Keywords
ADH, SIADH, ANP, hyponatremia
ADH, SIADH, ANP, hyponatremia
The referees pointed out that while the ADH level was "low", it was not zero, and thus the patient likely still had SIADH from ectopic ADH production. We agree with this assessment and have altered the case description.
Previously, we described SIANP as driving the hyponatremia in the absence of SIADH. Now, we have changed my assessment of the clinical situation to one where SIADH likely initiated the hyponatremia but a concomitant SIANP made it much more difficult to correct serum sodium levels. The fact that the patient had an elevated ANP level, in addition to the elevated ADH level, was likely why he was refractory to standard hyponatremia treatment and still makes this case report quite interesting.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
Hyponatremia is commonly found in patients that have been diagnosed with lung cancer – reportedly as high as 15–30% of patients with small cell lung carcinoma (SCLC) present with hyponatremia1,2. While it is clear that water and sodium homeostasis are abnormal in these patients, the complete pathogenesis controlling the hormonal mechanisms of renal sodium and water re-absorption in malignancy has not yet been completely elucidated. However, SCLC is associated with a variety of paraneoplastic syndromes characterized by the ectopic production of peptide hormones or centrally-active antibodies3,4. The most common of these paraneoplastic phenomena is hyponatremia of malignancy, traditionally thought to be caused by elevated levels of arginine vasopressin (AVP), otherwise known as anti-diuretic hormone (ADH), ectopically produced by the neoplastic cells. Inappropriately elevated ADH levels increase the permeability of water in the cells of the distal tubule and collecting duct of the kidney, increasing water re-absorption and decreasing free water clearance, thus resulting in subsequent hyponatremia. This condition is widely known as the syndrome of inappropriate ADH secretion or SIADH2,5,6, and antineoplastic therapy and methods to correct hyponatremia such as fluid and sodium restriction typically constitute effective treatment strategies.
While SIADH is clearly responsible for the majority of cases of hyponatremia of malignancy, there have been documented cases of SCLC patients with hyponatremia, but with no detectable levels of ADH in their plasma7,8 or produced by their cancer cells9–11. These interesting observations led a number of researchers to investigate potential alternative mechanisms to explain the low levels of sodium observed in these cases. An alternative hypothesis based on reports showing ectopic production of atrial natriuretic peptide (ANP) mRNA in tumor lines, would suggest the possibility of an analogous ectopic hormone syndrome: elevated levels of ectopically produced ANP, referred to here as the syndrome of inappropriate ANP or SIANP. This hypothesis has been attractive because ANP is thought to have physiologic effects that promote natriuresis: direct sodium wasting effects on the kidney through ANP receptors, inhibition of renin secretion and inhibition of aldosterone secretion12–14. Despite the evidence for ectopic production of ANP in tumor cell lines, there is no evidence revealing a causal link between ANP and hyponatremia.
Here we report the case of a middle-age man with known small cell carcinoma of the lung, who developed a progressive, profound hyponatremia that was refractory to treatment despite early initiation of strict fluid restriction and ADH-antagonist therapy. Laboratory analysis revealed elevated urine osmoles, low but non-zero levels of ADH, inappropriately low levels of aldosterone, and elevated serum ANP despite no known history of heart failure and no clinical signs of volume overload. We speculate that while the low but inappropriately non-zero levels of ADH likely initiated the hyponatremia, the elevated levels of ANP promoted further sodium wasting and blunted the aldosterone response, making the hyponatremia much more difficult to treat.
A 64-year-old man with limited-stage small cell carcinoma of the lung (Figure 1) undergoing concurrent chemoradiation therapy was admitted to hospital with a pulmonary embolism after collapsing en route to his seventh radiation treatment. At the time of admission, he was at 10 days status after his first cycle of carboplatin/etoposide chemotherapy and he had six treatments of radiation therapy. Admission laboratory values were notable only for a leukopenia and a serum sodium level of 138 mmol/L.
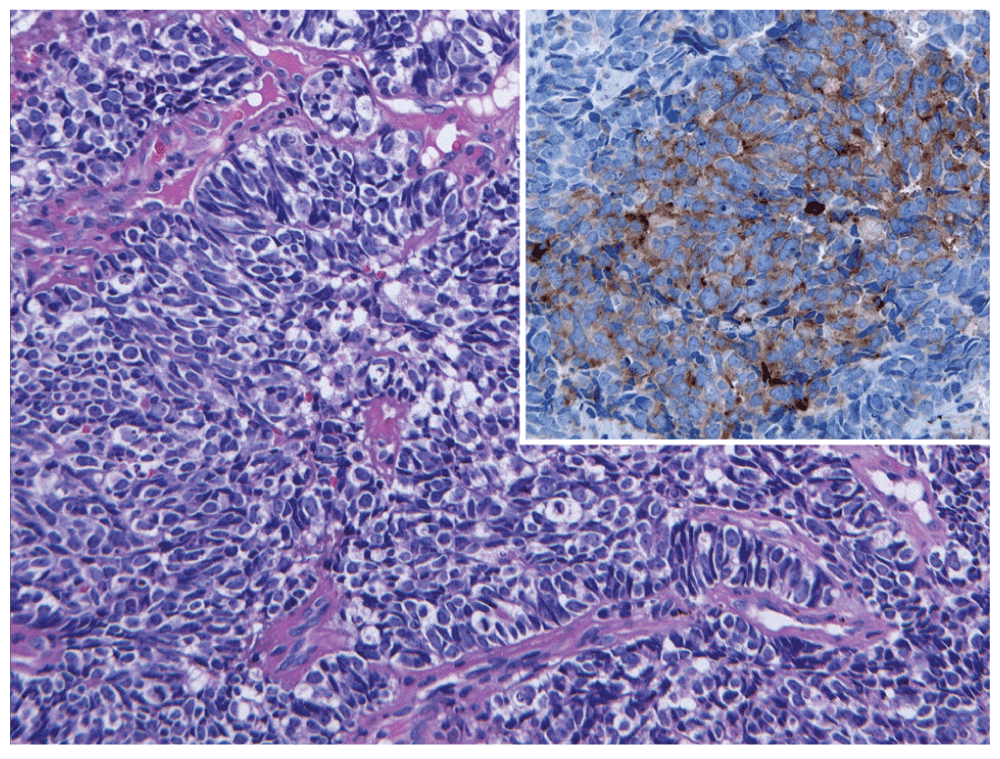
The numerous atypical small cells with minimal cytoplasm are characteristic of metastatic small cell carcinoma. Inset. Immunohistochemical staining is positive for the neuroendocrine marker, synaptophysin, further supporting the diagnosis.
Two months earlier, at the time of his small cell lung cancer diagnosis, he presented with an acute onset altered mental status. A work-up revealed a hypotonic, euvolemic hyponatremia (Na+ 116 mmol/L), with elevated urine osmolality (609 mOsm/Kg) and urine sodium (181 meq/L). He was presumptively diagnosed with SIADH syndrome, and was treated with a mild fluid restriction and ADH antagonist (demeclocycline at 300 mg PO BID) therapy (Figure 2A), which restored his normal serum sodium levels after < 3 weeks of therapy. Upon discharge from the hospital after his initial diagnosis, he was continued on 300 mg PO BID of demeclocycline, which he was taking at the time of the current admission for the pulmonary embolism. At home, he was not observing a fluid restricted diet.
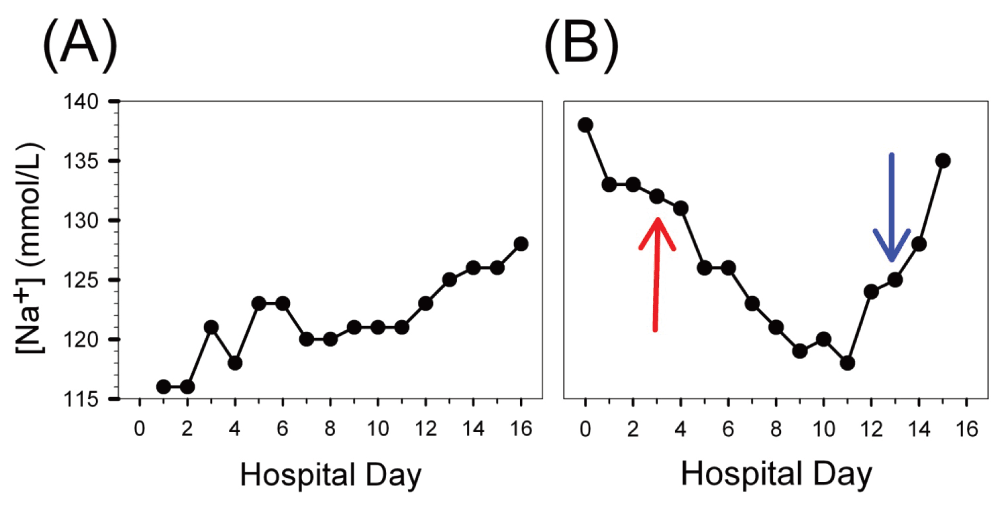
(A) Initial presentation and diagnosis of small cell carcinoma of the lung. (B) Two months later during the current hospitalization when he presented to our service. (A) At the time of initial hospitalization for altered mental status, the patient was found to have a sodium level of 116 and an elevated urine osmolality (609 mOsm/Kg). Mild fluid restriction and demeclocycline treatment resulted in a slow, steady rise in sodium back toward normal, consistent with pure SIADH. (B) On our service, a slow and steady decline in sodium level was observed despite the early initiation of demeclocycline treatment and sodium and fluid restriction (represented by red arrow). Starting on day 3, the patient resumed radiation therapy after going 5 days without receiving radiation. After reaching a minimum of 118 mmol/L, the sodium level began to rise toward normal. AVP, ADH, and aldosterone levels were drawn on day 13, represented by the blue arrow, and revealed an inappropriately non-zero level of ADH in addition to elevated AVP and low aldosterone (Table 1). This is consistent with hyponatremia initially caused by SIADH but made more difficult to treat because of concomitant SIANP.
The inappropriately non-zero ADH level suggests that SIADH likely initiated the hyponatremia but the elevated ANP level and low aldosterone level were making the hyponatremia refractory to treatment, consistent with a concomitant hyponatremia due to SIANP.
While in the hospital for treatment of the pulmonary embolism, his sodium level steadily declined from the admission value of 138 mmol/L to 118 mmol/L over 11 days (Figure 2B), despite severe fluid restriction and increases to maximal doses of demeclocycline (600 mg PO BID, initiated at the red arrow in Figure 2B). Of note, his physical exam remained consistent with a euvolemic hyponatremia – he did not have any evidence of jugular venous distention, pulmonary edema or lower extremity edema. His liver enzymes were within normal limits (AST = 22 IU/L, alkaline phosphatase = 88 IU/L, and total bilirubin = 0.4 mg/dL) and his kidney function remained normal (Cr = 0.7 mg/dL). Further, his thyroid stimulating hormone, free T4 and cortisol levels were normal (Table 1), and his urine osmolality (563 mOsm/Kg) and urine sodium (128 mmol/L) remained high. A computed tomography (CT) scan of the brain revealed no evidence of metastatic disease or intracranial hemorrhage.
During the initial period of hospitalization, the patient was not able to undergo radiation treatments – this therapy resumed on hospital day 3 after having gone five full days without a treatment. Starting on hospital day 12, sodium levels began to increase, returning to 135 mmol/L by day 15. On day 13, when the sodium levels were at 125 mmol/L, laboratory values were drawn in an attempt to qualify the etiology of the hyponatremia: ADH levels were 1.7 pg/mL (low < 7.0 pg/mL), aldosterone levels were < 3 ng/dL (low < 32 ng/dL) and ANP levels were at 140 pg/mL (normal 20 – 77 pg/mL) (see Table 1). The laboratory results, imaging data, and his physical exam findings suggested that neither pure SIADH alone nor any of the other common etiologies (hypovolemia, hypothyroidism, adrenal insufficiency, cerebral salt wasting) could explain this patient’s hyponatremia. Fortunately for the patient, once his sodium level began to trend upward, it stayed in the normal range for the duration of his hospital stay and was 137 mmol/L on discharge. Successful reversal of the hyponatremia was attributed to resumption of his radiation therapy with effective treatment of his malignancy.
In 1957, Schwartz et al. presented the first cases of hyponatremia of malignancy from inappropriate anti-diuretic hormone secretion in two patients with lung cancer who developed low serum sodium levels associated with continued urinary sodium losses15. The authors correctly postulated that the tumors were producing excessive levels of anti-diuretic hormone (vasopressin) via a feedback insensitive mechanism, a hypothesis that was later proven16–18. The same group subsequently went on to further characterize the SIADH and proposed three mechanisms which could theoretically explain the ongoing urinary sodium losses5: (i) an increase in the glomerular filtration rate leading to an increase in the filtered load of sodium, (ii) a suppression of tubular re-absorption of sodium secondary to expansion of the extracellular fluid volume, and (iii) a suppression of aldosterone secretion as a result of the elevated extracellular fluid volume.
It is well now known that ADH acts primarily to insert aquaporin channels into the apical membrane of the distal tubule and collecting ducts of the kidney, increasing the permeability of these cells to water, and allowing its re-absorption and excretion of concentrated urine19–21. This process results in an ADH-induced water retention; the subsequent volume expansion activates the mechanisms for secondary natriuresis mentioned above, resulting in both a sodium and water loss in an attempt to restore euvolemia. With chronic SIADH, sodium loss is much more prominent than water retention and hyponatremia ensues. There has been much discussion on the diagnosis, pathophysiology, and management of hyponatremia secondary to SIADH2,6.
However, it is interesting to note that as many as one-third of patients with documented small cell lung cancer and hyponatremia do not have elevated levels of serum ADH or ectopic production of ADH from their tumor cells1,7,22,23, leading some to propose the more appropriate umbrella term syndrome of inappropriate antidiuresis (SIAD), while reserving the term SIADH for only those cases where ADH levels are actually elevated6,24. An alternative etiology for hyponatremia of malignancy in patients without elevated ADH levels includes ectopic production of ANP. In the case presented in this report, we postulate that although ectopic ADH may have initially caused the patient’s hyponatremia, sodium levels were refractory to correction because of concomitant production of ectropic ANP. As mentioned above, there is evidence that ectopic production of ANP can contribute to hyponatremia.
ANP is a 28 amino acid peptide secreted from the atrial myocytes in response to local wall stretch and increased atrial volume, but also found in the hypothalamus, brainstem nuclei, pituitary and vascular tissue, kidney, and adrenal medulla12. It was identified and characterized after de Bold et al. demonstrated that a cardiac extract produced natriuresis in rats25. While the details surrounding all the physiologic roles of ANP are still under discussion, it seems that renal, vascular, and cardiac actions are important in maintaining the body fluid and sodium homeostasis12,13. The physiological effects of ANP are summarized in Figure 3. Overall, the main function of ANP is to counter increases in blood pressure and volume induced by the activation of the renin-angiotensin system. As such, the actions of ANP result in natriuresis.
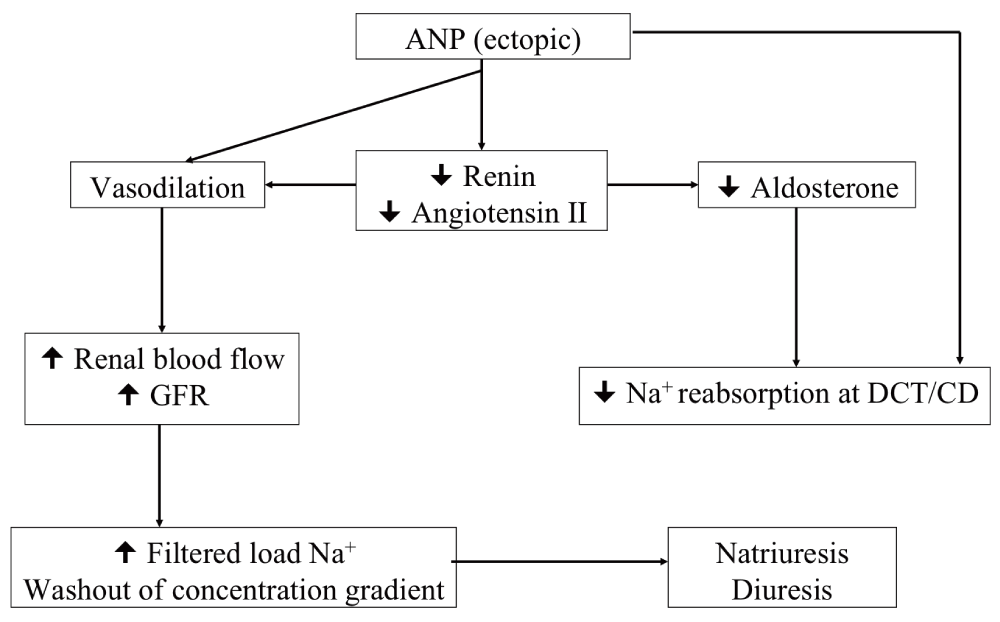
In response to local wall stretch and increased atrial volume, atrial myocytes secrete ANP, which dilates smooth muscle and blocks the action of norepinephrine and angiotensin II, causing a prolonged increase in glomerular filtration rate (GFR). This causes afferent arteriolar dilation with or without efferent arteriolar constriction and the increased GFR increases the filtered load of sodium, washing out the sodium gradient. It has also been demonstrated that ANP directly increases renal excretion of sodium, acting through ANP receptors in the kidney at the distal convoluted tubule (DCT) and the collecting duct (CD). ANP also directly decreases production of renin, angiotensin II and aldosterone contributing to hyponatremia through natriuresis, negative sodium balance, and non-osmotic release of arginine vasopressin (AVP) (from decreased intravascular volume).
This is accomplished through three distinct but inter-related mechanisms. First, ANP is known to dilate smooth muscle in arterioles and venules and block the action of norepinephrine, causing vasodilatation and increased renal blood flow12,13. This effectively increases the glomerular filtration rate (GFR) in the kidney leading to increases in the filtered load of sodium and subsequent natriuresis13. Radionucleotide studies performed in humans support these results by showing both a significant decrease in the mean blood pressure as well as an increase in GFR after intravenous infusion of ANP peptide26. Second, ANP acts directly on ANP receptors in the kidney to increase renal excretion of sodium9,12. This is thought to occur via receptor-induced increases in cyclic GMP which directly increases GFR, inhibits secretion of renin, and reduces sodium and water re-absorption in the collecting duct9,13. These data have been supported by experiments in rats that demonstrated that urine flow and sodium excretion decreases while plasma renin increases after administration of antibodies raised against ANP27. Finally, it is thought that ANP directly blocks the production of renin, angiotensin II, and aldosterone, as these hormones showed decreased levels following ANP intravenous infusions12,14,28. Suppression of aldosterone secretion from the adrenal medulla is yet another mechanism by which ANP can contribute to natriuresis and subsequent hyponatremia.
These physiologic effects suggest that ANP could feasibly be implicated to cause hyponatremia of malignancy without ectopic production of ADH or to perhaps worsen hyponatremia in cases where elevated ectopic ADH is also present. Further support for this hypothesis arose when researchers demonstrated ectopic production of ANP mRNA in both tumors and tumor cells of patients with hyponatremia of malignancy from small cell carcinoma. This has been shown using a number of techniques including northern blot, PCR, and nuclease protection assays1,7,23, and the ANP peptide itself has been detected in small cell lung cancer tumors7,22,29,30. Further, there are reports of the resolution of clinical hyponatremia following surgical resection of an ANP expressing tumor22. Interestingly, plasma levels of ANP have also been found to be elevated in patients with lung carcinoma and elevated plasma levels of ADH1,22,30. A recent case report presented a patient with SCLC, elevated levels of ANP, and elevated levels of ADH that varied in an oscillatory manner31.
Despite the reports demonstrating the association of ectopic ANP production and hyponatremia, there is still not overwhelming evidence proving a causal relationship. An early prospective study trying to establish whether ectopically produced ANP could contribute to hyponatremia through natriuresis failed to show a correlation between plasma ANP levels and levels of plasma renin, angiotensin II, and aldosterone. The authors concluded that ANP was unlikely to contribute to hyponatremia via suppression of the renin-angiotensin II-aldosterone axis1. In this study, there were 14 patients with elevated levels of ANP but only three had small cell carcinoma associated with hyponatremia. It is not clear why no correlation was found, however, the same group later published a study where four patients with SCLC and elevated ANP levels had inappropriately low levels of aldosterone9. In this second study, the patients had persistent natriuresis and low serum aldosterone despite decreasing serum sodium levels while being treated with a fluid- and sodium-restricted diet. Thus, the normal physiological aldosterone response failed to occur in patients with elevated ANP9. From this study, the authors were able to make three major conclusions: (i) hyponatremia in SCLC patients is often associated with inappropriate elevations of ANP instead of elevations in AVP; (ii) SCLC patients with elevated level of ANP do not seem to respond to fluid restriction, and in fact, fluid restriction may worsen the hyponatremia; and (iii) ANP appears to mediate some of its natriuretic effects through suppression of aldosterone. Interestingly, the patient presented in this manuscript displayed a disease process that exactly fits this description: elevated levels of ANP, a worsening hyponatremia despite strict fluid and sodium restriction, and inappropriately low levels of aldosterone.
Hyponatremia potentially caused or worsened by SIANP should not be expected to respond to the traditional treatments used for pure SIADH. As discussed above, water restriction, beneficial in SIADH, may worsen the hyponatremia of SIANP if sodium intake is not increased concurrently. Likewise, if ADH levels are already suppressed in SIANP, ADH antagonists such as demeclocycline or conivaptan will have no benefits. To date, no ANP antagonists have been developed and there is no specific treatment for SIANP except for addressing the underlying cause. However, it is still important to distinguish between pure SIANP and pure SIADH or to determine if they are both present. Certainly, any patient presenting with hyponatremia from SCLC should initially be placed on fluid and sodium restriction because SIADH seems to be more common. However, if the hyponatremia continues to worsen after 48–96 hours, alternative etiologies including ectopic ANP production should be considered and plasma levels of ANP and AVP should be measured to confirm the underlying etiology of the hyponatremia. It is very important to note that in the current case, the correction of hyponatremia starting on hospital day 12 was temporally associated with appropriate tumor response to radiation therapy and, perhaps, an associated reduction in circulating levels of ectopic ANP (and ADH). This suggests that for the time being, in the cases of SIANP, early treatment of the underlying malignancy might be the best way to correct the underlying hyponatremia.
SEM admitted the patient, challenged the original diagnosis of SIADH, and made the diagnosis of SIANP. SEM made all the figures and wrote the manuscript. DIK and DK were the Attending Physicians on service for the care of this patient. DIK and DK helped design Figure 3. All authors read and approved the final content of the manuscript.
We thank Dr. André M. Mansoor, Dr. Michael Bliziotes, Dr. Lynn Loriaux, and Dr. David B. Jacoby for the critical reading of this manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
References
1. Fenske WK, Christ-Crain M, Hörning A, Simet J, et al.: A copeptin-based classification of the osmoregulatory defects in the syndrome of inappropriate antidiuresis.J Am Soc Nephrol. 2014; 25 (10): 2376-83 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 16 Sep 14 |
read | read | |
|
Version 1 14 Aug 14 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)