Introduction
Biological invasions can threaten ecosystems1, economies2, and human health3. The Scientific Committee on Problems of the Environment (SCOPE) put biological invasions on top of its research agenda in 19834. Since then, the field of invasion ecology has rapidly gained momentum. The number of publications dealing with biological invasions has increased a hundredfold in less than two decades5. Several journals are partly (e.g. Diversity and Distributions, Natural Areas Journal) or fully (e.g. Biological Invasions, Invasive Plant Science and Management, NeoBiota) devoted to research, management and policy issues related to invasive species. However, despite a growing body of knowledge on biological invasions, difficulties remain in predicting invasion success6.
Within Europe, the distribution of people is strongly related to the number of alien species. Presumably, this reflects that biological invasions are aided by human transport and that species establishment is facilitated by human disturbance7. Nevertheless, at the global scale, the proportion of widely distributed alien plant species (relative to all species) is far lower in Europe than in North America – despite Europe’s long history of trade and therefore a longer residence time of alien plants8. The observation that Europe serves as a global contributor of alien plant species, whereas North America seems to be a better recipient, has sparked the concept of biological resistance, which explains invasion success or failure in relation to the traits of the native flora9. An additional important consideration, which has not been assessed to date, could be that Europe also has a higher proportion of landscapes that are actively managed by humans than, for example, the Americas, Australia and Africa10. To date, approaches to predict invasion patterns in response to anthropogenic global change have focused on (i) the extent of novel ecosystems11, and (ii) alien species richness12.
In this paper, we propose that the abundance of an alien species in a given landscape can be (at least partly) explained by the level of active landscape maintenance by humans. We term this hypothesis the Human Release Hypothesis. As discussed in detail below, the Human Release Hypothesis states that the abundance of invasive species may be partly explained by the level of human activity or landscape maintenance, with intermediate levels of human activity providing optimal conditions for high abundance. Unlike the Disturbance Hypothesis and the Intermediate Disturbance Hypothesis, which explain patterns of establishment of invasive species13 and patterns of native species diversity respectively14, the Human Release Hypothesis specifically addresses patterns in the abundance of alien species that are already established in particular areas outside their native ranges.
We first discuss how the Human Release Hypothesis fits into the context of other key hypotheses in invasion ecology. We then illustrate the hypothesis via a case study on a global invader, the sweetbriar rose (Rosa rubiginosa L.). Finally, we assess how the Human Release Hypothesis may be integrated into biological invasion research, and we hypothesize which locations worldwide may be particularly prone to supporting high abundances of invasive species.
The Human Release Hypothesis
According to Richardson et al. (2000)15, an invasive terrestrial plant species is a naturalized alien species that produces reproductive offspring, often in very large numbers, at considerable distance from parent plants, and thus has the potential to spread over extensive areas. A key question in invasion ecology is how the interaction of species traits with environmental characteristics predicts invasion success, including both establishment and abundance in the new environment6.
Catford et al. (2009)16 summarized 29 leading hypotheses predicting invasion success and integrated them into the PAB-framework (Figure 1). This framework considers the size and frequency of introductions (i.e. propagule pressure, P), ecosystem invasibility based on abiotic characteristics of the new environment (A), and biotic characteristics of an invasive species and its recipient community (B). In this framework, human influence on the invasion process is recognized primarily during the establishment stage. For example, human action can increase propagule pressure17 and multiple introduction events make establishment more likely, because species have a higher chance to encounter suitable environmental conditions18. Multiple introductions of the same species also can lead to higher genetic diversity19. However, examples exist of successful invaders with low genetic diversity20, and stemming from single or few introduction events, suggesting that propagule pressure is only one of many variables explaining invasion patterns21.
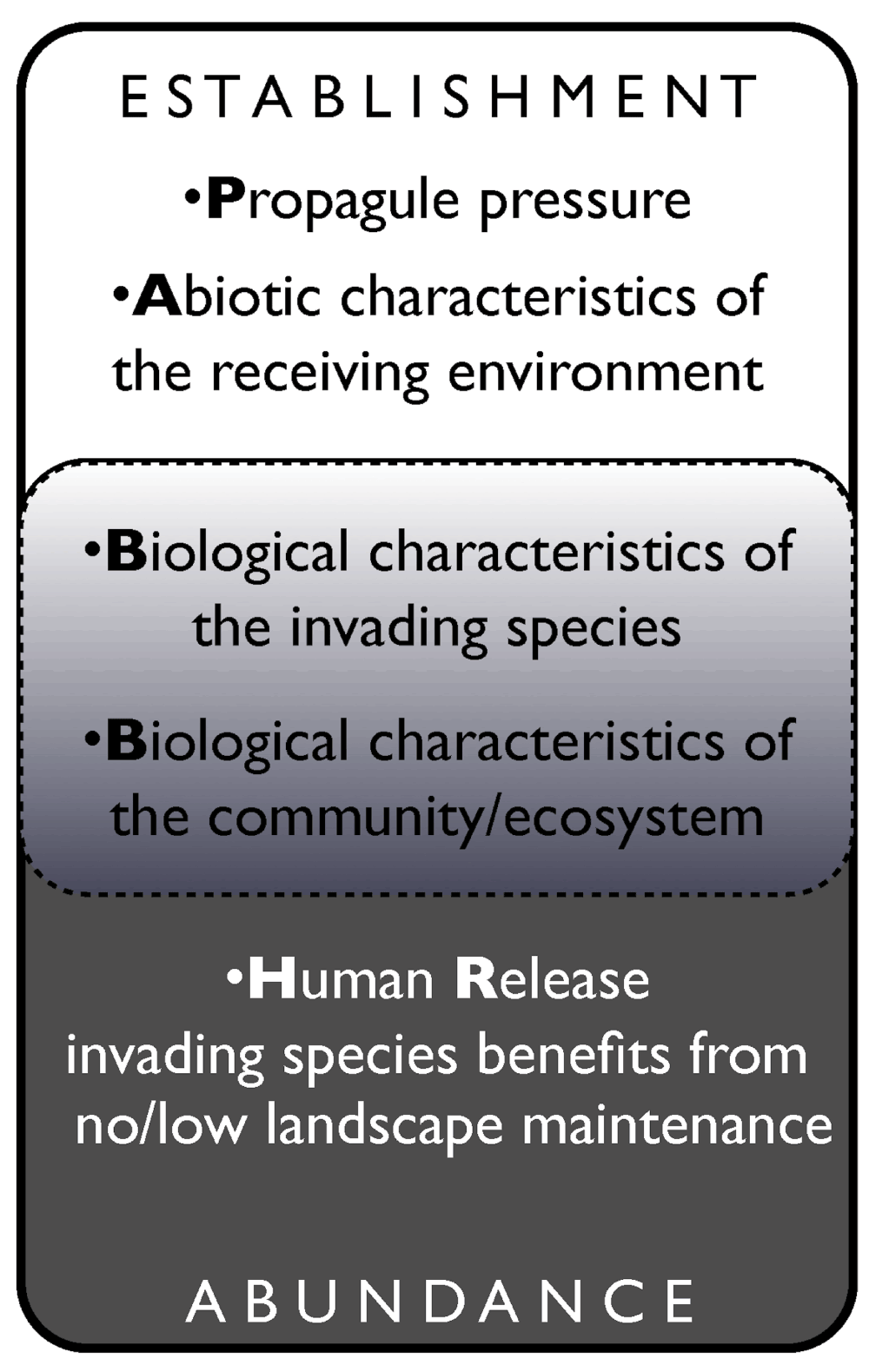
Figure 1. Incorporating our hypothesis into the PAB framework.
The establishment and abundance of invasive plant species are explained by different mechanisms, which have been summarized by Catford et al. (2009)16 in the PAB framework (see text for details). However, the biological characteristics of a given invading species and of its new environment only partly explain the abundance of established invasive populations. We argue that additional insights can be gained via the Human Release Hypotheses, which can complement the existing PAB framework.
With respect to abiotic conditions, invasion is facilitated if species are pre-adapted to their new environment, for example due to a similar climate in the new environment22. Like propagule pressure, pre-adaption is not a necessary precondition for successful invasion, because climatic niche shifts have been reported for invasive species23. Disturbance events also provide windows of opportunity for invasive species24. Many invasive plant species are adapted to exploit temporarily favourable conditions through their short life cycles, rapid growth, high reproductive allocation, persistent soil seed banks and rapid germination (the Ideal Weed Hypothesis)25.
Finally, biotic characteristics of the recipient community may involve the absence of natural enemies. The Enemy Release Hypothesis explains invasion success as a function of alien species having escaped their natural enemies, allowing them to allocate resources to growth and reproduction rather than defence26. This would make alien plants stronger competitors. In the context of the Intermediate Disturbance Hypothesis, which proposes higher species diversity at intermediate frequencies or intensities of disturbance (see Wilkinson, 1999)14, alien plants are likely to have the greatest impact on community diversity when resources become limited and plant diversity is highest, by co-opting more resources27.
In parallel to the Enemy Release Hypothesis, here, we propose the Human Release Hypothesis. It describes a situation where alien species have escaped relatively higher levels of human landscape maintenance that is characteristic within their native ranges. Changing patterns of land use are widely recognized to increase opportunities for introduced species to establish and spread28, but already prevailing patterns of land use intensity also should be expected to influence the populations of species – both in their native and introduced ranges. This is because highly intensive land use by humans (such as in many parts of Western Europe) often corresponds to high levels of active landscape maintenance – which translates into little available habitat for both native and introduced species, as well as high levels of active weed control. At the other end of the spectrum of human land use intensity, we hypothesize that pristine natural habitats also offer few windows of opportunity for alien species to establish (the Biotic Resistance Hypothesis)29. Thus, we hypothesize that the abundance of invasive species should be highest in between these two extremes – namely in extensively used landscapes characterized by frequent fallowing, low levels of weed control, high heterogeneity, and many disturbed edges of small farmland patches30. Such landscapes are where “human release” should contribute to optimal conditions for invasive species to establish large populations.
While existing hypotheses explain the establishment and naturalization process of invasions, little work has attempted to explain the (potential) abundance of invasive species in their new environments. Part of this gap may be effectively addressed by the Human Release Hypothesis (Figure 1).
Case study on an invasive rose
To illustrate the plausibility of the Human Release Hypothesis, we present findings at two scales on the invasion success of Rosa rubiginosa, a shrub native to Eurasia and invasive in Australia, New Zealand, South Africa, North and South America (see Dataset 1 and Supplementary Figure S1). First, we synthesize previous cross-continental case studies that compared plant performance between invasive populations in Central and Southern Argentina with native populations in Spain and Germany (for more details see Zimmermann et al., 2012)31. Second, we compare climatic conditions as well as land use and human population density between invasive and native R. rubiginosa populations at a global scale. In combination, our findings suggest the Human Release Hypothesis may be a useful complementary hypothesis to other existing hypotheses in invasion biology (Table 1).
Table 1. Hypothesis for the invasion success as they apply to Rosa rubiginosa in Argentina.
(aCavallero & Raffaele 2010, bZimmermann et al. 2010, c2011, d2012, eHirsch et al. 2011, fpresent publication).
| Hypothesis | Mechanism | Case study |
|---|
| Propagule Pressure | Multiple introductions into new range
make establishment more likely and
secure high genetic diversity | Genetic diversity in invasive populations very low,
small number of introduction eventsb,e
| REJECTED |
Favorable
environmental
conditions | Species benefits from climatic or
edaphic conditions, or vegetation
characteristics in new range | Structure of vegetation matrix did not differ between
ranges, edaphic conditions not favourable in
invasive populations and climatic conditions vary
greatly within the introduced ranged,f
|
| Enemy Release | Invasive species allocates resources
no longer needed for defence to
growth and reproduction | Damaged or infested leaf area high in invasive
and native ranged
|
Evolution of
Increased
Competetive Ability | Selection favours genotypes which
have allocated resources, which are
no longer needed for defence to
adapting and enhancing competitive
ability | Individuals from both ranges same growth rates
in common garden experimentsd
|
| Ideal Weed | Invasive species share traits that
facilitate invasions | Ideal weed traits of study species: high
phenotypic plasticity, clonal growth, asexual
reproductionb,d
| CONFIRMED |
| Disturbance | Disturbance events open window of
opportunity for invasive species | Species occurs in invasive range across
habitat types after anthropogenic or natural
disturbancea,c
|
| Human Release | Invasive species benefits from low
levels of landscape maintenance | Trimming or removal of individuals only in native
range, individuals in invasive range older, in
invasive range lower number of people/km2 as
well as less residential areas and less cropland
area than in native ranged,f
| PROPOSED |
Rosa rubiginosa has successfully invaded a range of ecosystems within Argentina, covering a major climatic gradient, but exhibiting low levels of genetic diversity32,33 (Figure 2a). Low genetic diversity suggests that multiple introduction events constituting particularly high propagule pressure cannot explain the species' invasion success. Despite lower genetic diversity, populations of R. rubiginosa are considerably smaller in Spain and Germany than in Argentina (Figure 3) – native populations consist of 5 to 20 individuals whereas invasive populations consist of hundreds of individuals31. In addition to propagule pressure, abiotic and biotic variables also cannot fully explain the invasion success of R. rubiginosa. In Argentina, the species neither benefits from favourable soil conditions nor from reduced biotic resistance31.
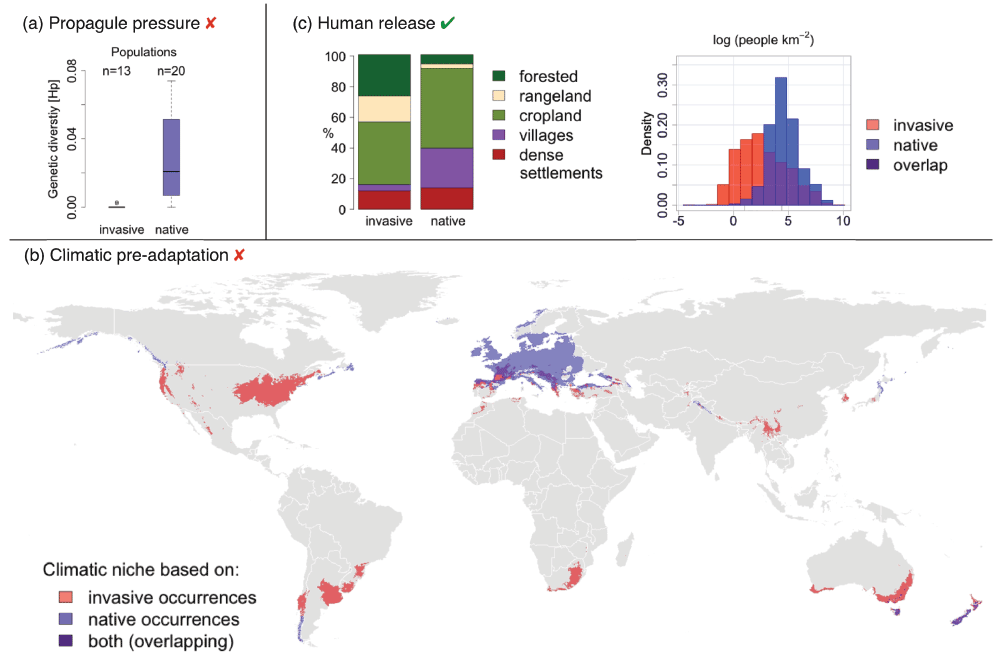
Figure 2.
Rosa rubiginosa benefits from human release.
(a) Genetic diversity in Rosa rubiginosa is higher in its native Spanish and German populations than in the introduced populations in Argentina, suggesting the species did not benefit from multiple introductions (for details see Zimmermann et al. 2010)32. (b) The species does not benefit from a climatic pre-adaptation to the new range. The world map shows the species' climatic niche based on the species’ native distribution (blue) and the invasive distribution (pink). Overlap of climatic niches (purple) is minimal. (c) Rosa rubiginosa appears to benefit from “human release” in its new range. The barplot shows the global proportions of different anthropogenic biomes10 according to the location of invasive and native sweetbriar rose populations. The native range has a larger proportion of residential areas and a higher human population density (log people/km2). Only 0.56% of the invasive range is wildlands, and only 0.03% of the native range.
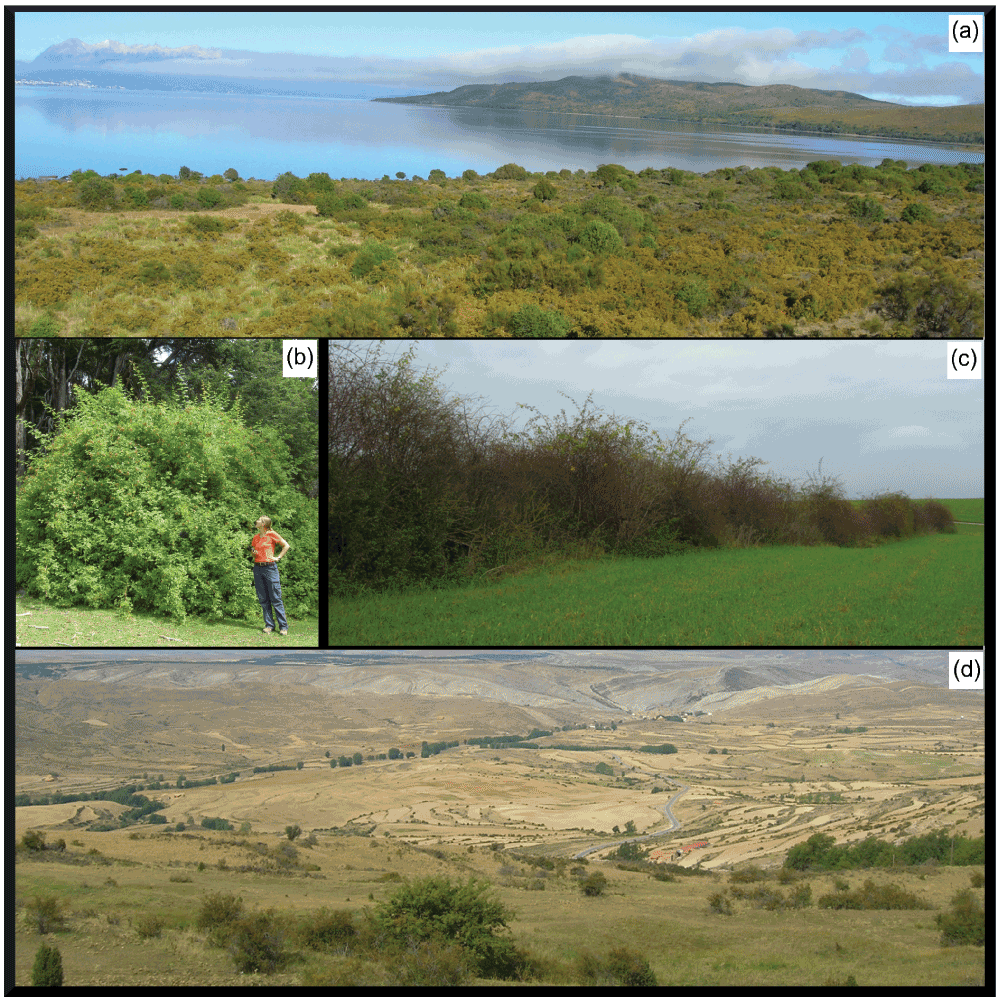
Figure 3. Invasive Rosa rubiginosa populations in Argentina (a, b) and native populations in Germany (c) and Spain (d).
In parts of Argentina, single disturbance events have offered windows of opportunity for the species to establish populations, some of which have remained undisturbed for 30 years or longer (a)31,36. The low level of human landscape maintenance means that populations can expand over vast areas and consist of hundreds of individuals (a, here along the whole visible lakeside in Patagonia). (a) For our study area in Patagonia we predicted that 36% of the area (5000 km2) was threatened by R. rubiginosa invasion, across a precipitation gradient from 1400 mm/annum (mountains in the far background) to 600 mm/a36. In Argentina R. rubiginosa shrubs have time to grow to their full size (b), by contrast, many native landscapes are regularly maintained; shrubs are regularly trimmed and mostly grow in hedgerows (c, Germany). Furthermore, in Germany and Spain, fewer habitats are available in landscapes dominated by agriculture and urban areas (d, Spain).
Moreover, a global climatic analysis shows that R. rubiginosa also does not depend or benefit from pre-adaptation to the climate of its new environment (Figure 2b). We developed two climatic envelope models based on BioClim parameters and the occurrence of native and invasive populations respectively using the maximum entropy method34 (MAXENT, see Appendix 1 and 2 in the Supplementary material). We detected a significant differentiation of realized niches between invasive and native populations (Schoener’s D=0.31, p<0.0001; Figure 2b). Furthermore, back-projection of the climatic niche based on invasive populations points to a southern European origin. However, genetic analyses tracked the native origin of invasive Argentinean, Chilean, Australian and New Zealand populations to Central Europe32,33. Key climatic predictors therefore do not point to a climatic advantage in the invasive range, but instead indicate that R. rubiginosa is able to thrive under a wide range of conditions (Supplementary Figure S2 and Supplementary Figure S3).
The Ideal Weed and Disturbance Hypotheses (Table 1) partly explain the invasion success of R. rubiginosa in Argentina31,35,36. However, the Enemy Release Hypothesis failed to explain abundance patterns – natural enemies appeared equally harmful to the species in the native and introduced ranges31 (Table 1). By contrast, in the invasive range, anthropogenic disturbances such as logging and burning create windows of opportunities for the rose to establish, but just as importantly, disturbance events are then followed by decades of abandonment that enable the species to become abundant.
Having considered a wide range of existing hypotheses (Table 1), we found that additional insights into the invasion patterns of R. rubiginosa may be gained by the Human Release Hypothesis. This is because a key difference between native and introduced environments appears to be the level of active landscape maintenance. In the case study, we observed frequent trimming or removal of individuals only in Spain and Germany and not in Argentina, and individuals and populations in Argentina were significantly older than their native counterparts31,36. At the global scale, our analysis revealed a similar pattern (albeit at a coarser resolution; 2.5 × 2.5 arc min, Figure 2c). Native R. rubiginosa populations occur in areas with higher proportions of cropland, residential areas and human population densities than invasive populations (Figure 2c). These conditions very likely correspond to a high degree of landscape maintenance, and hence little available habitat for R. rubiginosa in its native range.
Integrating the Human Release Hypothesis with other explanations
A key premise of this paper is that existing hypotheses that predict invasion success can be effectively complemented by the Human Release Hypothesis (Figure 1). Our own data, of course, focused only on one species – which is enough to pose a hypothesis, but far too little to test its general usefulness. To that end, we see two research priorities that should be addressed to further scrutinize the Human Release Hypothesis so that, if appropriate, it can be integrated into invasive species management. First, additional species should be studied in both their native ranges and in different parts of their introduced ranges. Such comparisons would be useful to test the drivers of invasive species abundance and to validate (or refute) invasion patterns derived from modelling approaches11,12. An important first clue that the Human Release Hypothesis may be relevant could be whether invasive individuals of a given perennial species are significantly older than individuals within the native range. Second, it may be useful to further investigate the relationship between landscape maintenance and human land use intensity, how it manifests in different regions, and if generalizations are possible at the global scale. The frequency of weeding and trimming, as well as the prevalence of fallowing, are just two of many potential indicators for the level of active landscape maintenance.
Evidently, the Human Release Hypothesis is still in its infancy, and it would be unwise to make bold management recommendations on its basis. Based on our analysis to date, preliminary insights that are relevant to managing invasive species are: (i) sparsely populated areas may face a higher risk of biological invasions than more densely populated areas; (ii) extensively managed rangelands may be more susceptible to high abundances of invasive species than intensively managed croplands; and (iii) high abundances of invasive species at landscape and regional scales could be facilitated by long periods of fallowing or land abandonment37.
Data availability
figshare: Dataset 1. Rosa rubiginosa L. occurrence data (occurrences_R.rubiginosa.csv, 416 kb). Doi: 10.6084/m9.figshare.100206738
Author contributions
HZ and HvW conceived the study. HvW and PB performed the climatic niche model and PB performed the climatic niche equivalency test. JF and HvW contributed substantially to the framing of the manuscript. EW compiled the geographic distribution of the study species. HZ wrote the first draft of the manuscript and contributed to the data analysis and data collection, and all authors contributed substantially to revisions.
Competing interests
No competing interests were disclosed.
Grant information
This study was funded by a Leuphana small research grant 73000787 (HZ) and through a Sofja Kovalevskaja Award by the Alexander von Humboldt Foundation (JF).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgements
The Human Release Hypothesis evolved during previous studies by H. Zimmermann, and we thank all co-authors involved in previous publications: D. Bran, M.A. Damascos, I. Hensen, H. Hirsch, D. Renison, C.M. Ritz, V. Wissemann, and K. Wesche.
Faculty Opinions recommendedReferences
- 1.
Crooks JA:
Characterizing ecosystem-level consequences of biological invasions: the role of ecosystem engineers.
Oikos.
2002; 97(2): 153–66. Publisher Full Text
- 2.
Born W, Rauschmayer F, Bräuer I:
Economic evaluation of biological invasions—a survey.
Ecol Econ.
2005; 55(3): 321–36. Publisher Full Text
- 3.
Mack RN, Smith MC:
Invasive plants as catalysts for the spread of human parasites.
NeoBiota.
2011; 9: 13–29. Publisher Full Text
- 4.
Drake J, Mooney HA, di Castri F, et al.:
Biological invasions: a global perspective. SCOPE 37. Chichester, UK: John Wiley and Sons; 1989. Reference Source
- 5.
Ricciardi A, MacIsaac H:
In Retrospect: The book that began invasion ecology.
Nature.
2008; 452: 34. Publisher Full Text
- 6.
Kueffer C, Pyšek P, Richardson DM:
Integrative invasion science: model systems, multi-site studies, focused meta-analysis and invasion syndromes.
New Phytol.
2013; 200(3): 615–33. PubMed Abstract
| Publisher Full Text
- 7.
Pyšek P, Jarošík V, Hulme PE, et al.:
Disentangling the role of environmental and human pressures on biological invasions across Europe.
Proc Natl Acad Sci U S A.
2010; 107(27): 12157–62. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 8.
Stohlgren TJ, Pyšek P, Kartesz J, et al.:
Widespread plant species: natives versus aliens in our changing world.
Biol Invasions.
2011; 13(9): 1931–44. Publisher Full Text
- 9.
Di Castri F:
History of biological invasions with special emphasis on the Old World. In: Drake JA, Mooney HA, di Castri F, Groves RH, Kruger F, Rejmánek M, et al., editors. Biological invasions: a global perspective. Chichester, UK: John Wiley and Sons; 1989. p. 1–30. Reference Source
- 10.
Ellis EC, Ramankutty N:
Putting people in the map: anthropogenic biomes of the world.
Front Ecol Environ.
2008; 6(8): 439–47. Publisher Full Text
- 11.
Perring MP, Ellis EC:
The Extent of Novel Ecosystems: Long in Time and Broad in Space. In: Hobbs R, Higgs E, Hall C, editors. Novel Ecosystems: Intervening in the new ecological world order. Chichester, UK: Wiley-Blackwell; 2013. p. 66–80. Publisher Full Text
- 12.
Ellis EC, Antill EC, Kreft H:
All is not loss: plant biodiversity in the anthropocene. Moen J editor.
PLoS One. Public Library of Science.
2012; 7(1): e30535. Publisher Full Text
- 13.
Sher AA, Hyatt LA:
The disturbed resource-flux invasion matrix: A new framework for patterns of plant invasion.
Biol Invasions.
1999; 1(2–3): 107–14. Publisher Full Text
- 14.
Wilkinson D:
The disturbing history of intermediate disturbance.
Oikos.
1999; 84(1): 145–7. Publisher Full Text
- 15.
Richardson DM, Pyšek P, Rejmánek M, et al.:
Naturalization and invasion of alien plants: concepts and definitions.
Divers Distrib.
2000; 6(2): 93–107. Publisher Full Text
- 16.
Catford JA, Jansson R, Nilsson C:
Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework.
Divers Distrib.
2009; 15(1): 22–40. Publisher Full Text
- 17.
Levine JM, Adler PB, Yelenik SG:
A meta-analysis of biotic resistance to exotic plant invasions.
Ecol Lett.
2004; 7(10): 975–89. Publisher Full Text
- 18.
Lockwood JL, Cassey P, Blackburn T:
The role of propagule pressure in explaining species invasions.
Trends Ecol Evol.
2005; 20(5): 223–8. PubMed Abstract
| Publisher Full Text
- 19.
Novak S, Mack R:
Genetic bottlenecks in alien plant species: influence of mating systems and introduction dynamics. In: Sax D, Stachowicz J, Gaines S, editors. Species Invasions: Insights into Ecology, Evolution, and Biogeography. Sunderland, Massachusetts: Sinauer Associates; 2005. p. 201–8. Reference Source
- 20.
Loomis ES, Fishman L:
A continent-wide clone: population genetic variation of the invasive plant Hieracium aurantiacum (Orange Hawkweed; Asteraceae) in North America.
Int J Plant Sci.
2009; 170(6): 759–65. Publisher Full Text
- 21.
Nuñez MA, Moretti A, Simberloff D:
Propagule pressure hypothesis not supported by an 80–year experiment on woody species invasion.
Oikos.
2011; 120(9): 1311–1316. Publisher Full Text
- 22.
Welk E, Schubert K, Hoffmann M:
Present and potential distribution of invasive garlic mustard (Alliaria petiolata) in North America.
Divers Distrib.
2002; 8(4): 219–33. Publisher Full Text
- 23.
Broennimann O, Treier UA, Müller-Schärer H, et al.:
Evidence of climatic niche shift during biological invasion.
Ecol Lett.
2007; 10(8): 701–9. PubMed Abstract
| Publisher Full Text
- 24.
Hobbs RJ, Huenneke LF:
Disturbance, diversity, and invasion: implications for Conservation.
Conserv Biol.
1992; 6(3): 324–37. Publisher Full Text
- 25.
Rejmánek M, Richardson DM:
What attributes make some plant species more invasive?
Ecology.
1996; 77(6): 1655–61. Publisher Full Text
- 26.
Keane RM, Crawley MJ:
Exotic plant invasions and the enemy release hypothesis.
Trends Ecol Evol.
2002; 17(4): 164–70. Publisher Full Text
- 27.
Catford JA, Daehler CC, Murphy HT, et al.:
The intermediate disturbance hypothesis and plant invasions: Implications for species richness and management.
Perspect Plant Ecol Evol Syst.
2012; 14(3): 231–41. Publisher Full Text
- 28.
Hobbs R:
Land-use changes and invasions. In: Mooney HA, Hobbs RJ, editors. Invasive species in a changing world. Washington DC: Island Press; 2000. p. 55–64. Reference Source
- 29.
Kennedy TA, Naeem S, Howe KM, et al.:
Biodiversity as a barrier to ecological invasion.
Nature.
2002; 417(6889): 636–8. PubMed Abstract
| Publisher Full Text
- 30.
Bartomeus I, Sol D, Pino J, et al.:
Deconstructing the native–exotic richness relationship in plants.
Glob Ecol Biogeogr.
2012; 21(5): 524–33. Publisher Full Text
- 31.
Zimmermann H, von Wehrden H, Renison D, et al.:
Shrub management is the principal driver of differing population sizes between native and invasive populations of Rosa rubiginosa L.
Biol Invasions.
2012; 14(10): 2141–57. Publisher Full Text
- 32.
Zimmermann H, Ritz CM, Hirsch H, et al.:
Highly reduced genetic diversity of Rosa rubiginosa L. populations in the invasive range.
Int J Plant Sci.
2010; 171(4): 435–46. Publisher Full Text
- 33.
Hirsch H, Zimmermann H, Ritz CM, et al.:
Tracking the origin of invasive Rosa rubiginosa populations in Argentina.
Int J Plant Sci.
2011; 172(4): 530–40. Publisher Full Text
- 34.
Elith J, Phillips SJ, Hastie T, et al.:
A statistical explanation of MaxEnt for ecologists.
Divers Distrib.
2011; 17(1): 43–57. Publisher Full Text
- 35.
Cavallero L, Raffaele E:
Fire enhances the “competition-free” space of an invader shrub: Rosa rubiginosa in northwestern Patagonia.
Biol Invasions.
2010; 12(10): 3395–404. Publisher Full Text
- 36.
Zimmermann H, von Wehrden H, Damascos MA, et al.:
Habitat invasion risk assessment based on Landsat 5 data, exemplified by the shrub Rosa rubiginosa in southern Argentina.
Austral Ecol.
2011; 36(7): 870–80. Publisher Full Text
- 37.
Cramer VA, Hobbs RJ, Standish RJ:
What’s new about old fields? Land abandonment and ecosystem assembly.
Trends Ecol Evol.
2008; 23(2): 104–12. PubMed Abstract
| Publisher Full Text
- 38.
Zimmerman H, Brandt P, Fischer J, et al.:
Dataset 1. Rosa rubiginosa L. occurrence data (occurrences_R.rubiginosa.csv, 416 kb). figshare. 2014. Data Source



Comments on this article Comments (0)