Case
There has been an explosion of interest in Nav1.7 as a potential therapeutic target for novel analgesics, as mutations in SCN9A are associated with profoundly altered pain thresholds1. Perhaps the greatest level of interest has been reserved for those very rare individuals with autosomal recessive mutations that truncate the protein Nav1.7 resulting in a complete lack of expression of the ion channel. The result is Channelopathy-associated Insensitivity to Pain (CIP) Syndrome: a complete absence of pain sensation, while all other sensory modalities apart from the sense of smell remain intact. Here we describe the experiences of a Caucasian 37-year-old patient with CIP whose older sister, but neither of her parents or other family members, is also affected. Other than a variety of injuries to the cornea and tongue, burns and relatively minor fractures sustained during childhood and now ascribed to CIP, there was no other medical history of note. Nonetheless, after childbirth she developed a symptom that she now readily describes as pain, and which has neuropathic features. We believe that this case report provides insights into the mechanisms of neuropathic pain, dissecting “positive” from “negative” symptomatology, and shows that it is possible to experience neuropathic pain in the absence of prior experience of acute pain.
Our patient had been recognized as having CIP aged 7, diagnosed at the same time as her older sister. This diagnosis was confirmed 18 years later by finding bi-allelic heterozygous null mutations of SCN9A in exon 29 (c.4975T>A p.K1659X) and exon 22 (c.3699-3709delATGGATAGCAT p.I1235LfsX2). The SCN9A gene on chromosome 2q24.3 encodes the alpha-subunit of the Nav1.7 voltage-gated sodium channel, which is expressed at high levels in small-diameter peripheral nociceptive neurons2.
The patient sustained painless pelvic fractures, presumably during labor, which were not recognized for two months. By then, examination revealed significant weakness in both legs, worse on the right, with absence of both ankle reflexes. We subsequently compared the results of formal quantitative sensory testing three months post-injury to those obtained four years pre-injury. Sensory thresholds to heat and cold in the foot dorsum were broadly similar on both sides (Table 1), and should be interpreted in the context of someone who has never felt pain. However, thresholds to mechanical (von Frey) stimulation of the dorsum of both feet were increased more than 10-fold bilaterally. Imaging studies revealed multiple fractures of both sacral wings and of the superior and inferior pubic rami bilaterally. Furthermore, there was an extensive hematoma extending into the left iliopsoas, right obturator externus and spinal canal, causing occlusion of the thecal sac at the level of the fifth lumbar (L5) and first sacral (S1) intervertebral space (Figure 1). The fractures were attributed to transient osteoporosis of pregnancy, and their severity to her continued walking in the face of CIP. However, shortly after the fractures were diagnosed, bone densitometry studies and all serum bone profile results were found to be normal.
Table 1. Temperature and mechanical thresholds of the dorsum of both feet before and after childbirth.
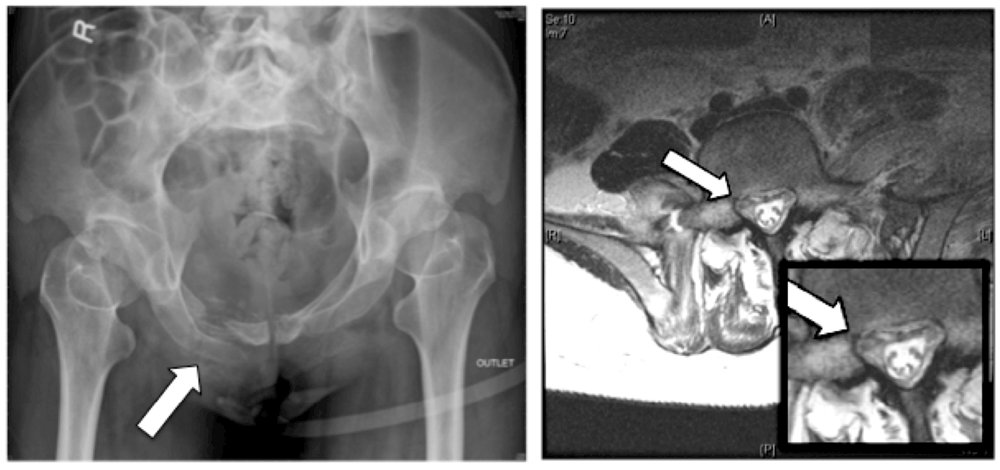
Figure 1. An anteroposterior X-ray outlet view of the patient’s pelvis.
The X-ray shows multiple fractures of the superior and inferior pubic rami (left) and an axial magnetic resonance image (right) plus magnification showing a hematoma impinging upon the right L5 nerve root at the exit foramen (arrow).
Two months later, four months after delivery, she reported troubling continuous buzzing in both legs and a vice-like squeezing in the pelvis when she walked: symptoms that are consistent with neuropathic pain3. These two symptoms did not respond to the anti-neuropathic drug gabapentin, and persist two years after the delivery. Further treatment has focused on physiotherapy and conservative measures such as pacing and activity management.
Neuropathic pain arises as a direct consequence of a lesion or disease affecting the somatosensory system, and is characterized according to four criteria: pain distribution; the link between distribution and history; confirmatory tests of neurologic status demonstrating sensory signs confined to the territory of the lesioned nerve, and further confirmatory diagnostic tests to identify the lesion or disease entity underlying the neuropathic pain3. The history, examination and investigations that we have described fulfill these criteria. We therefore believe that this patient has definite neuropathic pain, although it is manifested only by ‘negative’ symptoms, such as numbness and tingling, with an absence of the ‘positive’ symptoms such as stabbing or burning, but is nonetheless becoming increasingly debilitating.
The Nav1.7 channel plays a crucial role in pain transmission. However this case shows that neuropathic pain can be initiated and maintained in its absence in humans, as well as in knockout mice4, although we cannot rule out that Nav1.7 may mediate sharp, burning or electric shock sensations. Our data provide a further rational basis for seeking specific molecular substrates for neuropathic pain, some of which could act as mechanistic targets for new therapies for patients with symptoms of neuropathic pain.
Comments on this article Comments (0)