Introduction
Envelope glycoproteins E1 and E2 are essential for the initial binding and internalization of Hepatitis C Virus (HCV) into the host cells. Both glycoproteins have been shown to interact as a non-covalent heterodimer during biosynthesis1. Several conserved charged residues located in the TM domains of E1 and E2 were shown to function not only as membrane anchors, but were also essential for dimerization, endoplasmic reticulum retention and viral envelope formation2,3.
In our previous studies, we demonstrated the unfolding behavior of the E2 TM helix monomer was attributed to the charged Asp728 which was located in the hydrophobic core. The main contribution of Asp was postulated to be located at the helix-helix interface and involved the formation of a salt bridge with the Lys of the E1 envelope glycoprotein2. The ion-pair interaction of the E1–E2 heterodimer was captured in the molecular dynamics (MD) simulation studies based on the model that placed the charged Asp and Lys at the helix-helix interface4,5.
E2 envelope glycoprotein is known to be required for interactions with cellular receptors involved in endocytosis and membrane fusion6–8. E2 is composed of domain I-III, followed by the ST region and the TM domain7. Recent studies based on circular dichroism (CD) and nuclear magnetic resonance (NMR) spectroscopy revealed that the soluble region located adjacent to the TM domain of E2 was involved in the initial virus entry. This highly conserved ST region was showed to fold as a helix upon membrane binding9.
In this work, we carried out MD simulations for three E2 structures: (1) a model generated by the I-Tasser server, (2) an ideal helix model and (3) an NMR derived structure of the E2 segment (2KZQ.pdb). The first two models include the TM domain of E2 but the TM domain is not present in the NMR structure. We observed consistent structural stability in the ST region amphiphilic segment (residue 689–703) across all simulations suggesting that the contribution of the TM domain to the segment structure stability is minimal. In addition, we demonstrated the orientation and positional preferences of this amphiphilic segment.
Methods
Input structure preparation
The protein sequence of HCV E2 genotype 1a (H77 strain) (Uniprot ID P27958) used to prepare the models for MD simulations was obtained from UniProtKB/Swiss-Prot database (www.uniprot.org)10. The following is the sequence segment of HCV E2 used to prepare the first model for this work, referred to as an ideal helix model: 683PALSTGL 690IHLHQNIVDV 700QYLYGVGSSI 710ASWAIKWEYV 720VLLFLLLADA 730RVCSCLWMML 740LISQAEA. The ideal helix model was generated using Pymol (http://pymol.sourceforge.net) by orienting the ST segment (residue 683–714) perpendicular to the TM domain (residues 715–746). The protein structure images in this work were prepared with the Pymol program. The I-Tasser webserver11 was used to generate the second model. The complete sequence of E2 was submitted to the I-Tasser server. Five models were generated and the model with a low root mean square deviation (RMSD) with the available NMR structure (2KZQ.pdb) and consisting of a helical TM domain was selected. Only the same segment as examined with the ideal-helix model was used for further MD simulations. The third model studied was the NMR-derived structure of E2 (PDBID: 2KZQ) that was obtained from the Protein Databank (PDB)12. This E2 protein segment was based on the HCV genotype 2a (JFH-1) (Uniprot ID Q99IB8)9.
System preparation
Pre-equilibrated dipalmitoylphosphatidylcholine (DPPC) lipid bilayer was retrieved from the web of Prof. Tieleman (http://moose.bio.ucalgary.ca/). Peptide orientation in DPPC lipid bilayer was done by aligning hydrophobic belt of the peptide, parallel to the membrane plane using LAMBADA13. The optimal number of overlapping lipid molecules was subsequently calculated and removed followed by lipid expansion (inflation) and alternating twenty steps of deflation and energy minimization to allow the peptide to be embedded within the bilayer using inflateGRO213. A short 100 ps energy minimization was employed to relax possible steric conflicts. Ions and counter ions were added to neutralize the system followed by 20 ns position-restrained simulation allowing the bilayer to re-equilibrate around the protein. Production MD simulations were carried out for 100 ns in the I-Tasser model, and 20 ns for both the ideal helix model and NMR structure (2KZQ.pdb).
MD simulations
The DPPC lipid bilayer interactions were described using the Berger force-field parameters14. The TM helices were modeled with the united atom force-field GROMOS96 53a615. Simulations were performed with the Gromacs 4.5.5 package16 using 2-fs time steps. Periodic boundary conditions were used in all directions. Bonds to hydrogen atoms were constrained using the LINCS algorithms17. For the short-range van der Waals interactions, a cut-off distance of 1.0 nm was used. The long-range electrostatic interactions were treated using the particle mesh Ewald (PME) method with a grid spacing of 0.12 nm and cubic interpolation. The non-bonded pair list was generated every 10 steps with a cut-off of 1.0 nm. Water, lipid and peptide systems were coupled separately to temperature baths with 323 K for the DPPC using the Berendsen algorithm with a time constant of τT = 0.1 ps18. To maintain constant pressure, semi-isotropic coupling was employed separately for the lateral and for the normal directions with Berendsen weak coupling and a τp = 1 ps time constant. The compressibility was set to 4.5 × 10-5 bar-1 18.
Analyses of the trajectories were primarily performed with tools included in the Gromacs 4.5.5 suite16. RMSD analyses were based on the coordinates of all atoms of the peptides. The bilayer thickness was measured by averaging the distances between lipid headgroups in the upper and lower leaflets of the lipid membrane with the tool GridMAT-MD19.
Results and discussion
Stability of the amphiphilic region
A stable helical conformation of the E2 ST region (residue 689–703) was consistently observed with some uncharacteristic spikes in the early stages and towards the end of the MD simulation of I-Tasser model and the NMR derived structure (2KZQ.pdb), respectively. RMSD of this amphipathic segment was also consistently observed to be progressing within the commonly accepted 2 Å range for the ideal helix and NMR structures throughout the simulations. On the other hand, the I-Tasser model showed subtly higher RMSD progression over the simulation time (Figure 1, Figure 2 and Figure 3a, 3b).

Figure 1. Superimposition of the amphiphilic region of E2 (segment 689 to 703) of three molecular dynamics simulations.
The most stable helical region of the E2 amphiphilic segment during molecular dynamics simulations. 2KZQ.pdb in red, I-Tasser model in black and ideal helix model in gray.

Figure 2. Molecular dynamics simulation of E2 I-Tasser model.
Both amphiphilic region (segment 689–703) and TM domain located in the hydrophobic core of the bilayer. Length of residues 689–703 is plotted in red, root mean square deviation of the same residues with respect to the starting structure is plotted in black.
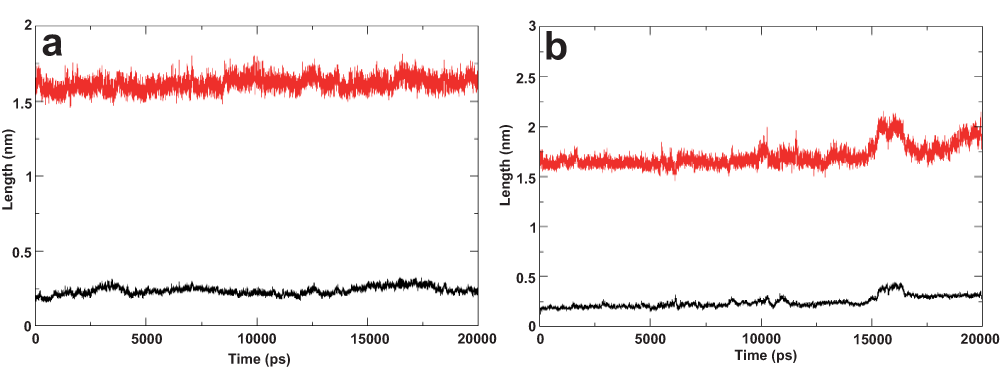
Figure 3.
(a) Molecular dynamcs simulation of the ideal helix model. The amphiphilic segment was positioned in water-lipid interface and the TM domain was oriented in hydrophobic core of the bilayer. The length of the helix of residues 689–703 is plotted in red, root mean square deviation of the same residues with respect to the starting structure is plotted in black. (b) Molecular dynamics simulation of nuclear magnetic resonance derived structure (2KZQ.pdb). 2KZQ was positioned in the water-lipid interface. The length of the helix segment is plotted in red, root mean square deviation of the same residue with respect to starting structure is plotted in black.
Secondary structure stability observed in these three contrasting simulation systems can be attributed to the amphiphilic nature of the residues allowing the helix to retain its structure on both the hydrophobic core of the lipid bilayer and the hydrophilic environment of the solvent. This amphiphilic characteristic of the residues has been discussed and described to great extent by Albecka et al. in a previous study9. Interestingly, the presence of the TM domain does not appear to contribute significantly to the helix stability of the amphiphilic region.
The helical length of this region does not vary much between the I-Tasser and the ideal-helix models (Figure 2 and Figure 3a), which include both the ST region and TM domain, compared with the 2KZQ.pdb (Figure 3b) which does not include the TM domain. This observation suggests that lipid-peptide interactions play a larger role in stabilizing the secondary structure of this amphipathic segment compared with the TM domain. These data provide evidence of the contribution of lipid to structural stability modulation and are in good agreement with the hypothesized lipid and/or protein contribution to structural stability9. The higher RMSD value observed in the I-Tasser model simulation was well anticipated and was mainly attributed to the relative positioning of the ST region, which was sandwiched in between lipid leaflets, forcing the segment to reorganize its structure conformation and having only a minimal effect on the helical integrity of the secondary structure. Examining this reorganization further by monitoring the distance of the amphiphilic segment to lipid leaflets led to another interesting observation described in the next section of this article.
Orientation and positional preference of the amphipathic segment
Monitoring the movement of the amphiphilic segment during the simulations led to another interesting observation. Segment of residues 689–703 in the I-Tasser model appeared to move towards the hydrophobic core of the lipid bilayer as depicted by steady progression in the distance to both the upper and lower lipid leaflets depicted in Figure 4. This reorganization is surprisingly interesting because we would have previously assumed that the amphiphilic region exposed to the solvent would hold the structure steady, despite some part of the amphiphilic region being initially vertically positioned in the hydrophobic core of the lipid (Supplementary Figure 1). In addition, given the amphiphilic nature of the residues in this segment, one could postulate that the residues would remain at this position. However, over the period of the simulation the segment reoriented by moving away from the lipid leaflets, while at the same time retaining structural integrity. The structural stability of the segment, as discussed in the previous section of this article, is attributed to the amphiphilic nature of the residues but this does not explain the movement towards the hydrophobic core of the lipid. The systematically orchestrated movement towards the hydrophobic core of the lipid leaflets indicates a strong orientation preference of the amphipathic segment, which in this specific case was parallel to the lipid leaflets. We then monitored the segment movement relative to the lipid leaflets with the other two simulations (the ideal helix model and the 2KZQ structure). The segment was initially positioned horizontally to the lipid leaflets. The results showed that the distance of the amphiphilic segment in both simulations was consistently within 4 Å to the lipid phosphate head group throughout the simulation (Figure 5). These data further clarify the orientation preference of the amphipathic segment with respect to the lipid leaflets and suggest that the residues are positioned in the membrane interface in a very stable manner. Interestingly, Albecka et al.9 speculated that these residues could have an in-plane topology or orientation and suggested that the ST region would ideally be positioned in the membrane interface, which is again in agreement with our data9. We have described the behavior and provided a detailed insight into the dynamics of this amphipathic segment in a lipid bilayer environment.

Figure 4. Distance between the helix and lipid leaflets during molecular dynamic simulation with the I-Tasser model.
The distance to the upper lipid leaflet is plotted in red and lower leaflet is plotted in black.

Figure 5. Distance of the amphiphilic segment to the lipid leaflets.
The 2KZQ structure is colored in black while the ideal helix model is colored in red. Amphiphilic residues remain within 4 Å from phosphate head group throughout the simulations.
Conclusion
In this study, the atomistic MD simulations provide insightful structural data for the E2 segment. The amphiphilic segment of E2 was able to remain as a stable helix in a lipid bilayer environment even without the respective TM domain. The results also revealed the orientation and positional preferences of the amphiphilic segment in relation to the water-bilayer interface that further clarify speculations from experimental studies.
Author contributions
SAJ analyzed the sequences. RA performed the molecular dynamics simulations. SAJ and RA carried out the research, were involved in the drafting of manuscript and have approved the final content.
Competing interests
No competing interests were disclosed.
Grant information
This work was supported by the Universiti Teknologi MARA (UiTM) Dana Cluster 600-RMI/DANA 5/3/CG (2/2012).
Acknowledgments
We are grateful to Faculty of Pharmacy, Universiti Teknologi MARA (UiTM) for providing the computational facilities in the Bioinformatics Lab. We acknowledge financial and administrative support from the Research Management Institute (RMI), UiTM and Ministry of Science and Technology Malaysia (MOSTI).
Supplementary figure
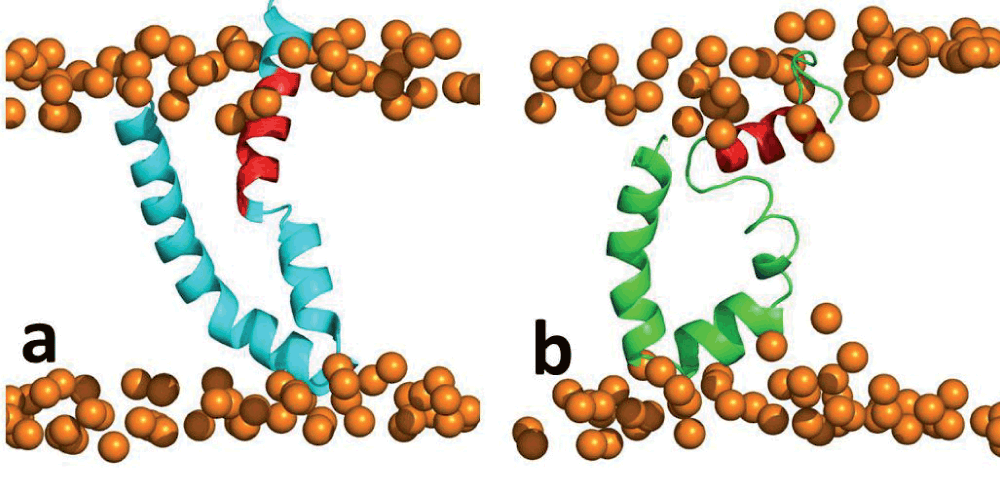
Supplementary Figure 1. Molecular dynamics simulation of I-Tasser model.
(a) Initial structure; (b) 100 ns snapshot. (Red – residues 689–703).
Faculty Opinions recommendedReferences
- 1.
Dubuisson J, Duvet S, Meunier JC, et al.:
Glycosylation of the hepatitis C virus envelope protein E1 is dependent on the presence of a downstream sequence on the viral polyprotein.
J Biol Chem.
2000; 275(39): 30605–30609. PubMed Abstract
| Publisher Full Text
- 2.
Cocquerel L, Wychowski C, Minner F, et al.:
Charged residues in the transmembrane domains of hepatitis C virus glycoproteins play a major role in the processing, subcellular localization, and assembly of these envelope proteins.
J Virol.
2000; 74(8): 3623–3633. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 3.
Op De Beeck A, Montserret R, Duvet S, et al.:
The transmembrane domains of hepatitis C virus envelope glycoproteins E1 and E2 play a major role in heterodimerization.
J Biol Chem.
2000; 275(40): 31428–31437. PubMed Abstract
| Publisher Full Text
- 4.
Jusoh SA, Welsch C, Siu SW, et al.:
Contribution of charged and polar residues for the formation of the E1–E2 heterodimer from Hepatitis C virus.
J Mol Model.
2010; 16(10): 1625–1637. PubMed Abstract
| Publisher Full Text
- 5.
Jusoh SA, Helms V:
Helical integrity and microsolvation of transmembrane domains from Flaviviridae envelope glycoproteins.
Biochim Biophys Acta.
2011; 1808(4): 1040–1049. PubMed Abstract
| Publisher Full Text
- 6.
Op De Beeck A, Voisset C, Bartosch B, et al.:
Characterization of functional hepatitis C virus envelope glycoproteins.
J Virol.
2004; 78(6): 2994–3002. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 7.
Krey T, D’Alayer J, Kikuti CM, et al.:
The disulfide bonds in glycoprotein E2 of hepatitis C virus reveal the tertiary organization of the molecule.
PLoS Pathog.
2010; 6(2): e1000762. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 8.
Penin F, Dubuisson J, Rey FA, et al.:
Structural biology of hepatitis C virus.
Hepatology.
2004; 39(1): 5–19. PubMed Abstract
| Publisher Full Text
- 9.
Albecka A, Montserret R, Krey T, et al.:
Identification of new functional regions in hepatitis C virus envelope elycoprotein E2.
J. Virol.
2011; 85(4): 1777–1792. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 10.
Magrane M, Consortium U:
UniProt Knowledgebase: a hub of integrated protein data.
Database (Oxford).
2011; 2011: bar009. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 11.
Zhang Y:
I-TASSER server for protein 3D structure prediction.
BMC Bioinformatics.
2008; 9: 40. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 12.
Berman HM, Westbrook J, Feng Z, et al.:
The Protein Data Bank.
Nucl Acids Res.
2000; 28(1): 235–242. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 13.
Schmidt TH, Kandt C:
LAMBADA and lnflateGRO2: efficient membrane alignment and insertion of membrane proteins for molecular dynamics simulations.
J Chem Inf Model.
2012; 52(10): 2657–2669. PubMed Abstract
| Publisher Full Text
- 14.
Berger O, Edholm O, Jähnig F:
Molecular dynamics simulations of a fluid bilayer of dipalmitoylphosphatidylcholine at full hydration, constant pressure, and constant temperature.
Biophys J.
1997; 72(5): 2002–2013. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 15.
Oostenbrink C, Soares TA, van der Vegt NF, et al.:
Validation of the 53A6 GROMOS force field.
Eur Biophys J.
2005; 34(4): 273–284. PubMed Abstract
| Publisher Full Text
- 16.
Hess B, Kutzner C, Van der Spoel D, et al.:
GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation.
J Chem Theory Comput.
2008; 4(3): 435–447. Publisher Full Text
- 17.
Hess B, Bekker H, Berendsen H, et al.:
LINCS: A linear constraint solver for molecular simulations.
J Comput Chem.
1997; 18(12): 1463–1472. Publisher Full Text
- 18.
Berendsen HJC, Postma JPM, Van Gunsteren WF, et al.:
Molecular dynamics with coupling to an external bath.
J Chem Phys.
1984; 81(8): 3684–3690. Publisher Full Text
- 19.
Allen WJ, Lemkul JA, Bevan DR:
GridMAT-MD: A grid-based membrane analysis tool for use with molecular dynamics.
J Comput Chem.
2009; 30(12): 1952–1958. PubMed Abstract
| Publisher Full Text





Comments on this article Comments (0)