Keywords
adipocyte, obesity, cytokine, β-adrenergic, TNFα, IL-6, lipolysis
adipocyte, obesity, cytokine, β-adrenergic, TNFα, IL-6, lipolysis
Obesity often leads to increased systemic inflammation which is now thought to play a causative role in the development of atherosclerotic disease and insulin resistance1–4. Increasing adiposity due to excessive weight gain sets up a chronic inflammatory response within adipose tissue which is promoted by recruitment and infiltration of classically activated, type-1 macrophages from the circulation5. Although the reasons for sustained adipose inflammation remain unclear, the large number of macrophages residing in obese adipose tissue leads to significant increases in secretion of interleukin-6 (IL-6) and tumor necrosis factor-α (TNFα); achieving levels sufficient to elevate circulating plasma concentrations6. IL-67 and TNFα8,9 are multifunctional cytokines that have well-established roles in inflammatory responses. In adipose tissue, these cytokines share a common activity in delivering potent signals for stimulation of lipolysis10–13. Increased lipolytic activity in obese adipose tissue increases free fatty acid (FFA) flux into the circulation. From a lipocentric view, elevated plasma levels of non-esterified fatty acids (NEFA) leads to increased amounts of atherogenic lipoproteins in the circulation resulting in unmanaged hyperlipidemia14, often accompanied by reduced systemic insulin responsiveness15,16.
The metabolic actions of IL-6 are diverse. Determination of which actions take precedence is largely dictated by tissue and metabolic context17. For example, IL-6 is an important mediator of the acute phase response which includes hepatic effects to increase glucose output and elevate CRP levels17,18. IL-6 secretion is also increased as a result of exercise19,20, which in turn increases glucose oxidation21 and insulin sensitivity22 in skeletal muscle. IL-6 infusion in humans increased circulating FFA levels11,23, and when adipose tissue or isolated adipocytes were treated with IL-6, lipolytic activity was increased17. In adipocytes, IL-6 binds to a cell surface heterodimer composed of the IL-6 receptor and gp13024,25, and activates two intracellular signaling pathways; the Janus kinase/Signal Transducer and Activator of Transcription (JAK/STAT) pathway, and the p44/42 Mitogen-activated protein kinase (MAPK) pathway17,26.
TNFα is a potent metabolic effector that, in adipocytes, signals primarily through TNFα receptor-127. Intracellular signaling in adipocytes is mediated by p44/42 MAPK and Jun N-terminal kinase (JNK)12,28. Once activated, these pathways induce phosphorylation of perilipin to recruit hormone sensitive lipase for triacylglycerol hydrolysis and the release of FFA, and to downregulate perilipin expression29,30. Perilipin is a phosphoprotein that coats intracellular lipid droplets in adipocytes to maintain minimal lipolytic activity. Phosphorylation of perilipin serves a dual purpose: to release bound CGI-58 to activate adipose triglyceride lipase and to relocate perilipin away from the lipid droplet permitting catalytic access for activated lipases31. However, signaling the sequence of events leading to lipolytic activation through JAK/STAT, p44/42 MAPK and JNK pathways is not the normal physiologic response to increased energy demands requiring the release of fatty acid fuel stores from adipocytes. Normal physiologic activation of lipolysis by β-adrenergic and glucagon signaling during periods of increased systemic energy demands is mediated through heterotrimeric G-protein activation followed by increased intracellular cAMP and protein kinase-A (PKA) activation. Obese adipose tissue is subject to normal β-adrenergic and glucagon stimuli to regulate energy balance; however, this tissue is also subject to additional lipolytic stimuli by IL-6 and TNFα. With the presence of multiple pathways for lipolytic activation, i.e. the normal endocrine/neural pathway and cytokine-mediated pathways, it is unclear whether the effects of multiple signaling events on lipolysis are additive or coincident; that is, do IL-6 and TNFα stimulate lipolytic activities that are in excess of that provided by maximal β-adrenergic activation, or do the pathways activated by these cytokines merge into the downstream β-adrenergic pathway and are they unable to add significantly beyond maximal β-adrenergic activation. To address this question, we have measured lipolytic activity in 3T3-L1-derived adipocytes activated by a β-adrenergic agonist, IL-6 or TNFα, both individually and in combination as co- and tri-stimulation experiments.
Mouse 3T3-L1 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA), and grown and differentiated in complete Dulbecco’s Modified Eagle Medium (DMEM; Irvine Scientific, Santa Ana, CA) with high-glucose and supplemented with 10% fetal bovine serum (FBS; Irvine Scientific, Santa Ana, CA) and incubated at 37°C in a 5% CO2 environment. Twelve-well culture plates (Costar Tissue-culture Treated; Corning, Tewksbury MA) were coated with 1% gelatin (Sigma-Aldrich, St. Louis, MO) prior to cell seeding. After reaching confluency, cells were differentiated into mature adipocytes by incubating with 450 µM 3-isobutyl-1-methylxanthine, 250 nM dexamethasone and 167 nM insulin (Sigma-Aldrich, St. Louis, MO) diluted into DMEM for 3 days, followed by 4 days supplementation with 167 nM insulin alone. Differentiation was confirmed by visual examination (MicrosOpics, IV900 Series inverted microscope) of cells to assess for lipid droplet formation and morphological changes. Prior to each experiment, cells were incubated with complete DMEM without insulin supplementation for a period of 24 hours. Cells were then treated either with isoproterenol (25 nM to 2.5 µM) (Sigma-Aldrich, St. Louis, MO), TNFα (0.1 nM to 25 nM) (Cell Signaling, Danvers, MA) or IL-6 (0.5 nM to 8 nM) (eBioscience, San Diego, CA) for 24 hours to identify an optimal concentration of effector necessary for induction of fatty acid hydrolysis and glycerol release. Once this concentration was determined, subsequent experiments were performed by incubating cells with this identified concentration for 24 hours.
Lipolytic determinations were made by quantifying glycerol released into the culture medium following treatments. Culture media were collected and processed using Free Glycerol Reagent according to the manufacturer’s instructions (Sigma-Aldrich, St. Louis, MO). Following the prescribed incubation, the resulting quinoneimine dye in samples was measured by spectrophotometry at 540 nm (Molecular Devices, Spectramax 384 Plus, Sunnyvale, CA). Glycerol amounts in the culture media were determined through comparison with standard curves that were generated by parallel quantification assays using known concentrations of glycerol.
Our primary objective for this study was to determine if cytokine stimulation (IL-6 or TNFα) heightens lipolytic activity (as measured by glycerol release) over and above what is achieved by normal β-adrenergic signaling. To obtain this quantitative evaluation, we first incubated mature 3T3-L1-derived adipocytes with varying concentrations of either isoproterenol (a β-adrenergic agonist), IL-6 or TNFα individually in order to determine the concentration of ligand that provided maximal lipolytic stimulation. This will ensure that individual ligands will be used at concentrations that maximally activate their respective signaling pathways in co- and tri-stimulation experiments. From our titration study, we have determined that maximal lipolytic stimulation for each ligand is achieved with the following concentrations: 1 µM for isoproterenol, 2 nM for IL-6 and 0.58 nM for TNFα (data not shown).
Co- and tri-stimulation experiments were performed using the ligand concentrations determined above. When adipocytes were incubated with the individual ligands, different levels of lipolytic activation were noted with IL-6 < isoproterenol < TNFα (Figure 1. compare bar 2 < bar 1 < bar 5). These differences are likely due to activation of different signaling pathways which have varying quantitative effects on lipolytic activation. Co-stimulation of adipocytes with isoproterenol and IL-6 resulted in lipolytic activity that was greater than that achieved by stimulation with the individual ligands (Figure 1. compare bar 3 with bars 1 and 2), suggesting parallel activation of different signaling pathways that merge into downstream lipolytic activation. The level of increased lipolytic activation from isoproterenol and IL-6 co-stimulation is approximately the sum of the individual ligands, suggesting an additive effect of contributions from two independent pathways, likely cAMP/PKA and p44/42-JAK/STAT, respectively. When isoproterenol and TNFα were combined, again an additive effect on lipolytic activation was observed (Figure 1. compare bar 6 with bars 4 and 5), similarly suggesting a summing of the effects caused by the activation of two separate signaling pathways, cAMP/PKA and p44/42-JNK, respectively.
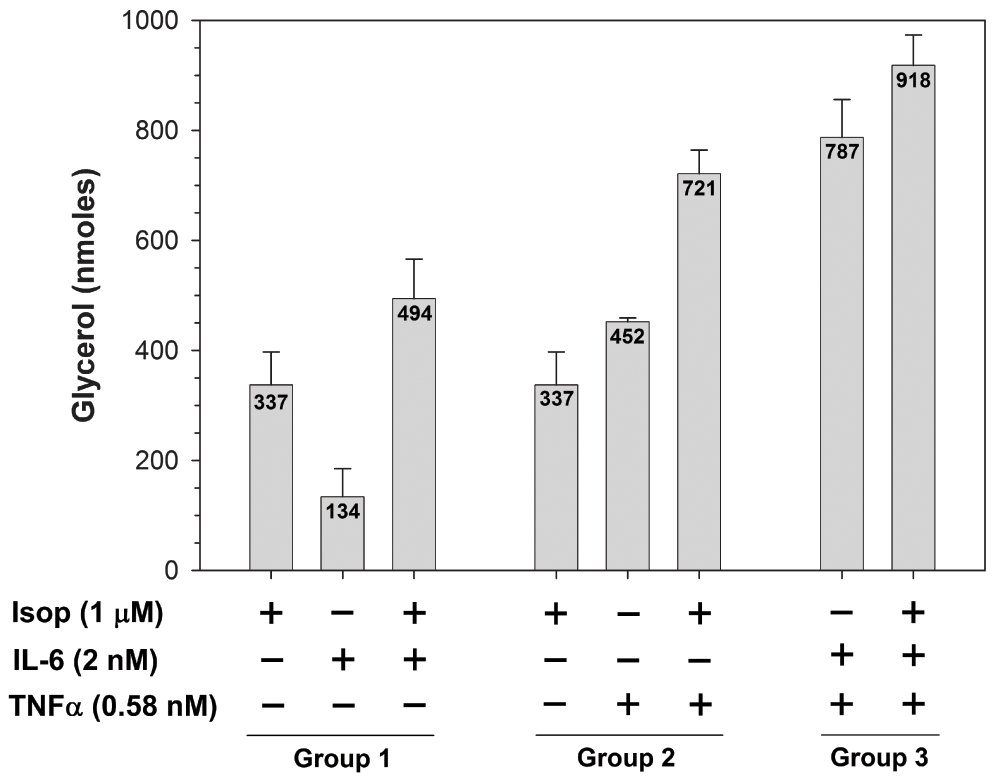
3T3-L1 cells were differentiated into mature adipocytes and incubated for 24 hours in the absence of insulin to diminish insulin-dependent anabolic signals. Cells were then incubated for an additional 24 hours in the presence (+) or absence (-) of the indicated concentrations of β-adrenergic agonist (isoproterenol) or cytokine (IL-6 or TNFα) for individual, co- and tri-stimulations. Medium was removed from cells and glycerol was quantified as described in the Materials and methods section. Experimental points were measured in triplicate to determine mean values (shown within bars) and standard deviations (error bars). Data shown are mean values determined using replicate experiments (n = 3). Mean values obtained for each experimental treatment group (1, 2 or 3) were compared by a one-way analysis of variance (ANOVA). Statistical significance is reported when P-values were < 0.05.
In vivo, obese adipose tissues express both IL-6 and TNFα when they are inflamed and susceptible to concurrent stimulation by both cytokines. To determine the effects of dual cytokine stimulation on lipolysis, adipocytes were incubated with both IL-6 and TNFα. In this case, lipolytic activation was somewhat more than an additive response (Figure 1. compare bar 7 with bars 2 and 5), suggesting that independent, as well as overlapping, signaling pathways were activated. The independent pathways include JAK/STAT for IL-6 and JNK for TNFα, while both cytokines are capable of activating the p44/42 pathway. The greater than additive response is likely due to dual stimulation of the common p44/42 pathway. Finally, in order to simulate a physiological environment where obese, inflamed adipose tissue undergoes normal β-adrenergic stimulation during fasting and/or exercise, we treated adipocytes with a triple combination of isoproterenol, IL-6 and TNFα. This condition resulted in the highest level of lipolytic activation with a value slightly greater than adding isoproterenol stimulation to a combined treatment of IL-6 and TNFα (Figure 1. compare bar 8 with bars 1 and 7).
The level of lipolytic activation from the triple stimulation with isoproterenol, IL-6 and TNFα far surpassed that of normal β-adrenergic stimulation alone, and provides mechanistic evidence for the cause of hyperlipidemia in obese individuals. Under normal circumstances (in lean individuals), β-adrenergic signaling is activated during fasting and exercise to mobilize fatty acids from adipose tissue and compensate for a negative systemic energy balance. Once the energy balance has returned to homeostasis, β-adrenergic stimulation is inactivated and the release of fatty acids is halted to prevent excessive plasma lipid levels. Obese individuals are also subject to normal β-adrenergic, epinephrine and norepinephrine stimulation of adipose tissue due to stress responses or negative energy balance, and this stimulation signals through the heterotrimeric G-protein, adenylyl cyclase, cAMP, PKA network32. In addition to this signaling, cytokines produced in inflamed obese adipose tissue also activate additional pathways that make a cumulative addition to the normal lipolytic response. Evidence provided here suggests that IL-6- and TNFα-activated pathways in adipose contribute to increased lipolysis through both independent and common signaling pathways (Figure 2). In considering therapeutic options for obese individuals, maintaining normal β-adrenergic signaling is vital to manage routine changes in energy balance that occur due to cyclical variations in physical activity. However, the contributions of IL-6 and TNFα to increased lipolytic activity being additive to normal β-adrenergic stimulation indicates that these pathways (p44/42, JAK/STAT and JNK) represent excellent therapeutic targets that will prevent excessive lipolysis, yet minimally interfere with maintaining normal responses to varying energy demands.
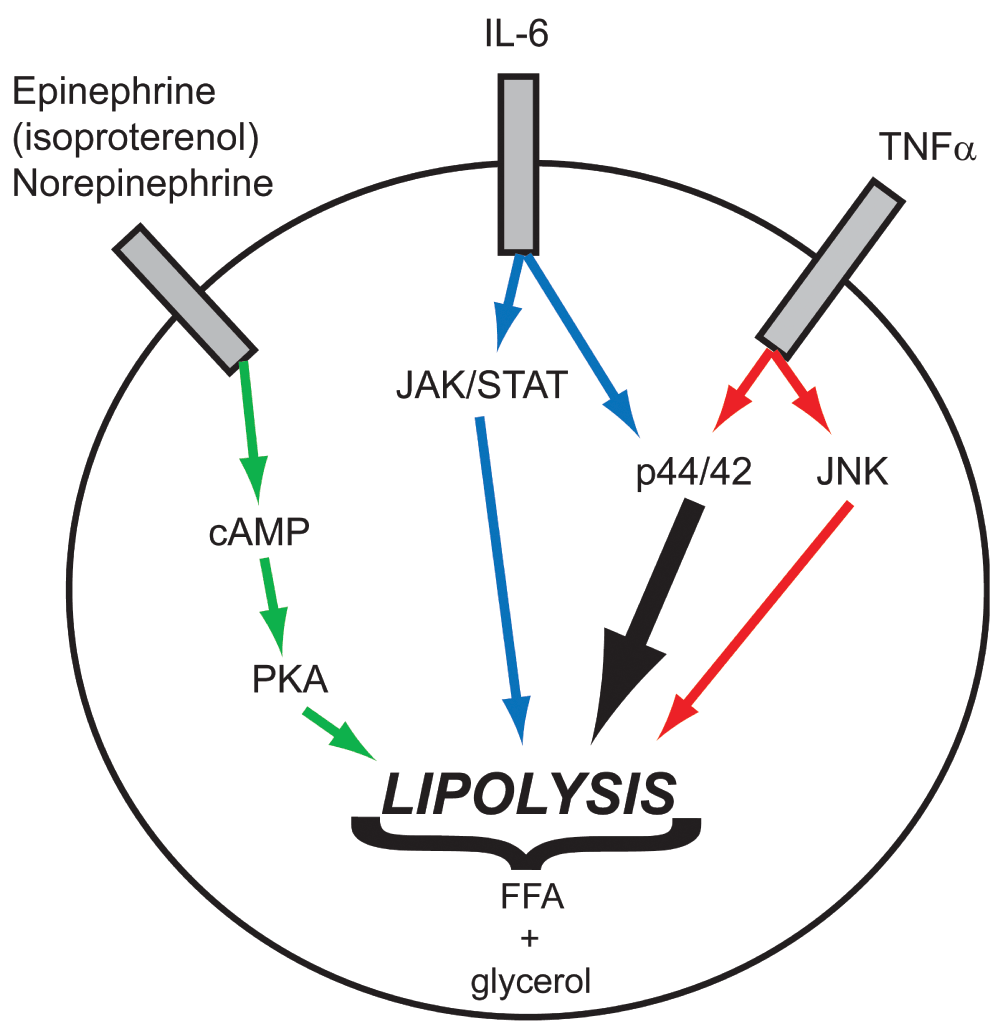
β-adrenergic (epinephrine and norepinephrine) stimulation of adipose tissue to induce activation of lipolysis proceeds through heterotrimeric G-protein and adenylyl cyclase activation, which elevates cytosolic cAMP levels (green arrows). Cyclic-AMP-dependent protein kinase-A (PKA) then phosphorylates downstream regulatory proteins and lipases to initiate release of fatty acids from triacylglycerol stores. Inflamed obese adipose tissue is also subject to additional stimuli originating from secreted cytokines. In adipocytes, IL-6 activates the JAK/STAT and p44/42 pathways (blue/black arrows), while TNFα activates the JNK and p44/42 pathways (red/black arrows), which are independently sufficient to stimulate lipolysis. Based on the magnitude of fatty acid release when cytokine stimulation is concurrent with normal adrenergic signaling, it appears that the activation of combined pathways provides an additive response to lipolytic activation leading to an excess of fatty acids released.
F1000Research: Dataset 1. Glycerol release following isoproterenol, IL-6 and TNFα stimulation, 10.5256/f1000research.4151.d2771133
NC and RO conceived the study. NC and RO designed the experiments. NC carried out the research. NC and RO prepared the first draft of the manuscript. NC, WG and RO contributed to the experimental interpretations and preparation of the manuscript. All authors were involved in revising the draft manuscript and have agreed to the final content.
We thank the University of New Mexico, School of Medicine, Office of Research for providing generous funding for this work.
We thank Drs. Carolina Franco Nitta and Yijuan Sun for their thoughtful contributions to this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
The authors evaluate the β-adrenergic pathwaywhich is an interesting target for modulation of inflammatory and metabolic pathways. This paper investigates the potential (additive) effects of β-adrenergic and cytokine stimulation on lipolysis in mouse adipose cells. Additive effects of isoproterenol, TNF-α and IL-6 on lipolysis in 3T3-L1 cells are demonstrated in vitro.
A major question concerns the applied statistics:
Other questions concerning this paper:
These questions should be addressed in an updated version of the paper.
Competing Interests: No competing interests were disclosed.
The paper investigates the additive effects of isoproterenol, interleukin-6 and TNF-alpha on lipolysis in mature mouse 3T3-L1 adipocytes. For this, the maximal lipolytic doses of each of the three agonists were established and 3T3-L1 adipocytes were incubated for 24h with these doses, alone or in combination. Lipolysis was assessed as glycerol release into the medium over the 24h incubation period.
The main conclusion to be drawn from the study is that IL-6 and TNF-alpha signaling are additive to the beta-adrenergic signaling with respect to 3T3-L1 adipocyte lipolysis. The investigators furthermore conclude "that this suggests that therapeutic inhibition of cytokine signaling will prevent excessive lipolysis, yet minimally interfere with maintaining normal responses to varying energy demands". This addition seems to be too far-fetched for several reasons: 1. confirmation in human adipocytes is required, because there may be species differences; 2. the study tested maximal lipolytic doses of the three lipolytic agents and under physiological conditions such a combination is unlikely to occur; 3. it is well-known that in obesity responsiveness to beta-adrenergic stimulation is blunted, therefore it could also be that the increased lipolysis due to increased cytokine levels in obesity helps to maintain normal lipolytic responsiveness to increased energy demand rather than result in excessive lipolysis.
Other comments:
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 24 Jun 14 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)