Keywords
alpine, cushion plant, pollination, climate induced mismatch, pollinator decline
alpine, cushion plant, pollination, climate induced mismatch, pollinator decline
We have addressed all suggestions made by the three referees. No major findings have changed but the flow and clarity of the paper has been improved.
See the authors' detailed response to the review by Ernesto Badano
See the authors' detailed response to the review by He Qiang
See the authors' detailed response to the review by Fabio Bulleri
Two future scenarios in which pollination rates may be directly reduced include pollinator declines (Potts et al., 2010) and plant-pollinator mismatch (Hegland et al., 2009). Recently there has been concern over general global trends of reduced pollinator species abundance and diversity that are both predicted to reduce pollination rates to plants (Memmott et al., 2007; Potts et al., 2010). Climate induced plant-pollinator mismatch can reduce pollination rates by creating a temporal mismatch in pollinator emergence and plant flowering times (Hegland et al., 2009). Pollinator emergence is regulated by temperature, whereas plant bloom time is regulated by photoperiod (Hegland et al., 2009). If climate warming shifts pollinator emergence but not plant bloom time, then a temporal mismatch between plants and pollinators occurs (Hegland et al., 2009). This scenario is likely more pronounced in alpine and polar environments that are experiencing a more rapid increase in annual temperature than the global average (very high confidence; Intergovernmental Panel on Climate Change [IPCC], 2013). Conceivably, both of these reductions in pollination rates occur simultaneously and thus adaptability of different sexual morphs in alpine plants can be an important consideration in predicting responsiveness and variation in reproductive output.
Hypotheses associated with pollen availability in alpine environments are controversial. It has been assumed that pollination rates are inherently low in alpine environments (Larson & Barrett, 2000; Totland & Sottocornola, 2001; Torres-Díaz et al., 2011). This is attributed to low temperatures, overcast conditions, strong winds, and relatively unpredictable weather being challenging for insect pollinators (Körner, 1999). These harsh conditions generally lead to lower pollinator diversity, abundance, and activity in alpine ecosystems relative to milder ecosystems (Kevan, 1972; Primack, 1978; Moldenke & Lincoln, 1979; Arroyo et al., 1982; Primack, 1983; Billings, 1987; Totland, 1993). Alternatively, pollination rates can increase with elevations suggesting adequate pollen availability under current conditions (Arroyo et al., 1985; Arroyo & Squeo, 1990; Bingham & Orthner, 1998; Utelli & Roy, 2000).
The reproductive assurance hypothesis (RAH) and the stress gradient hypothesis (SGH) are thus highly relevant hypotheses to explore in the alpine. The RAH proposes that when pollen supply is low, self-compatible plants are favored over self-incompatible plants (Lloyd, 1992; Lloyd & Schoen, 1992). This is because self-compatible plants create their own pollen thereby being more adapted to low or variable pollination rates (Larson & Barrett, 2000; Muñoz & Arroyo, 2006; García-Camacho & Totland, 2009). Further, it has been proposed that self-compatible plants are less likely to become extinct if pollinators drastically decrease or disappear from a given system (Richards, 1997; Morgan et al., 2005). Therefore, self-compatible plants may become favored in the future if pollen supply declines. The SHG is also an important ecological theory to consider in the possibility of reduced pollination rates and therefore reduce pollen supply. The SHG states that facilitation between two plant species is more common when resources are limited (Bertness & Callaway, 1994). Typically, the stress gradient hypothesis is tested using a gradient of environmental condition such as altitude (Cavieres & Badano, 2009) or moisture (Cavieres et al., 2006), but has not been applied to the concept of pollen supply limitations which could be categorized as a quantifiable limiting resource (Maestre et al., 2009). Taken together, these sets of ecological theories provide a solid platform to build pollen limitation studies upon and provide a set of potential ecological drivers that can help predict pollination rate changes in the alpine.
Here, we use a gynodioecious population of Silene acaulis to assess the sensitivity of different genders to pollen limitation and the potential effect of beneficiary plant species on these cushions. We test the following predictions associated with the reproductive assurance hypothesis: (1) that the reproduction of self-incompatible plants is more sensitive to reduced pollen deposition than that of self-compatible plants and (2) self-incompatible plants will be less viable under experimentally reduced pollen deposition. In doing so, we also explore whether pollen supply can be viewed as a resource stress within the stress gradient hypothesis. Specifically, we predict that facilitation between cushions and beneficiaries to increase when pollen supply is reduced.
S. acaulis (L.) Jacq. (Caryophyllacae), commonly known as moss campion, is a common long-lived evergreen cushion that is found throughout the northern hemisphere (Hitchcock & Maguire, 1947). Each plant has a single strong taproot, and there is no clonal reproduction (Morris & Doak, 1998). Small pink flowers can be abundant. S. acaulis is visited by bumblebees (Shykoff, 1988; Shykoff, 1992; Marr, 1997; Delph et al., 1999; Delph & Carroll, 2001), moths, beetles, ants (Marr, 1997; Delph & Carroll, 2001), flies (Totland, 1993; Delph & Carroll, 2001), butterflies and Osmia bees (Reid & Lortie, 2012). S. acaulis is a nurse plant species that, like many other cushion forming plants, benefits other plant species (called beneficiaries) by reducing abiotic stress (Arroyo et al., 2003; Bertness & Callaway, 1994; Callaway & Walker, 1997; Cavieres et al., 2006). Recent beneficiary removal studies suggest by facilitating the beneficiaries, cushions bear a cost in reduced reproductive success (Cranston et al., 2012; Schöb et al., 2013).
S. acaulis is sexually polymorphic (Hitchcock & Maguire, 1947) with this study population of S. acaulis is gynodioecious with plants that only have hermaphrodite flowers and other that only have female flowers. Female flowers have three styles with stigmatic lobes and hermaphrodite flowers have ten stamens. Male-sterility in female morphs of S. acaulis is predominantly under nuclear-cytoplasmic control (Delph & Carroll, 2001); the gene for male-sterility is passed on through the female gamete (Lewis, 1941). In addition to S. acaulis, the flowering plant species Antennaria alpina, Antennaria sp., Arnica sp., Carex sp., Erigeron sp., Luzula sp., Phacelia sericea, Phlox diffusa, Phyllodoce sp., Poa alpina, Potentilla diversifolia, P. heptaphila, P. villosa, Ranunculus eschsoltzii, Salix sp., Saxifraga bronchialis, and Solidago multiradiata were present at relatively high densities on and around the cushions.
The experiments were conducted on Whistler Mountain in British Columbia 50°03′31.68″N, 122°57′22.53″W, 2168m elevation), Canada during the snow-free season from June 26th to August 29th, 2010. This area is classified as alpine tundra with 10-months of snow cover a year (Pojar et al., 1987). A total of 271 S. acaulis plants were measured. Three S. acaulis plants were excluded from the study because they were infected with the pollinator-transmitted anther smut-fungus Microbotryum violaceum that renders the flowers of both genders sterile (Baker, 1947; Alexander & Antonovics, 1988; Hermanutz & Innes, 1994; Marr, 1997).
To experimentally reduce plant pollination, plants were covered with mesh and hand pollinated three times over the duration of the snow-free season. Before bud-burst, 60 S. acaulis plants were covered with fine cloth mesh to prevent pollinators contacting the flowers (Donnelly et al., 1998). As plant gender was unknown when initially covering, 60 plants were covered to ensure that there would be 20 replicate plants of each gender. Plant gender was established after bud-burst, and at that time, plants were randomly assigned a treatment and marked with a unique identification code. Reduced pollination treatments were conducted on 20 hermaphrodite and 20 female plants. The first 20 female and hermaphrodite plants found where used as replicates with the additional 20 plants being uncovered. The 40 plants (20 of each gender) selected for the reduced pollination treatments remained covered with mesh for the entire flowering season to exclude all insect pollination. It is assumed that plants that are covered and hand pollinated three times receive less pollen than the plant open to natural pollination during the 33 snow-free days.
All flowers of the reduced-pollination treatment plants were hand pollinated with pollen collected from S. acaulis plants within 10 m from the treatment plants. Hermaphrodite flowers with mature anthers were collected in the morning of the hand pollination days. Pollen was then applied using small paintbrushes or by directly touching the anthers to the stigmas of all the treatment-plant flowers. We found direct contact of the anthers to the stigmas the most effective method of hand pollination. The exact amount of pollen applied to each flower at each hand-pollination event was not quantified. Hand pollination was repeated three times between July 20th and August 1st, 2010.
Reproductive output measures were collected from the 40 hand-pollinated treatment plants as well as 231 naturally pollinated S. acaulis plants of which 124 were female and 107 were hermaphrodites. These measures include total number of flowers, total number of fruits, percent fruit-set, seeds per fruit, fruit weight, and seed weight. Percent germination was calculated on a subset of 60 plants, including the 40 treatment plants and 20 naturally pollinated plants.
Total number of flowers was counted during fruit collection including both successfully and unsuccessfully pollinated flowers. Fruit were collected when mature but not yet dehiscing so that the seed remained in the fruit capsule. This occurred between August 11th and 25th. All fruits were place in small-labeled paper envelopes and allowed to dry at room temperature to avoid decomposition. Percent fruit-set was calculated using the measures of total number of fruit and total number of flowers. Mean fruit weight (g) was calculated by averaging the weight of five randomly selected fruits per plant. These five fruits were dissected and the seed counted. Mean seed number per fruit was calculated from the seed counts. Total seed number per plant was estimated by multiplying total fruit with mean seed number per fruit. Mean seed weight (mg) was calculated by averaging the weight of 10 randomly selected seeds per plant. All weighing was done to four significant digits. When a plant produced less than five fruits or 10 seeds, the average was based on the maximum number of fruit or seed produced. Weighed seed was stored separately and cold stratified at 4°C for two months. A test germination trial was conducted with limited success likely because the cold stratification was not sufficient. Therefore, seeds were then stored at 0°C for two additional months in preparation for germination trials.
Germination trials were conducted on the weighed and cold stratified seeds from the 40 S. acaulis plants in reduced pollination treatments and the remaining 20 labeled plants that were left open to natural pollination. Growth chambers were set to standard optimum growing conditions of 20°C and light for 12 hours then 10°C and dark for the remaining 12 hours of the day (Baskin & Baskin, 1998). Relative humidity was set to 90%. The 10 seeds from an individual plant were placed on a labeled filter paper in a Petri dish. Seeds were checked weekly for three months, after which germination is rare (Milbau et al., 2009). Germination was considered to have occurred when the radical breaks open the seed (Milbau et al., 2009). Germinated seeds were removed to speed-up counting during the subsequent weeks and reduce counting errors (Milbau et al., 2009). Percent germination was expressed as the fraction of total number of germinated seeds with respect to the total number of seeds per Petri dish.
Cushion area and floral density were measured because of their possible effect on reproductive output. Cushion area was defined by the external boundary of vegetation and calculated as an ellipse with the formula,
cushion area = (a/2)*(b/2)*π
where a is the longest diameter of the plant and b the diameter perpendicular to a. We calculated floral density by dividing total flower number by cushion area.
To test if facilitation became more common under reduced pollen loads (stress gradient hypothesis) we measured beneficiary abundance on all cushion plants. Beneficiary abundance is the total number of individual plants living on the cushions.
To assess the sensitivity of female and hermaphrodite reproductive output under reduced pollen deposition, we calculated percent change of reproductive success measures within each gender between current and reduced pollen deposition. Percent change was calculated using the following equation:
percent change = (T-C)/C*100
where T is the reproductive output measures under the reduced pollination treatments and C is the reproductive output measures under the current pollination rates (Ayres, 1993). Negative numbers indicate that reduced pollination treatments decrease reproductive success and positive numbers indicate that reduced pollination treatments increase reproductive success. This method facilitates comparisons of the direction and magnitude of change.
To statistically test if reduced pollination, gender, and their interaction effects significantly explained variation in the reproductive output measures, we used a generalized linear model (GLM) with Poisson distribution and a log link function. Covariate measures of included S. acaulis surface area, S. acaulis floral density, and beneficiary abundance.
Hermaphrodites provide one-half of the genetic material to the population through pollen production. Therefore, female plants must produce at least twice the number of seeds as hermaphrodite plants to persist in the population (Charnov, 1982) or have offspring that are more fit (Lewis, 1941). To statistically analyze the viability of females under current and reduced pollen deposition levels we compared female reproductive output to twice that of hermaphrodite reproductive output. In this way, if female reproduction (F) is greater than two times hermaphrodite reproduction (2H), then females are viable in the population. For females to be viable, not all measures of reproductive success need to be twice that of hermaphrodites, but all measures are shown to be comprehensive. GLMs were also used to test if gender significantly affected reproductive output measures under the current ambient and experimentally reduced pollination regimes.
Instead of testing the effect of beneficiaries on measures of reproductive success over a range of environmental gradients (Bertness & Callaway, 1994) we test this effect under two pollen regimes: current and reduced. As there was no experimental removal of beneficiaries, interactions are inferred by association. To test the stress gradient hypothesis in this plant-pollinator system we conducted correlation analysis and tested for significance in the interaction term between the effect pollination and beneficiary abundance on percent fruit set in a GLM. A significant p-value (p<0.05) indicates that the response of percent fruit set to beneficiary abundance significantly differs between plants in the current and reduce pollination regimes. All analyses are appropriate for dealing with the unbalanced number of replicates between the current and reduced pollination regimes and were done in JMP 10 (SAS, 2012).
Pollination regime significantly influenced percent fruit-set, seeds per fruit, fruit weight and percent germination (Table 1). Percent fruit-set, seeds per fruit, fruit weight, and percent germination were decreased in both genders with reduced pollen deposition (Figure 1). There was a significant interaction effect between gender and pollination regime for percent fruit set, and percent germination (Table 1) indicating that these measures differed in their response. The direction of these differences is illustrated in the percent change calculations. Percent fruit-set reduced to a greater in females compared to hermaphrodites (Figure 1). Whereas, percent germination reduced to a greater in hermaphrodites compared to females (Figure 1).
Summary of GLM results testing the effect of gender, pollination regime, and the gender by pollination regime interaction on measures of reproductive success with covariate measures of S. acaulis surface area (SA), S. acaulis floral density, and beneficiary abundance indicated by *. Significance is considered at <0.05 and indicated in bold.
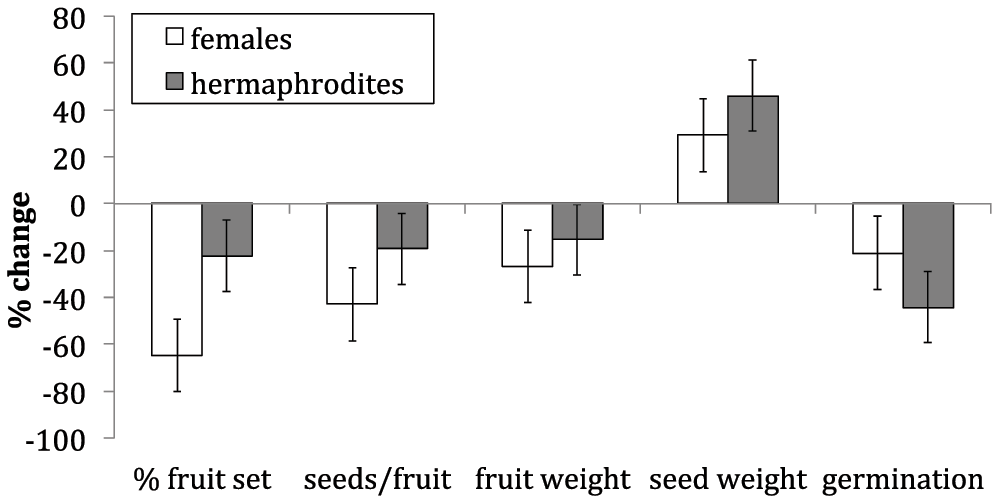
± 1 standard error bars shown.
Under current pollination rates, female plants had more than twice (2.98 times) the percent fruit-set compared to hermaphrodites (Figure 2). Female plants had less than twice the seeds/fruit, fruit weight, seed weight and percent germination compared to hermaphrodites (Figure 2). Under current pollination rates, all reproductive output measures were significantly different between females and two times hermaphrodite reproductive measures (Table 2).
Under reduced pollination rates, none of the female reproductive measures were greater than two times that of hermaphrodites (Figure 2). Under reduced pollination rates, all reproductive output measures, except seed weight and percent germination, were significantly different between females and two times hermaphrodite reproductive measures (Table 3).

± 1 standard error bars shown.
Summary of GLM results testing the effect of gender on measures of reproductive success under the current pollination regime with covariate measures of S. acaulis surface area (SA), S. acaulis floral density, and beneficiary abundance indicated by *. Reproductive measures for hermaphrodites are doubled. Significance is considered at p<0.05 and indicated in bold.
Summary of GLM results testing the effect of gender on measures of reproductive success under the reduced pollination regime with covariate measures of S. acaulis surface area (SA), S. acaulis floral density, and beneficiary abundance indicated by *. Reproductive measures for hermaphrodites are doubled. Significance is considered at p<0.05 and indicated in bold.
Beneficiary abundance had a significant effect on percent fruit-set (Table 1). Under the current pollination rates, percent fruit-set and beneficiary abundance are negatively related (slope = -0.48, R2 = 0.04, Figure 3). In contrast, under reduced pollination rates, percent fruit-set and beneficiary abundance are positively related (slope = 0.24, R2 = 0.06, Figure 3). The slopes of these lines significantly differ (Chi2 161.25, p-value <0.0001).
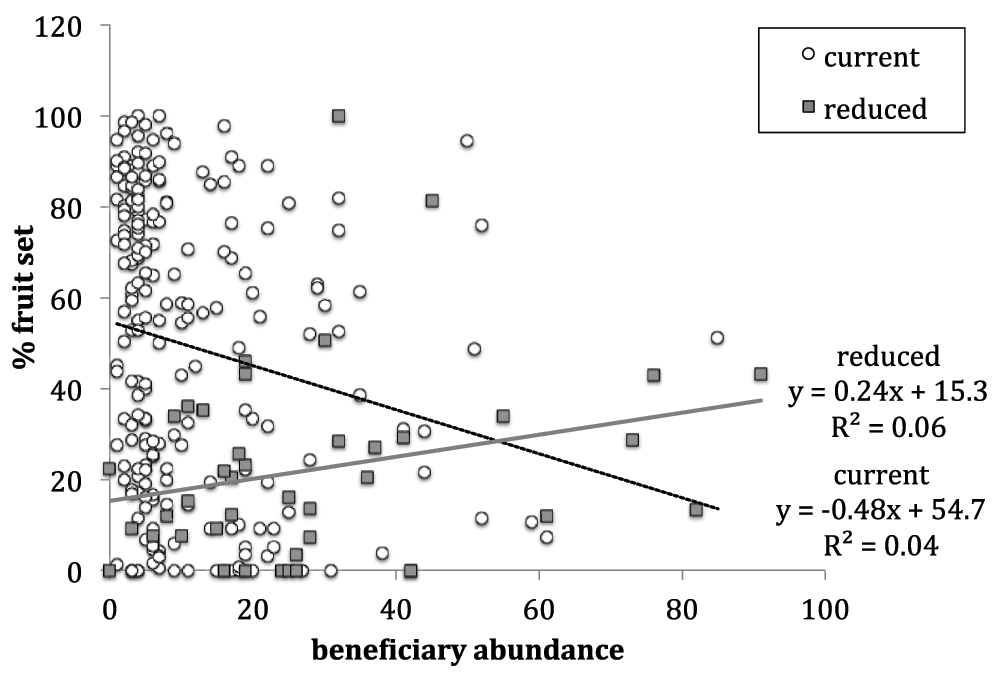
The linear best-fit line for plants under current pollination regime (black dashed line) has a negative slope of -0.48 and R2 value of 0.04. The linear best-fit line for plants under reduced pollination regime (grey line) has a positive slope of 0.24 and R2 value of 0.06. The slopes of these lines significantly differ (Chi2 161.25, p-value <0.0001).
Pollinator declines and climate mismatches are important in understanding the capacity for alpine plants to respond to possible future scenarios with reduced pollen loads. Using the cushion plant S. acaulis, we tested two predictions associated with the reproductive assurance hypothesis and more broadly that pollination stress may influence plant-plant interactions. All predictions were supported. Females were more sensitive than hermaphrodites to reduced pollen loads resulting in reproductive output dropping to below twice that of hermaphrodites. As the pollen supply conditions became more stressful (i.e. reduced), beneficiary plant species on these cushions positively related to percent fruit-set of the cushions. Hence, the reproductive assurance hypothesis and use of pollen reduction experiments can be important tools for ecological experiments on the responsiveness of alpine plant-pollinator systems to future changes in pollen availability.
Pollinator declines and plant-pollinator mismatches are important potential drivers of broad plant-community dynamics in the alpine if dominant cushion plant species are impacted because they often function as keystone plant species (Arroyo et al., 2003; Bertness & Callaway, 1994; Callaway & Walker, 1997; Cavieres et al., 2006). Current trends of decreasing native pollinator populations are a pressing concern globally (Memmott et al., 2007; Potts et al., 2010; Bartomeus & Winfree, 2013). In these alpine environments bumblebees in particular are suggested to be critical because they are the most effective alpine pollinator (Bingham & Orthner, 1998; Chittka et al., 1999; Gegear & Laverty, 1998) and because some bumblebee populations in BC are in decline (Colla & Ratti, 2010). The future scenario that pollinators emerge before flowers are in bloom due to a warming climate has also been proposed (Hegland et al., 2009) and shown in the alpine of Japan (Kudo, 2013). Although it may not mimic the exact future pollen deposition rates, the experimental design tested herein begins to explore how alpine plants may respond to reduced-pollen loads whilst concomitantly testing the reproductive assurance hypothesis. Differences in reproduction between alpine plant genders are thus a critical avenue of research.
Female cushion plants became less viable under reduced pollen loads indicated by the decrease in percent fruit-set. Although the other measures of reproductive success were not equally decreased under reduced pollination, the decrease in percent fruit-set more than compensates for female plants reproductive disadvantage over hermaphrodite plants. Hence, a reasonable proxy or single measure to consider in similar future studies using dominant cushion plants is percent fruit-set only.
Interestingly, our results also supported the application of the stress gradient hypothesis to pollen limitation in addition to its original formulation for environmental stress or consumer pressure. That beneficiary abundance explains little variation in percent fruit-set (low correlation coefficients), suggests that other variables are of greater importance in determining percent fruit-set. Yet, the relationship between beneficiary abundance and percent fruit-set shifted from negative to positive as the pollination rates were reduced. This supports previous findings that under current pollination to the plant community, beneficiary plants living on cushions generally have a cost associated with the cushion plants reproductive fitness (Cranston et al., 2012; Schöb et al., 2013). The findings here however also further suggest that under reduced pollen loads this cost of facilitation can be diminished likely because competition between cushions and the other species is significantly reduced. Clearly, additional research is needed to identify the causal mechanisms between plant-plant interactions and plant-pollinator interactions with dominant plant species that host other species.
F1000Research: Dataset 1. The reproductive effects of reduced pollen deposition via exclosures and hand pollination on the cushion plant Silene acaulis, 10.5256/f1000research.4382.d29313 (Reid et al., 2014).
AR and CJL wrote the paper and analyzed the data. AR, RH and OM performed the experiments and collected the data. All authors read and approved the final content of the manuscript.
This research was funded by an NSERC DG and an NSERC Canadian Pollination Initiative (CANPOLIN) grant to CJL. This is publication #26 of NSERC-CANPOLIN. AR was funded by an Ontario Graduate Scholarship and two York University Faculty of Graduate Studies research grants. RH was funded by NSERC URSA. OM was funded through York University Department of Biology.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
This is an interesting study dealing with the effects that facilitated plants may have on the performance of the reproductive success of the nurse species. As far as I am aware, this is the first study reporting such effects. The article is well written and is easy to read. The arguments provided in the introduction section support the hypotheses raised by the authors, and the methods used to test these hypotheses are sound. I believe that this paper makes an important contribution to the conceptual framework of positive interactions among plants along environmental gradients, mainly because it helps one to understand the outcome of these interactions in future climate change scenarios. I just have a few comments and queries about the methods used by the authors. The other sections of the article are clear and would not require major improvement.
Materials and Methods: Specific comments
Competing Interests: No competing interests were disclosed.
This is a well conducted experiment and well written manuscript. It is the first experimental test of the stress-gradient hypothesis under pollen limitation stress, showing how we can further our understanding of plant-plant interactions with a new perspective. In my view, this is a neat paper, though I have some concerns that I hope the author can address in a revision.
The main limitation of this paper is that plant-plant interactions are inferred by association, not experimental removal of neighbours. Although a number of studies from alpine habitats suggest that facilitation can be indicated by positive association relationships, this limitation I feel should be clarified in the paper.
It is also a bit confusing to me why the authors did hand pollination for reduced-pollination treatment plants - was this because reduced-pollination treatment plants did not receive pollination at all without hand pollination? If so, the question would be how the authors controlled the magnitude of hand pollination as compared to natural pollination.
A third issue is that the pollen reduction treatments lacked procedural control. For example, one cannot tell the effect of mesh on the data. Also surely, the authors need to provide some details about the cloth mesh they used, such as mesh size etc.
It is unclear what can be the potential mechanisms for facilitation under pollen limitation. Facilitation in physically stressful habitats is produced by neighbours’ relief of abiotic stress, and in biologically stressful habitats by associational defence. It is not a must for the authors to experimentally demonstrate this in the paper but I think they should at least discuss some of the possibilities. The authors may also want to include descriptions to encourage future research that experimentally tests for these possibilities.
I have several more specific comments:
Overall, this is a great paper. The publication of this paper can be a stimulator of a new direction of positive species interactions with environmental stress.
Competing Interests: No competing interests were disclosed.
This is an interesting article reporting on a study investigating how reduced pollen supply influences reproduction of self-compatible versus self-incompatible plants of an alpine cushion species and its interaction with associated species. The main strength of the study is that of providing hints on the potential role that factors other than environmental conditions can play in modulating species interactions. In a relatively recent work by Maestre et al. (2009), stress has been divided into two major types, resource and non-resource. I am not sure whether pollen availability can be seen as a resource, but I would not have major issues with viewing pollinator availability as a quantifiable, limiting resource. Maybe, these concepts could be touched upon somewhere in the Introduction.
The main weakness of the study is the lack of quantification of pollen delivery in different treatments: I think the authors should try to give the reader, at the very least, a gross idea of the order of magnitude of differences between natural and artificial pollen supply. The second point is that there is not a formal gradient of pollen supply, as this implies more than two levels. This does not impinge on the robustness of the results, but limits the insight the study offers into the Stress Gradient Hypothesis.
Finally, both in the Abstract and M&M I did not get clearly who the benefactors and beneficiary were. Actually, only from the Results did I understand that you were analyzing the effects of species hosted by the cushion on the cushion-forming species. I sense that referring to the cushion and associated species might be a better solution.
SPECIFIC COMMENTS
Introduction
Material & Methods
Results
Discussion
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 07 Aug 14 |
read | ||
|
Version 1 19 Jun 14 |
read | read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)