocardia or mesocardia are likely to be involved in the response of the embryonic heart tube to the left/right asymmetry signals. This is not a completely reliable interpretation, for example mutations in the transcription factor Pitx2 lead to mice with situs inversus totalis, however, Pitx2 is expressed in the left lateral plate and its continued asymmetric expression is necessary for asymmetric morphogenesis of most visceral organs44. The mouse knockout consortia data47 will continue to help with the identification of additional early heart development genes, and informed interpretation of these phenotypes will make it possible to separate those genes likely to be associated with the node from those with functions within the heart tube.
In humans, defects in early heart development are likely to result in spontaneous abortion and therefore many genes required for early heart development will go undetected48. Consequently, human embryos with heart defects, which develop to full term, represent the less severe end of the spectrum. Mutations in several human genes have now been identified as causative of abnormal heart looping, such as ACVR2B, LEFTY2, GJA1 and ZIC349–52, and some of the ‘jogging ortholog’ genes (CCDC103, CCDC40, DNAAF1, LRRC6, NPHP3 and PKD2) are also associated with heart looping defects. Thus providing evidence to support an involvement of these genes in left-right asymmetry determination in the heart. Furthermore, mutations in some of the ‘jogging ortholog’ human genes, FOXH1 and PTPN11, are associated with heart septal defects in humans, which seems to imply that in individuals with these mutations early heart developmental processes have proceeded normally, suggesting that, contrary to their role in zebrafish, these genes may not be involved in the early stages of human heart development. However, there are other possible reasons why there is a poor association of heart defects with the ‘jogging ortholog’ gene list. This may simply be due to the lack of detection of situs inversus totalis53, or reflect a redundancy in gene function, or it may be that the majority of mutations in these genes are simply not detected in humans because they are masked by first trimester spontaneous abortions, which are known to have a high level of heart defects48.
The impact of lethal mutations on detection of genes associated with heart development would suggest that mutations in these genes would only be detected in individuals with mutations with relatively minor impact on gene function. This idea is supported by the recent identification of multiple ‘minor’ heterozygous mutations within a functional network in three patients with transposition of the great arteries. All of these genes either participate or cooperate within the Nodal signaling pathway54 and the carriers of single mutations exhibit no heart or laterality defects. The impact of ‘minor’ mutations, such as these, may explain the contribution of ‘genetic modifiers’ to congenital heart defects with variable penetrance within a family55, or may suggest a polygenic basis for some of these diseases56. This is supported by model organism data, which provides evidence of multigenic origins for congenital heart disease56. However, model organisms are rarely used to examine the impact of genetic modifiers on heart development, as the majority of model organisms are inbred and examination of mutations leading to ‘minor’ phenotypic variations is often not viewed with the same level of interest as the more extreme heart development defects.
Next Generation Sequencing (NGS) has the potential to identity many more instances of multiple mutations in genes which are functionally linked through a specific pathway. However, teasing out which gene mutations are contributing to a disease, as a genetic modifier or as the causative gene variant, and which are not involved in the disease, is likely to take considerable time. Gene Ontology, KEGG and Reactome pathways, along with protein interaction networks have the potential to inform the process of identifying genetic variants associated with heart defect risk through the identification of pathways and networks which are common to the genes associated with the risk gene variants. Consequently, interpretation of NGS data will be greatly improved with full annotation of the candidate genes involved. The identification of these risk gene variants is likely to be of considerable value to those patients seeking prenatal diagnosis. In addition, the identification of more genes associated with heart defects will also help clarify the conserved and divergent heart development pathways that exist between humans and key model organisms.
This study demonstrates that full annotation, using GO, of a set of genes known to be associated with early stages of heart development in zebrafish can be used to confirm functional conservation of the role of these genes in a variety of developmental processes. While this study supports the assertion of gene function based on orthology between genes, it also identifies that for some genes there is no direct evidence for their conserved involvement in specific developmental processes through evolution. Consequently, for evolutionary studies, manual annotation of the genome of individual species will be necessary to enable a bioinformatics approach to investigating the evolution of developmental processes.
VKK conceived and designed the study, undertook curation of the prioritized human genes, analysed the datasets and drafted the manuscript. DH participated in the design of the study, undertook curation of the prioritized zebrafish genes, and drafted the manuscript. PJT and RB participated in the study design and helped to draft the manuscript. RCL participated in the design of the study, analysed the datasets and drafted the manuscript. All authors read and approved the final manuscript.
The authors declare that they have no conflict of interests. VKK is currently employed by F1000Research. Her role at the journal does not include any involvement in the pre-publication editorial checks, or with the refereeing process.
The Cardiovascular GO Annotation Initiative is funded by the British Heart Foundation (SP/07/007/23671 and RG/13/5/30112) and PJT is supported by the British Heart Foundation (RG08/014). The Zebrafish Model Organism Database is funded by the National Human Genome Research Institute (P41 HG002659) of the National Institutes of Health.
Many thanks to Dr. Jim Hu and Dr Mary Dolan for their help with the creation of Figure 1 and Dr Fotios Drenos for creating the Venn diagrams in Figure 2.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 10 Feb 14 |
read | |
|
Version 1 13 Nov 13 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)