Keywords
posttraumatic stress disorder, PTSD, major depressive disorder, MDD, insula, ACC, resting state, functional connectivity, comorbidity, fMRI
posttraumatic stress disorder, PTSD, major depressive disorder, MDD, insula, ACC, resting state, functional connectivity, comorbidity, fMRI
Posttraumatic stress disorder (PTSD) is an anxiety disorder that can develop after a traumatic event. It is characterized by re-experiencing the traumatic event, avoidance of trauma reminders and emotional numbing symptoms, and increased arousal1. PTSD frequently co-occurs with other Axis I psychiatric disorders, such as major depressive disorder (MDD2). Patients with both PTSD and depression were found to have more psychological distress and are also more treatment resistant than patients with PTSD or depression alone3–5. About 48% of PTSD patients were found to have comorbid MDD in a large national survey in the United States2. Therefore, studies investigating the neurobiology of PTSD often comprise patients with and without comorbid MDD. Neuroimaging studies have demonstrated dysfunction of similar brain regions in both PTSD and MDD. That is, PTSD and MDD are both associated with alterations in structure and function of the medial prefrontal cortex (mPFC), amygdala, insula, and anterior cingulate cortex (ACC6–8). To what extent comorbid MDD contributes to the reported neurobiological alterations of PTSD is yet to be determined.
Thus far, two neuroimaging studies have directly investigated differences in PTSD patients with and without comorbid MDD. First, reduced activity of the mPFC and amygdala was found in PTSD patients with comorbid MDD versus PTSD patients without MDD, when fearful faces were presented9. Second, during a symptom provocation paradigm PTSD patients with comorbid MDD had decreased activity in the insula, and increased ACC and posterior cingulate cortex (PCC) activation versus PTSD patients without MDD10. In addition, decreased insula activation remained significant after controlling for PTSD severity. One other study has investigated the effects of depressive symptoms in PTSD patients. A positive correlation between depressive symptoms and (para) hippocampal and ventral ACC activity during an emotional memory task was observed in PTSD patients11. A fourth fMRI study involving PTSD patients versus both controls and MDD patients found increased activity in several brain areas of PTSD patients including the insula when emotional pictures were presented12.
However, these four studies were limited by small sample sizes (8 PTSD-MDD, 8 PTSD+MDD9, 11 PTSD-MDD and 15 PTSD+MDD10, 21 PTSD+MDD and 12 PTSD-MDD11, 16 PTSD and 16 MDD12). In addition, these studies investigated neurobiological alterations during emotional tasks, potentially inducing PTSD (and/or depressive) symptoms. It is expected that PTSD and/or MDD symptom provocation induces an altered state in PTSD with or without MDD, which is reflected by alterations in brain activity. Whether regular functioning of the brain in the absence of symptom-inducing stimuli deviates in PTSD with versus without comorbid MDD remains unclear. To our knowledge, functioning of the brain during resting state, without presenting stimuli or requiring task performance, has not been investigated in PTSD patients with and without comorbid MDD. Thus, the effect of comorbid MDD on brain functioning at baseline of PTSD patients deserves further investigation.
Here, we investigate the effects of comorbid MDD on resting state functional connectivity in PTSD patients. Since the studies described above indicated that functioning of the ACC distinguishes PTSD with and without MDD during emotional tasks9–11, this brain area was chosen as a region of interest. MDD has been associated with alterations in structure13, function14, structural connectivity15, and reduced resting state functional connectivity16–18 of the subgenual ACC in particular, which is a subdivision of the ventral ACC. In addition, subgenual ACC activation and cortical thickness have been associated with symptom improvement in PTSD19,20. Therefore, the subgenual ACC was selected as a more specific region of interest. Second, alterations in activation of the insula also differed between PTSD patients with and without PTSD, even when controlling for PTSD severity10. Furthermore, insula activation distinguished PTSD patients from MDD patients12. Thus, the insula was chosen as a second region of interest. As increased ACC activity was found in PTSD with comorbid MDD, as well as a positive correlation of ACC activity with depressive symptoms, we hypothesize that functional connectivity of the subgenual ACC is increased in PTSD with versus without comorbid MDD. Since insula activity is increased in PTSD versus MDD and insula activity was reduced in PTSD with comorbid MDD versus PTSD without MDD, we expected to find lower insula functional connectivity in PTSD with MDD as compared to PTSD without MDD. In summary, in order to provide more insights into the potential effects of MDD on the neurobiology of PTSD, the present study examined the effects of comorbid MDD on subgenual ACC and insula resting state functional connectivity in PTSD patients.
In total, 30 male veterans with PTSD with comorbid MDD (PTSD+MDD, mean age 34.2 ± 8.5), and 25 male veterans with PTSD without comorbid MDD (PTSD-MDD, mean age 37.4 ± 10.1) were included in this study. All patients were recruited from the Military Mental Health Care Center, the Netherlands. PTSD and MDD diagnoses were confirmed using the Clinician Administered PTSD scale (CAPS21) and the Structural Clinical interview for DSM-IV (SCID22). Several patients were medication naive (PTSD+MDD; n=13, PTSD-MDD; n=10), some patients were currently taking antidepressants (e.g. selective serotonin reuptake inhibitors; PTSD+MDD; n=6, PTSD-MDD; n=6), and some patients used benzodiazepines (PTSD+MDD; n=5, PTSD-MDD; n=2), or both antidepressants and benzodiazepines (PTSD+MDD; n=1, PTSD-MDD; n=3). One patient from the PTSD+MDD group used both antipsychotics and benzodiazepines. Most of the veterans had been deployed to Afghanistan (n=28) and to the former Yugoslavia (n=10). After receiving a complete written and verbal description of the study, all participants gave informed consent. Participants received financial compensation for their participation. The Medical Ethical Committee of the UMC Utrecht approved the study (protocol number NL29550.041.09), and the study was performed in accordance with the Declaration of Helsinki23.
Functional and structural images were obtained using a 3.0 Tesla magnetic resonance imaging scanner (Philips Medical System, Best, the Netherlands). Before the resting state scan, a ten minute T1-weighted high-resolution image (TR = 10 ms TE = 4.6 ms flip angle 8, 200 slices sagittal orientation, FOV 240 × 240 × 160, 304 × 299 matrix) was acquired. This image was utilized for co-registration and segmentation purposes and also allowed the participants to adapt to the scanner environment. During the nine minute resting state scan participants were asked to relax, to let their mind wander and to focus on a fixation cross. Three hundred and twenty T2* echoplanar interleaved images were collected (TR = 1600 ms, TE = 23 ms, flip angle = 72.5°, 30 transverse slices, FOV 256 × 208 × 120, 64 × 51 matrix).
Pre-processing was conducted with SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/), which included slice-timing correction, realignment, co-registration with the anatomical scan, normalization, and spatial smoothing (8 mm FWHM). Five participants (2 PTSD+MDD, 3 PTSD-MDD) were excluded due to excessive motion (more than 2 mm displacement in any direction (x, y or z) or 2 degrees rotation (pitch, roll or yaw)).
The Data Processing Assistant for Resting-State fMRI (DPARSF) was utilized for further analyses (restfmri.net24), which is based on MRIcroN (http://www.mricro.com), SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/), and the Resting-State fMRI Data Analysis Toolkit24. Resting state images were band-pass filtered (0.08-0.01 Hz) to reduce low-frequency drift and high-frequency noise, and detrended to correct for general signal drift. In order to correct for physiological processes and motion, the motion parameters from the realignment step, mean global signal, white matter signal, and cerebral spinal fluid signal were included as covariates in the analysis. In addition, motion scrubbing was applied to scans that surrounded a minimum of 0.5 mm frame displacement (one scan before displacement, two scans after displacement), using nearest neighbour interpolation25. A minimum of approximately 5 minutes of resting state (183 unscrubbed resting state images) was set as a required threshold for correct scrubbing. One participant was excluded due to excessive scrubbing, resulting in the following groups: 27 PTSD+MDD, and 22 PTSD-MDD.
For the subgenual ACC two spherical seeds (left and right, 3.5 mm radius) were created around two seed point coordinates, as previously described by Kelly et al. (2009)26. The anterior insula seed was created from two distinct anterior insula subdivisions that were described as the insula regions involved in emotion and cognition, as reported by Kelly et al. (2012)27. The mean time series for each of those seeds was extracted for all individuals and correlated with the time series of every voxel in the brain in order to create functional connectivity maps. These correlation maps were normalized using Fishers z-transform, resulting in a z-map for each ACC network per participant. The individual z-maps were used for second-level group analysis (full factorial design, SPM). A general effect of group (F-test) was investigated to determine group differences within the positive and negative network of the seed pairs.
Cluster-level multiple comparison correction was applied according to Gaussian Random Field theory28. A height threshold of p<0.001 was applied and combined with an extended cluster threshold of k>11, that corresponds to corrected p<0.05 (as determined with 1000 Monte Carlo simulations using Alphasim, implemented in the REST toolbox; FWHM 8 mm, cluster connection radius 7 mm).
Post-hoc analyses were performed including the total CAPS score as a covariate, in order to assess whether the results were due to differences in PTSD severity (F-test, height threshold p<0.001 extended threshold k>11, resulting in false discovery rate (FDR) corrected p<0.05). In addition, the positive affect (PA) score from the mood and anxiety questionnaire (MASQ29), which has been reported to reflect a core feature of MDD30, was also investigated as covariate. In addition, functional connectivity values (z-values) were extracted from the peak voxels of clusters of significant differences in order to perform post-hoc correlations with PTSD and MDD symptoms. Total CAPS scores, CAPS symptom cluster B, C, and D scores, as well as MASQ PA scores were investigated. Correlations between whole brain functional connectivity and CAPS and PA scores were calculated respectively.
Groups did not differ significantly in age, handedness, the number of times they were deployed, the time since their last deployment, and educational level as measured with the international standard classification of education (ISCED;31). The PTSD+MDD group differed from the PTSD-MDD group in total PTSD severity (CAPS score; p=0.008), which appeared to be largely driven by differences in avoidance and emotional numbing symptom scores (cluster C; p=0.001). In addition, the PTSD+MDD group had lower PA scores versus the PTSD-MDD group (p=0.012), while negative affect and somatic anxiety did not differ between groups. In the PTSD+MDD group 10 patients were diagnosed with a comorbid anxiety disorder (n=10), and one patient had a comorbid somatoform disorder. In the PTSD-MDD group seven patients met the current diagnostic criteria for a comorbid anxiety disorder, one patient had a somatoform disorder only, and one patient was diagnosed with both a comorbid anxiety and somatoform disorder. An overview of demographical and clinical data is presented in Table 1.
Spatial connectivity maps. Figure 1 shows the positive and negative networks for the bilateral insula and the bilateral subgenual ACC. Positive functional connectivity of the subgenual ACC was found with the ventromedial PFC, temporal regions (including the hippocampus) and a posterior cluster comprising the PCC/precuneus. Positive functional connectivity of the insula was found around the insular lobe, extending into the temporal and parietal lobe. A medial cluster around the dorsal ACC showed positive functional connectivity with the insula.
Subgenual ACC. Reduced functional connectivity of the PTSD+MDD group versus the PTSD-MDD group was found in functional connectivity of the subgenual ACC with the bilateral thalamus (Left thalamus; 29 voxels; peak value F=25.71; peak MNI-coordinates x=-12, y=-16, z=4. Right thalamus; 16 voxels; peak value F=34.37; peak MNI-coordinates x=20, y=-12, z=4). Increased functional connectivity was found between the subgenual ACC and perigenual regions of the ACC (peak in left perigenual ACC; 100 voxels; peak value F=25.71; peak MNI-coordinates x=-12, y=40, z=-4) in the PTSD+MDD group versus the PTSD-MDD group (see Figure 2, Figure 3 and Table 2).
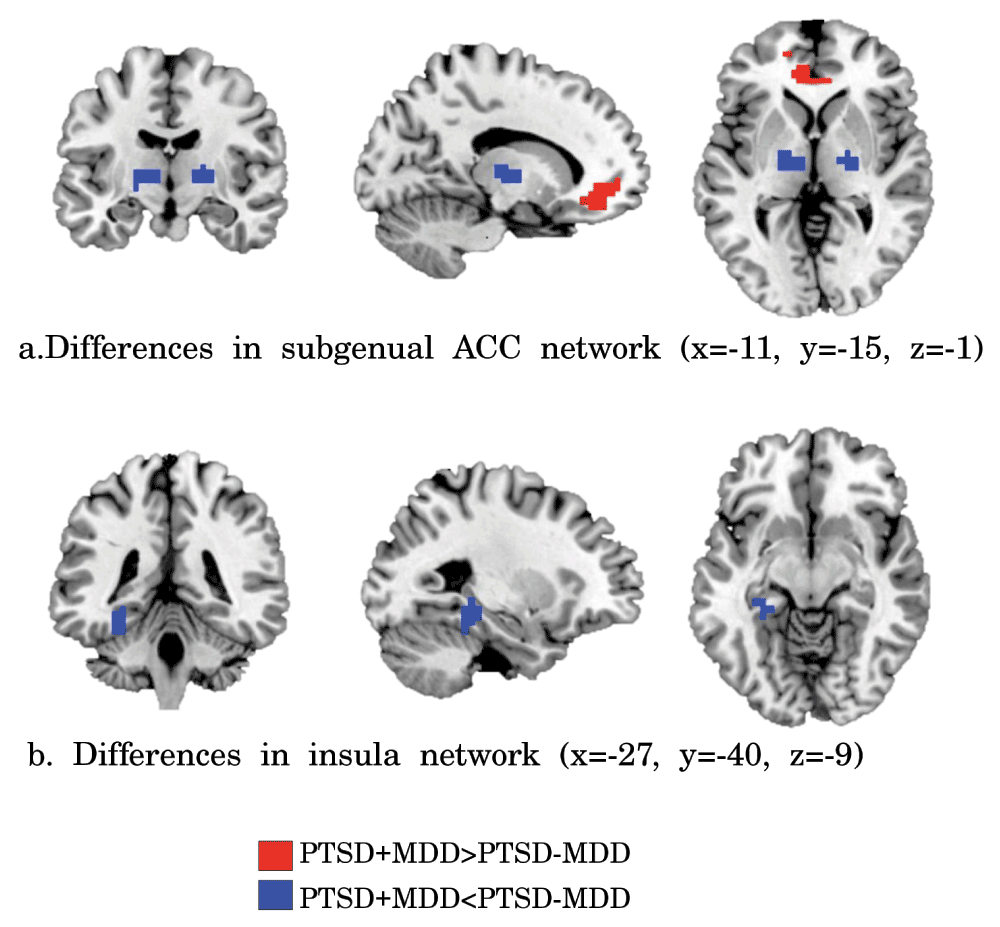
Clusters of significant different functional connectivity of the insula (a) and subgenual ACC (b) seeds. Increased functional connectivity in PTSD+MDD versus PTSD-MDD is shown in red and reduced connectivity in blue (height threshold p<0.001, extended threshold k=11, resulting in FDR corrected p<0.05).
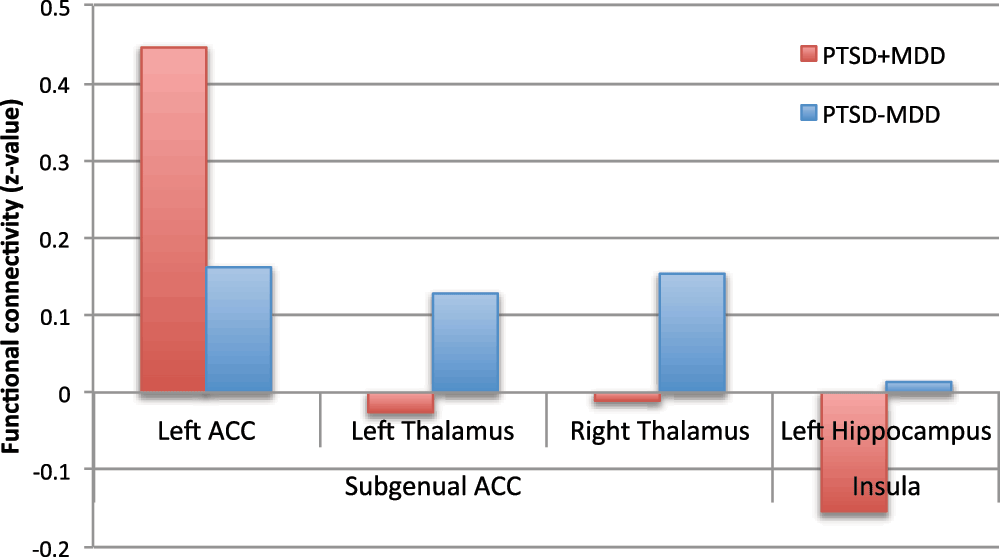
Z-values of the peak voxels for the PTSD-MDD group (red) and the PTSD+MDD (blue) group are presented.
Insula. Functional connectivity of the bilateral insula with the left hippocampus (17 voxels; peak value F=19.05; peak MNI-coordinates x=-28, y=-32, z=-8) was reduced in the PTSD+MDD group as compared to the PTSD-MDD group, which showed no functional connectivity between these regions (see Figure 2, Figure 3, and Table 2).
Because of group differences in PTSD severity, we repeated the analyses after including total CAPS scores as a covariate. This did not affect significance of the subgenual ACC connectivity differences in the bilateral thalamus (cluster in left thalamus 12 voxels (p=0.022) and right thalamus 15 voxels (p=0.005)) and perigenual ACC (cluster increased to 124 voxels). However, the cluster of altered connectivity between insula and hippocampus was no longer significant after controlling for total CAPS scores (cluster size reduced to 4 voxels (p=0.813)).
Post-hoc correlation analyses of the peak voxel of significant difference with CAPS total, CAPS symptom cluster, and PA scores were performed within both groups separately. CAPS cluster C scores correlated negatively with connectivity of the subgenual ACC with the peak voxel of significant difference in the left thalamus (r = -0.523, p=0.012) within the PTSD-MDD group. Within the PTSD+MDD group CAPS cluster B scores correlated negatively with connectivity of the subgenual ACC with the peak voxel of significant difference in the perigenual ACC (r = -0.396, p=0.041). No correlations were found between CAPS cluster D scores or PA scores and the peak voxels of difference in subgenual ACC and insula connectivity.
Exploring the relation of whole brain subgenual ACC connectivity with CAPS and PA scores revealed a negative correlation of CAPS and PA scores with subgenual ACC-PCC/precuneus connectivity, amongst other regions (see Supplementary Figure S1). In addition, a negative correlation was found between CAPS and PA scores and negative functional connectivity of the insula with the PCC/precuneus (see Supplementary Figure S1).
This study showed that resting state functional connectivity of the subgenual ACC and insula differs between PTSD patients with and without comorbid depressive disorder. PTSD+MDD patients had increased functional connectivity between the subgenual ACC and the left perigenual ACC compared to PTSD-MDD patients. Reduced functional connectivity was found in PTSD+MDD between the subgenual ACC and the bilateral thalamus, and between the insula and left hippocampus. The subgenual ACC results remained significant after controlling for PTSD severity. This study complements previous task-based studies9,10 by showing that differences in the subgenual ACC/insula between PTSD patients with and without comorbid MDD are also apparent using resting state functional connectivity.
Increased subgenual ACC connectivity with the perigenual ACC was found in PTSD+MDD versus PTSD-MDD, which is in line with neuroimaging studies in MDD that demonstrated specific alterations in these regions8,13. Furthermore, increased resting state functional connectivity between the subgenual ACC and perigenual ACC has been previously reported in MDD16–18, while reduced functional connectivity of the medial PFC, including the subgenual and perigenual ACC, has been shown in PTSD patients versus controls32. Our results complement these results, by showing increased connectivity of the subgenual ACC with the perigenual ACC in PTSD+MDD versus PTSD-MDD. In addition, the perigenual ACC has been related to self-referential processing33, which underlies depressive symptoms such as helplessness, self-reproach and guilt rumination17,34. During self-referential processing tasks a reduced negative blood oxygen level dependent (BOLD) response (thus an increase in BOLD signal) has been found in medial PFC regions, including the perigenual ACC, in MDD patients versus controls34–36. On the other hand, reduced medial PFC activation, including the perigenual ACC, has been observed in PTSD versus controls during a self-referential processing task37. It can thus be suggested that the increases in subgenual-perigenual ACC connectivity in the PTSD+MDD group versus the PTSD-MDD group reflect a difference in self-referential processing. A negative correlation between re-experiencing symptoms and functional connectivity of the subgenual ACC and perigenual ACC was also found within the PTSD+MDD group. This may suggest that when more specific PTSD symptoms (re-experiencing, cluster B) are prevalent, the balance of symptoms is tilted towards a PTSD only state, and the more functional connectivity resembles the PTSD-MDD group.
The perigenual ACC is part of the default mode network, which is the network that is active during rest and deactivated during task performance. Connectivity of the perigenual ACC with default mode network regions (PCC/precuneus and medial PFC) has been negatively correlated with general symptom severity in PTSD, even when correcting for depression diagnosis38 and depression severity39. This was confirmed in our post-hoc results, as we also found a negative correlation between total CAPS and PA scores and subgenual ACC connectivity with the PCC/precuneus. In addition, a negative correlation was found between total CAPS and PA scores and negative functional connectivity between the insula and PCC/precuneus (see Supplementary Figure S1). Specific correlations between CAPS scores and subgenual ACC-PCC/precuneus connectivity were also present, whilst controlling for PA scores. Connectivity in these regions did not differ between the PTSD+MDD and PTSD-MDD groups. Thus, it seems that subgenual ACC connectivity with the perigenual ACC in particular has the capacity to distinguish PTSD with and without comorbid MDD. The importance of differences in connectivity of the perigenual ACC in particular in MDD patients has been previously described for the default mode network, the affective network, and the salience network18. Instead, functional connectivity of other regions of the default mode network may be related to general (PTSD and MDD) symptom severity.
Previous fMRI studies including resting state paradigms, have reported decreased thalamus connectivity with the ACC in both depression40 and PTSD41 versus healthy controls. The thalamus is the relay station of the brain42, and can modulate attention and arousal43. The thalamus is also implicated as a target for surgical treatment of severe MDD44. In line with these findings we found that connectivity between the thalamus and subgenual ACC is more reduced in PTSD+MDD versus PTSD-MDD, suggesting that MDD effects add up to the PTSD effects in these regions. Furthermore, the results were not influenced by general PTSD severity, which may indicate that reduced thalamus connectivity is specific for comorbid MDD. In addition, functional connectivity between the subgenual ACC and thalamus was negatively correlated with cluster C symptoms in the PTSD-MDD group only. Thus, individuals with less avoidance and reduced interest, a shared PTSD and MDD symptom, have a stronger connection between the subgenual ACC and thalamus. A possible explanation of the correlation may be that the more the clinical image shifts toward more depressive symptoms, the more the connectivity pattern resembles PTSD+MDD. However, this interpretation needs further investigation, since cluster C symptoms both include avoidance and reduced interest symptoms.
Insula connectivity with the hippocampus was reduced in the PTSD+MDD group versus PTSD-MDD. However, the cluster was no longer significantly associated with comorbid MDD when PTSD severity was added as a covariate. Thus, hippocampus-insula connectivity does not seem to be specifically related to depressive symptoms, but rather to PTSD severity. The hippocampus is a brain region that is often associated with PTSD7,45,46 and is involved in memory47. Therefore, differences found in connectivity between the insula and hippocampus may be related to trauma-related memory. Thus, alterations in connectivity between the insula and hippocampus between the PTSD+MDD and PTSD-MDD group may be due to differences in PTSD severity and not to comorbid MDD diagnosis per se.
This study has some limitations. First, no MDD only group was included in the current study. Thus, this study does not show whether subgenual ACC and insula connectivity differs from patients with MDD only. The results only give insights in the effects of comorbid MDD in the context of PTSD, and not on general effects of PTSD or MDD. Including this group in future research can provide more insights in PTSD, MDD, and their neurobiological overlap or differences. Second, both groups included participants that were currently using antidepressants. Further studies should investigate the effect of medication on the neurobiology of PTSD with or without MDD. Third, no validated measure of the severity of all MDD symptoms was included in the study. If MDD severity was measured, it would have been possible to determine common and distinct factors of PTSD symptom severity and MDD symptom severity by including both measures in a single model (as attempted in the Supplementary Figure S1). Here, MDD diagnosis was determined with the SCID, and depressive symptom severity were approximated with the positive affect scale of the MASQ, which is only representative of a subset of symptoms (reduced positive affect). Future studies should investigate the specific effect of MDD symptom severity in the occurrence of PTSD, measured with more sensitive and comprehensive instruments.
This study revealed differences between PTSD+MDD and PTSD-MDD in resting state functional connectivity of the subgenual ACC, even when controlling for PTSD severity. Increased functional connectivity of the subgenual ACC with the perigenual ACC and bilateral thalamus was found in the PTSD+MDD group versus the PTSD-MDD group. Functional connectivity of the left thalamus was negatively correlated with cluster C in the PTSD-MDD group. Differences in connectivity of the insula and hippocampus were also found, but this seemed to be related to PTSD severity and not to the presence of comorbid MDD per se. Unraveling the neurobiological features of MDD and PTSD during rest can provide insights in which specific brain areas could be targeted for effective treatments. For example, tasks or therapy methods that increase functional connectivity between the regions with dysfunctional connectivity may be effective. Future studies should investigate long-term effects of training that is associated with functional connectivity alterations. This is in particular relevant for treatment of PTSD patients with comorbid MDD, since patients with this combination of psychological problems tend to be more treatment resistant.
EG and AR have made a substantial contribution to the conception and design of the study. MK and SvR have made a substantial contribution to the acquisition of data. MK performed the analyses and prepared the first draft of the manuscript. EG, AR, SvR and RK were involved in the interpretation of the data, and critically reviewing the article. All authors have agreed to the final content of the article.
This study was funded by the Dutch Ministry of Defence.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
In addition, we thank Jonathan van Leeuwen for his help with data acquisition and preprocessing.
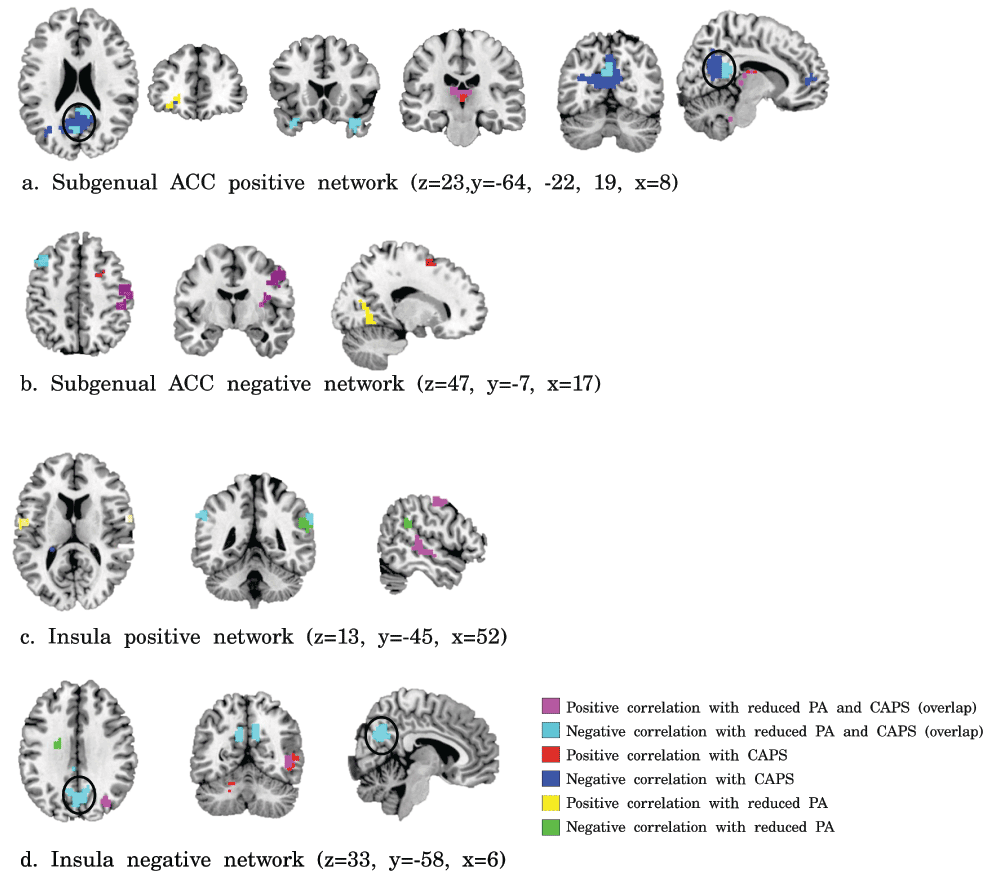
Correlations of PTSD symptom severity and reduced positive affect with subgenual ACC (a, b) and insula (c, d) functional connectivity. Violet = positive correlation with both CAPS scores and reduced PA, cyan = negative correlations with both CAPS scores and reduced PA, red = positive correlations with CAPS scores, blue = negative correlations with CAPS, yellow = positive correlations with reduced PA, and green = negative correlation with reduced PA (p<0.001, k>11, resulting in FDR-corrected p<0.05).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 16 Apr 14 |
read | read |
|
Version 1 30 Dec 13 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)