Keywords
Mycobacterium tuberculosis, Immune Reconstitution Inflammatory Syndrome, HAART
Mycobacterium tuberculosis, Immune Reconstitution Inflammatory Syndrome, HAART
We have made several minor changes to the new version. We added more details regarding the treatment regimen and incorporated suggestions made by the referees.
See the authors' detailed response to the review by Susana N. Asin
See the authors' detailed response to the review by Eric Daar
A 39-year-old man with a history of Acquired Immune Deficiency Syndrome (AIDS) presented to the emergency room with fever, productive cough, fatigue, diarrhea, and weight loss. Three weeks prior, he had been initiated on antiretroviral therapy (ART) with darunavir, ritonavir and combination tenofovir and emtricitabine. At that time, he had a CD4 count of 85 cells/μL (9%) and HIV-1 viral load of 336,950 RNA copies/mL. He was now febrile (41.0°C), with a heart rate of 100 beats/min and respiratory rate of 24/min. Physical examination revealed oral thrush and palpable cervical, supraclavicular and axillary lymphadenopathy. His laboratory evaluation was significant for a CD4 count of 28 cells/uL (10%), HIV-1 viral load of 3,410 RNA copies/mL, and hemoglobin of 6.6 g/dL. Chest radiograph on admission (not shown) demonstrated a 2.9 × 4.4 cm soft tissue mass in the anterior mediastinum.
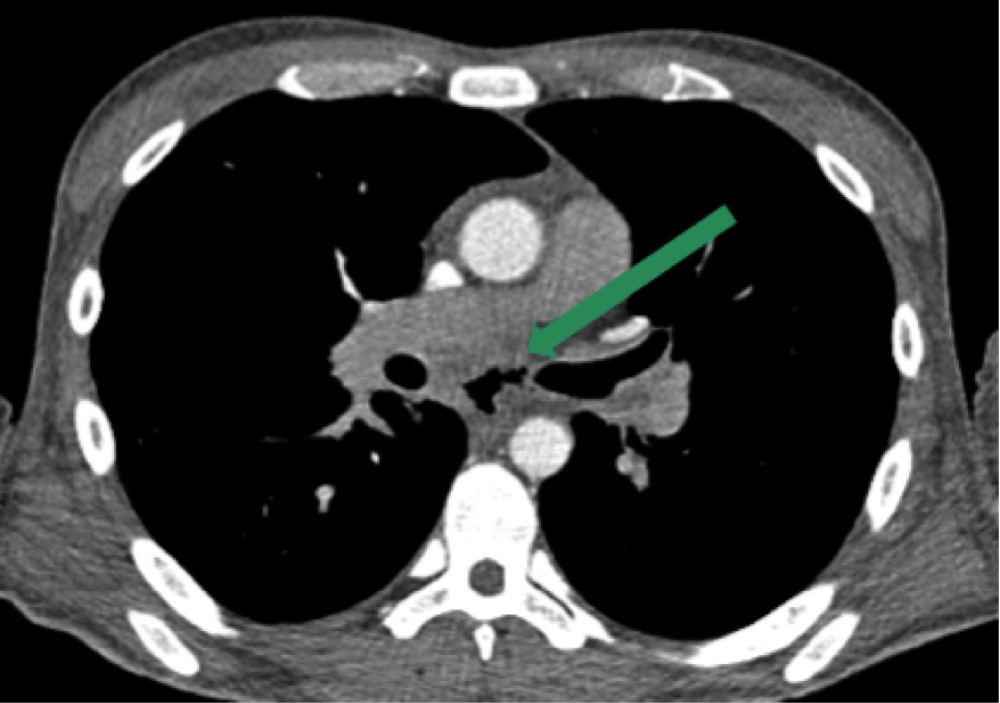
The initial computed tomography (CT) scan of the chest showed multiple low-attenuation mediastinal lesions, indicative of abscesses or necrotic lymphadenopathy (Figure 1), as well as esophageal discontinuity in the subcarinal region, a sign of esophageal fistula or perforation (Figure 2). Multiple cavitary lung nodules were also present (Figure 3).
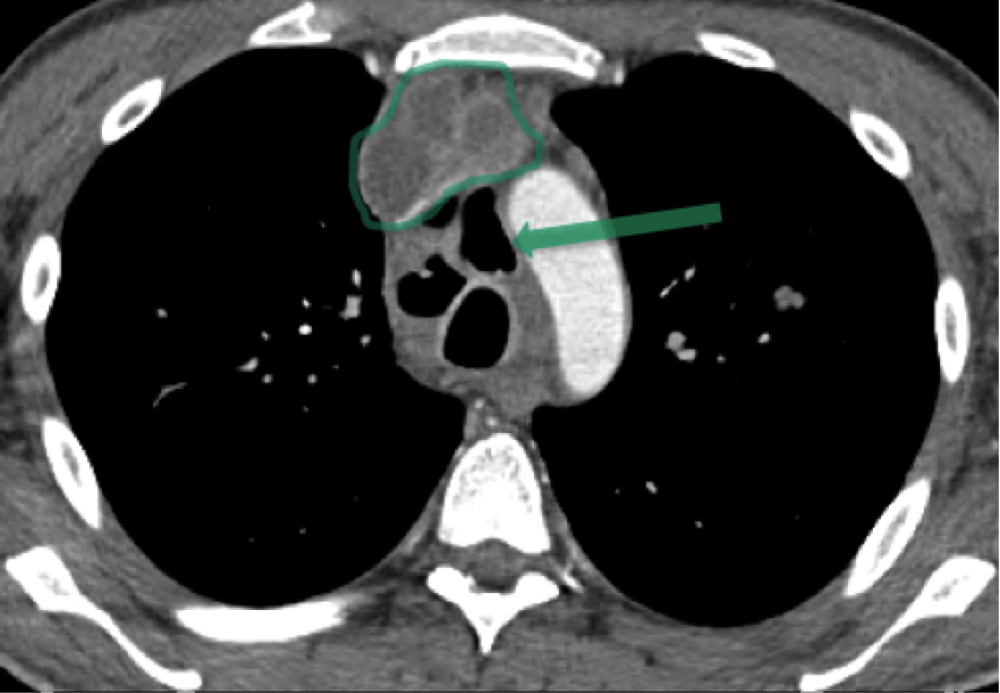
Mediastinoscopy revealed purulent fluid drainage from necrotic lymph nodes on day 3 of hospitalization. An esophagogastroduodenoscopy (EGD) demonstrated a 2 cm linear tear in the esophagus with proximal perforation at the 29–31 cm level.
The mediastinal fluid was found to be 4+ acid-fast bacilli (AFB) smear positive. PCR of the mediastinal fluid was also positive for Mycobacterium tuberculosis (MTB) complex on day 5 of hospitalization and the patient was started on IV linezolid, amikacin, rifampin and levofloxacin and transitioned to standard 4 drug anti-tuberculosis therapy (isoniazid, rifabutin, ethambutol and pyrazinamide) on day 8 when gastrointestinal access was obtained. Antiretroviral therapy had been held on day 2 and was subsequently resumed on day 9. The clinical presentation, recent initiation of ART, current 2-log decrease in viral load, and thoracic CT findings suggested a diagnosis of unmasking MTB immune reconstitution inflammatory syndrome (IRIS).
IRIS, previously known as immune restoration disease (IRD) and immune reconstitution syndrome (IRS) is a paradoxical deterioration in the clinical status of a patient after initiation of antiretroviral therapy1,2. The pathophysiology is related to the inflammation that occurs when the recovered immune system targets either live microorganisms or antigens from dead microorganisms3–7. Although recently proposed criteria for IRIS differ slightly, most criteria include the evidence of a recovered immune system along with a decrease in HIV viral load and/or increase in CD4 cell count. IRIS may occur as a paradoxical worsening of a known disease that has been under control with treatment, or an unmasking of a previously unsuspected disease4. Common pathogens associated with IRIS include tuberculous and non-tuberculous mycobacteria, cytomegalovirus, Pneumocystis jirovecii, JC virus, Cryptococcus neoformans, herpes simplex virus, hepatitis B virus, hepatitis C virus and Kaposi sarcoma4,5. Non-infectious diseases such as sarcoidosis, Grave’s disease and thrombotic thrombocytopenic purpura have also been described, suggesting that IRIS is not only an exuberant reaction to live or non-viable organisms, but also a manifestation of an unbalanced immune system8. While IRIS can occur acutely for up to 18 months after initiation of ART, most cases occur within the first two weeks to two months after initiation. IRIS is more likely to occur in the setting of high viral loads and low CD4 counts at the time of initiation of ART9–11.
In pre- and early Highly Active Antiretroviral Therapy (HAART) studies, the most common cause of lymphadenopathy (as seen on imaging) for an HIV patient with a CD4 count less than 50 cells/μL is mycobacterial infection12. Determining the presence of IRIS is not always straightforward; however, several key features help support correct diagnosis. The most common imaging feature in MTB-IRIS includes lymph node enlargement with central necrosis, most commonly located in the abdominal, axillary and mediastinal distributions13. The marked mediastinal lymphadenopathy in our patient is of particular interest, as this is common in patients with MTB-related IRIS14.
This patient initiated ART with a low CD4 count of 85 cells/uL (9%) and a high HIV viral load of 336,950 RNA copies/mL. After initiation of ART, his viral load decreased by 2 logs to 3,410 RNA copies/mL. While the absolute CD4 count decreased, the percentage increased. The absolute CD4 decrease was likely related to mycobacterial bone marrow invasion and subsequent inflammation causing pancytopenia and total leukocytosis. Because of this common phenomenon, some authors question the efficacy of CD4 rise as part of a proposed standardized IRIS diagnostic criteria3.
The exaggerated immune response to our patient’s mediastinal mycobacterial burden resulted in extension of inflammation from necrotic lymphadenopathy to the esophageal wall, which underwent necrosis and perforation. This resulted in a gas collection replacing the necrotic lymph nodes (Figure 1). Esophageal perforation can occur from extensive coughing and retching in an MTB patient, with or without an underlying infectious esophagitis.
Although definitive management of IRIS has not been established by carefully controlled studies, current management may include the addition of corticosteroids and in severe cases, temporarily withholding ART. Case reports suggest non-steroidal anti-inflammatory drugs (NSAIDS) may offer symptomatic relief, however randomized evidence of this effect is lacking15. Future management may include evaluation for a combination of cytokines and inflammatory markers such as interleukin 7, interleukin 6 and/or C-reactive protein to predict who is at higher risk of developing IRIS, which can be assessed prior to initiation of ART16,17. Future therapies may include immunomodulatory medications (C-C chemokine receptor 5 inhibitors, TNF antagonists or interleukin 6 receptor inhibitors) to temper the vigorous immune reconstitution.
Our patient had a complicated hospitalization including recurrent pneumothoraces (Figure 4), empyema, and unmasking of cutaneous Kaposi sarcoma. Antiretroviral therapy was continued, except for a brief interruption between hospital days 2 and 9, throughout the hospitalization. As previously mentioned, antituberculous treatment was started after active M. tuberculosis infection was confirmed and the treatment regimen included isoniazid, rifabutin, ethambutol, and pyrazinamide.
The esophagus in a patient with a low CD4 count is vulnerable to infection18. Our case illustrates a mediastinal infectious process in which TB-IRIS was the etiology and causative factor for an esophageal perforation that further complicated the treatment of this patient with AIDS.
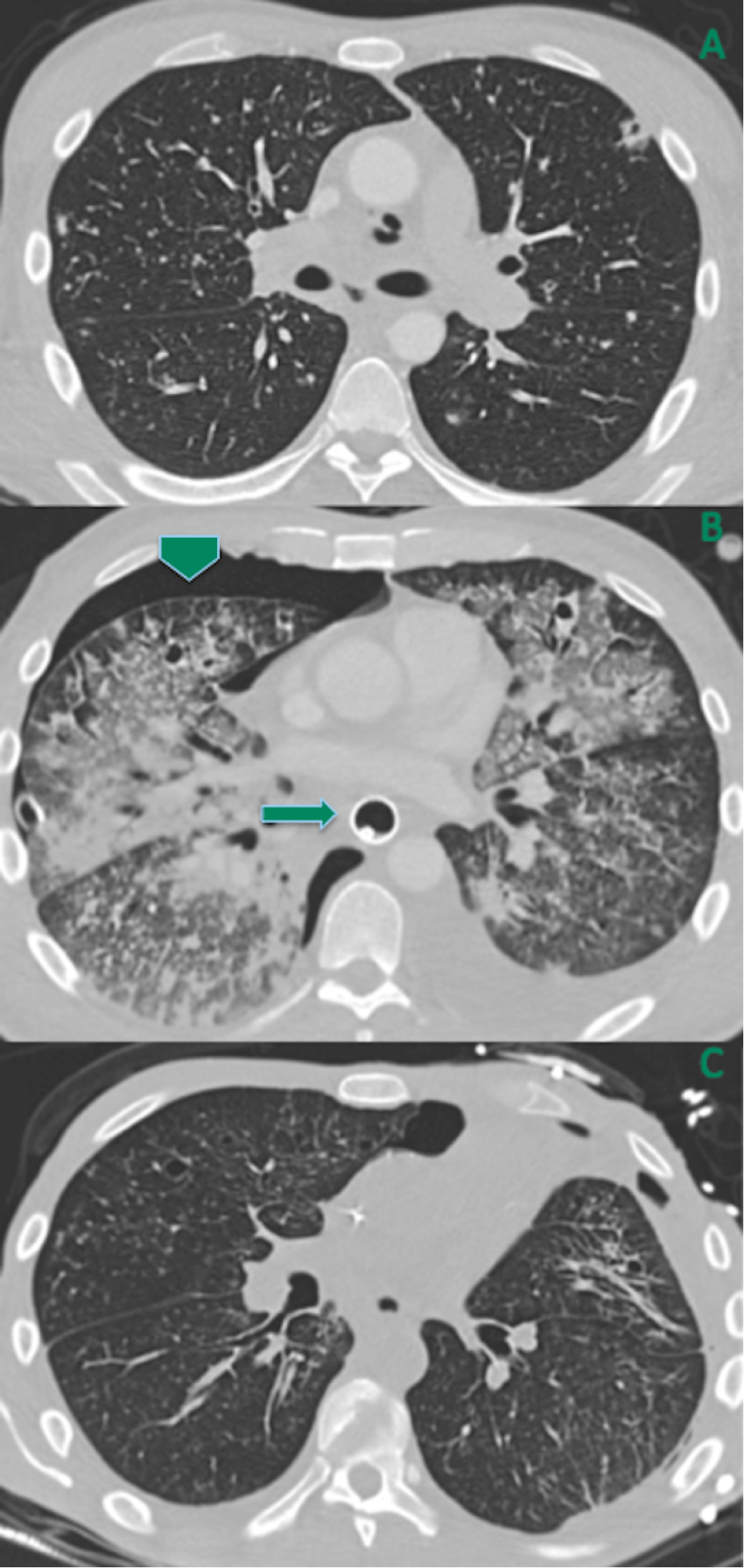
(A) Multiple cavitary and non-cavitary lung nodules (same as Figure 3). (B) Hospital day 6: increased pulmonary tree-in-bud nodules and consolidations, new small right-sided pneumothorax (arrowhead), and new esophageal stent (arrow). (C) One month follow-up: nearly-resolved pulmonary opacities decreased tiny right pneumothorax, and removal of esophageal stent.
Written informed consent for publication of clinical details and clinical images was obtained from the patient.
Leonardo Valentin and Andrew DiNardo wrote the manuscript. Elizabeth Chiao, Laila Woc-Colburn, and Arun Nachiappan revised the manuscript. Arun Nachiappan selected the images. All authors approved the final manuscript for publication.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (update) 07 Aug 13 |
read | read | read |
|
Version 1 18 Feb 13 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)