Keywords
human embryonic stem cells, neural differentiation, electrophysiology, neuronal maturation, synaptic activity, action potential.
human embryonic stem cells, neural differentiation, electrophysiology, neuronal maturation, synaptic activity, action potential.
Only minor changes were made according to the comments by the referees - see the first part of the Results in the description of the morphology of the neurons and the last part of the Conclusions, at the end of the Discussion section.
See the authors' detailed response to the review by Shauna Yuan
See the authors' detailed response to the review by Maija Castren
In-vitro neural differentiation (IVND) of human pluripotent stem cells (hPSCs), is a promising vehicle for disease modeling and regenerative medicine1–3. Several protocols for IVND of hPSCs including embryonic stem cells (hESCs) or induced pluripotent stem cells (hiPSCs), are used worldwide, resulting in different neuronal types4,5. Upon IVND, confirmation of the neuronal fate is commonly analyzed by the expression of neuron-specific genes, including those of cytoskeletal components (e.g.; TUJ1, MAP2), transcription factors (e.g.; NeuN, NeuroD1) and synaptic proteins (e.g.; synaptotagmin, synaptophysin)6. The expression of these genes indicates that the cell has acquired the machineries needed to build a neuron, but the ultimate indication that these are genuine neurons involves the analysis of their electrical properties. Most studies that do include electrophysiological recordings of hPSCs-derived neurons focus at one specific final time-point along the differentiation cascade, to demonstrate the neuronal identity that is related to the disease in study7–9. However, the analysis of the dynamics of electrical maturation at several time points can provide valuable insights into the pathology of neurodevelopmental disorders. Moreover, in the context of human embryonic neurogenesis, analyzing electrical maturation on hPSCs during IVND can shed light over molecular and cellular mechanisms that so far have been studied only using animal models.
Currently, only a few studies on IVND of hPSCs employed electrophysiological recordings at consecutive time points during neural differentiation, so far with inconsistent results, in terms of timing, frequency of action potentials and formation of spontaneous synaptic activity10–13. Although the timing of the development of neuronal electrical properties following IVND is extremely important, it currently remains poorly understood due to high variability in differentiation protocols, culture conditions and hPSC lines used. For this reason, the aim of this study was to systematically analyze, by electrophysiological tools, the developing neurons derived in-vitro from hESCs, in order to define predictive parameters for their electrical maturity, as well as to model the dynamics of neural development.
hESCs culture conditions and IVND: The hESC line HUES-13 (kindly provided by the Melton Lab, Harvard University), was used in all experiments. hESCs were cultured on feeder layers of mitomycin C (Sigma)-inactivated mouse embryonic fibroblasts in hES-medium supplemented with bFGF (R&D), as previously described7. Before induction of IVND, hESCs were cultured on Matrigel (BD)-coated wells for two passages. The dual SMAD inhibition IVND protocol was applied as previously described14, including minor modifications. Figure 1A illustrates the actual IVND protocol used. Briefly, neural induction was achieved by gradually changing the medium from hES to N2 while adding dorsomorphin and SB431542 for 10 days; neuronal induction was achieved by changing the medium to N2/B27 and adding BDNF, GDNF, ascorbic acid, dbcAMP and DAPT for 10 additional days. At day 20 cells were dissociated using Accutase (Life Tech.) and seeded on 13 mm glass coverslips previously coated with 50 µg/ml Poly-D-Lysine and 20 µg/ml Laminin (Sigma). Seeding density was ~1.0×105 cells/cm2. From day 20 and on, neurons were continuously grown in N2/B27 medium supplemented with 20 ng/ml BDNF, GDNF and NT3. Concentrations of reagents and growth factors used were as follows: 5 µM dorsomorphin (Stemgent), 10 µM SB431542 (Stemgent), 20 ng/ml BDNF (PeproTech), 20 ng/ml GDNF (PeproTech), 0.2 mM ascorbic acid (Sigma), 0.5 mM dbcAMP (Sigma), 10 µM DAPT (Tocris), 20 ng/ml NT3 (PeproTech). N2 medium was composed of DMEM:F12 (Life Tech.), supplemented with 1% N2 (Life Tech.), 1% non-essential amino acids (BioInd.), 1% Glutamax (Life Tech.) and 100 µg/ml Primocin (InvivoGen). N2/B27 Medium was a 1:1 mixture of N2 and B27 media. B27 medium was composed of Neurobasal (Life Tech.), supplemented with 1% B27 (Life Tech.), 1% non-essential amino acids (BioInd.), 1% Glutamax (Life Tech.) and 100 µg/ml Primocin (InvivoGen).
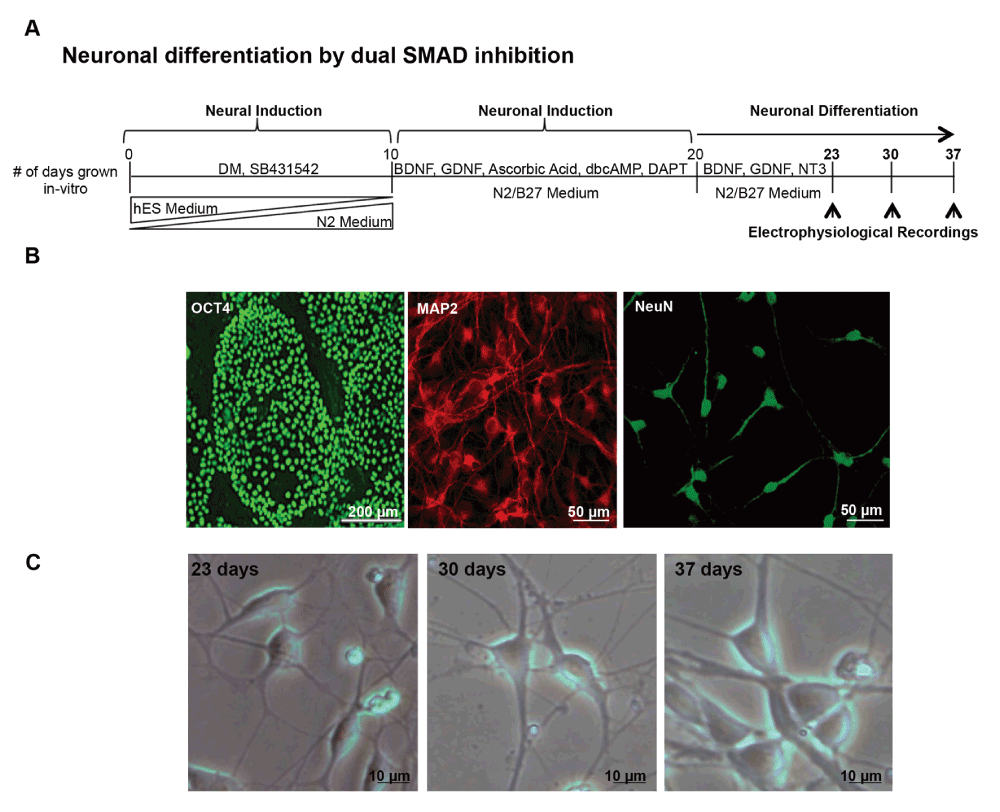
(A) Schematic representation of the protocol for in-vitro neural differentiation using dual SMAD inhibition. The protocol includes 3 steps: 1) Neural induction (days 0–10), by blocking SMAD signaling using Dorsomorphin (DM) and the TGF-β inhibitor SB431542 while reducing the relative amounts of hES medium and concomitantly increasing the relative amounts of N2 medium. 2) Neuronal induction (days 10–20), by incubating cells with brain-derived neurotrophic factor (BDNF), glia-derived neurotrophic factor (GDNF), ascorbic acid, dibutyryl-cyclic-AMP (dbcAMP) and the NOTCH1-inhibitor DAPT; 3) Neuronal differentiation (days 20–37), by dissociating cells and re-plating them on poly-lysine/laminin-coated glass coverslips in the presence of BDNF, GDNF and neurotrophin 3 (NT3). The specific time-points for electrophysiological recordings are indicated with arrows. (B) Representative images of HUES-13 hESC line undifferentiated colonies stained positive for OCT4 (green); hESCs-derived neurons at day 21 stained positive for MAP2 (red) and hESCs-derived neurons at day 37 stained positive for NeuN (green). (C) Bright field representative images of neurons at days 23, 30 and 37 of in-vitro neural differentiation.
Cell patch-clamp: Electrophysiological recordings were conducted as previously described7. In brief, neurons on glass coverslips were transferred to a custom-made recording chamber adapted for an inverted microscope (Olympus XI-50), in standard recording medium, containing (in mM):10 HEPES, 4 KCl, 2 CaCl2, 1 MgCl2, 139 NaCl, 10 D-glucose (340 mOsm, pH 7.4). Cells were patch-clamped with glass pipettes (Sutter Instruments, 1.5 mm OD, 0.75 mm ID), pulled using a P87 Puller (Sutter Instruments). Pipettes contained intracellular medium composed of (in mM) 136 K-gluconate,10 KCl, 5 NaCl,10 HEPES, 0.1 EGTA, 0.3 Na-GTP, 1 Mg-ATP, and 5 phosphocreatine, pH 7.2 (pipette tip resistance was 5–8 MΩ). Action potentials were evoked (in current clamp mode) by injecting depolarizing current pulses. Membrane potential was held at -60 mV. Spontaneous synaptic currents were recorded in 2 minute sessions in voltage clamp mode with a 50 µs sampling rate. Signals were amplified with a Multiclamp 700B amplifier and recorded with Clampex9.2 software (Axon Instruments). Data were subjected to a 500-Hz low-pass filter and analyzed using Clampfit-9 and SigmaPlot.
Immunofluorescence: Immunostaining was performed as previously described7. Briefly, cells were fixated for 15 minutes at R.T. using Cytofix (BD) and washed with PBS. Primary antibodies were applied at 4°C, overnight, in a PBS solution containing 2.5% BSA and 0.1% Triton. Staining with secondary antibody was performed for 1 hour at R.T., in the dark. The pluripotent gene Oct4 was detected with monoclonal mouse anti-human OCT4 (Santa Cruz, #sc-5279, RRID: AB_628051, Lot C1308, dilution – 1:200). Neurons were stained using polyclonal rabbit anti-human MAP2 (Santa Cruz, #sc-20172, RRID: AB_2250101, Lot D2710, dilution 1:250), and monoclonal mouse anti-human NeuN (GeneTex, #GTX30773, RRID: AB_1949456, Lot 27334, dilution 1:20). Primary antibodies were detected using sheep anti-mouse Cy2-conjugated and goat anti-rabbit Cy3-conjugated secondary antibodies (Jackson Labs).
Imaging: Bright field and fluorescence images of cells were obtained using an Olympus IX51 inverted light microscope, and a Zeiss LSM 700 confocal microscope. Images were processed using Olympus CellA for XP (2006) and ImageJ (NIH, v. 1.49) software.
Statistical analysis: Data were collected from 15–25 cells for each time point, in 2 different experiments. ANOVA was performed on data using SPSS (v. 19).
We have used a slightly modified version of an established protocol for IVND of hESCs5,14, that is based on the dual inhibition of the SMAD pathway (Figure 1A). SMADs are the mammalian homologues to drosophila mad and C. elegans sma, and function as cytoplasmic mediators of TGFβ signaling15. The process of IVND implemented here includes three major steps: (i) Neural induction by blocking SMAD signaling using Dorsomorphin and SB43154216,17; (ii) Neuronal induction by incubating cells with pro-neuronal factors (BDNF, GDNF, ascorbic acid, dbcAMP) and the NOTCH1-inhibitor DAPT18; (iii) Neuronal differentiation, by dissociating cells and re-plating them on poly-lysine/laminin-coated glass coverslips in the presence of BDNF, GDNF and NT3. Our hESCs selected for IVND were pluripotent as shown by the expression of OCT4 and the neurons derived were stained positive for MAP2 (>90% of cells), already at day 21 of IVND (Figure 1B). At day 37, >90% of cells expressed the neuronal transcription factor NeuN (Figure 1B). Typical neuronal morphology with 10–15 µm phase bright somata displaying pyramidal shape, extending an ‘apical’ dendrite and a few ‘basal’ dendrites was visible already at day 21 (MAP2 and NeuN in Figure 1B and Figure 1C). Importantly, the neurons did not undergo any significant morphological changes during later stages of differentiation (Figure 1C, days 23, 30 and 37). Taken together, these results suggest that the cells analyzed during the three recording time-points (Figure 1A) are probably early human embryonic neurons, which are practically impossible to study in-vivo.
Current clamp recordings of neurons at days 23, 30 and 37 of IVND (corresponding to days 3, 10 and 17 days following induction of neuronal differentiation), showed a steady increase in the excitability of hESCs-derived neurons (Figure 2A), while their input resistance remained similar for every time-point (Figure 2B-input resistance). At days 23 and 30, these neurons could discharge only single action potentials (APs; 21 and 25 neurons were recorded at days 23 and 30 respectively). However, multiple spikes were observed in all neurons recorded at day 37 (Figure 2A, B-spike frequency; 23 neurons were recorded). In addition, spike amplitude was significantly increased at day 30 and 37 as compared to day 23. Spike duration (measured at half-width of the action potential (AP), became shorter with prolonged differentiation, from 3.31±0.16 msec at day 23, to 2.57±0.10 msec at day 30, to a mean duration of 1.95±0.20 msec at 37 days (Figure 2B). On the other hand, spike threshold was significantly lower for day 37 only. After hyperpolarization (AHP) potentials were not detected at day 23, but were present at day 30 and 37, in which the drop from spike threshold was ~-7.9 mV for both time-points. These results clearly show a steady ongoing process of electrical maturation for human in-vitro developing neurons, in which sequential firing of multiple spikes is achieved by day 37 following induction of IVND with the dual SMAD inhibition protocol. Furthermore, our results show that spike frequency, amplitude, duration, threshold and after hyperpolarization can serve as the best predictive measurements for electrical maturation.
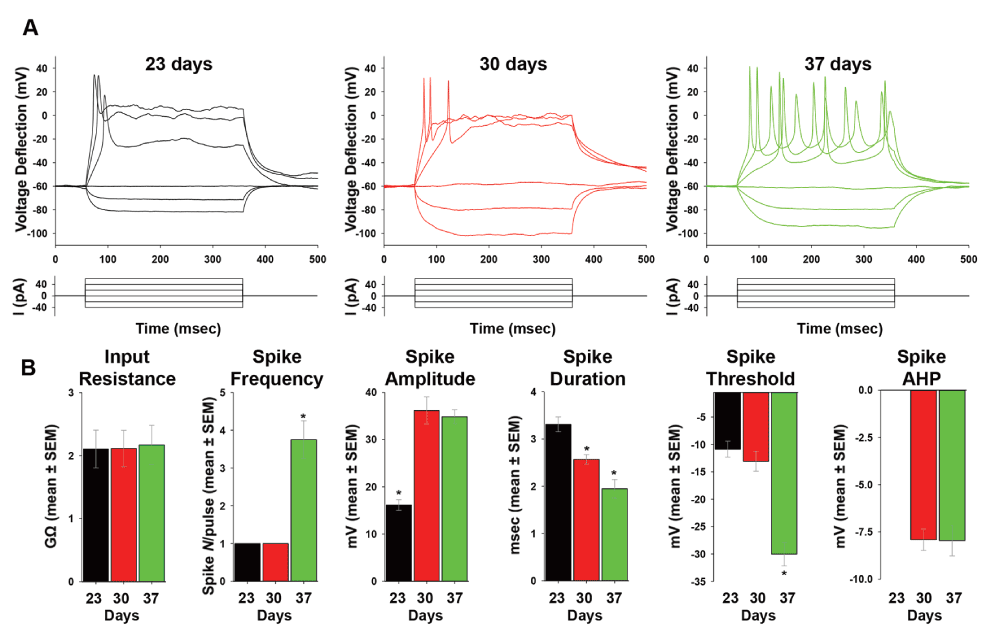
(A) Representative traces for current clamp recordings at day 23 (black), 30 (red) and 37 (green). Membrane potential was held at ~-60 mV and voltage deflections (mV) are shown following 6 consecutive pulses of ~20 pA current injection from -40 to +60 pA. (B) Data analysis showing (from left to right): input resistance (GΩ), spike frequency (spike number per pulse), spike amplitude (mV), spike duration (msec), spike threshold (mV) and spike after hyperpolarization (‘AHP’, mV), for the same recordings days as in (A). Sample size: n=21 at day 23; n=25 at day 30; n=23 at day 37. Values are mean ± SEM. *P<0.05, ANOVA.
In order to further explore the dynamics of time-dependent development of spike discharges, we examined properties of the derived neurons in voltage clamp mode at the same time-points listed above (Figure 3A). We measured K+ currents evoked by successive 20 mV voltage commands. The results show a significant difference between day 23 and 30 as compared to day 37 in the I-V curves of both the transient K+ (IA) current and the sustained (IK) current (Figure 3B; number of neurons recorded in each day was 21, 25 and 23 respectively). The sigmoid regression function that fits these curves is given by the equation: = , where ‘a’ is the slope of the curve. Our calculations show that the slope of the curve in IK increased from 1.36 (day 23), to 1.64 (day 30), and finally to 2.34 (day 37). Moreover, the value of a in IA increased from 1.49 (day 23), to 2.01 (day 30), and finally to 3.50 (day 37). This robust increase in the slope of both K+ currents from day 30 to day 37 is indicative that during this period the membrane of hESCs-derived neurons probably undergoes important changes in their expression of K+ channels. In addition to the parameters we have measured in current-clamp for APs characteristics, the slope of I-V curves of K+ currents could also be used as a tool to measure electrical maturity during IVND of hPSCs.
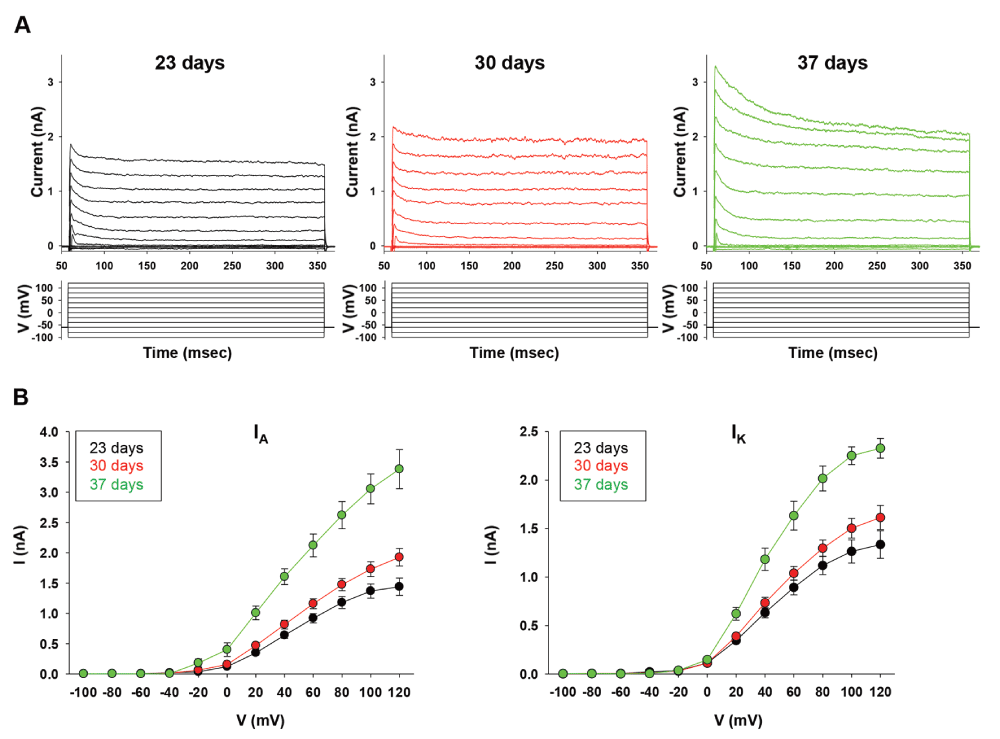
(A) Representative traces for voltage clamp recordings at day 23 (black), 30 (red) and 37 (green). Membrane potential was held at -60 mV and current (nA) is shown following 12 consecutive pulses of 20 mV voltage steps from -100 to +60 mV. (B) I-V curves for IA and IK currents for the same time-points as in (A). Sample size: n=21 at day 23; n=25 at day 30; n=23 at day 37. Values are mean ± SEM.
Finally, we examined the time-course of formation of spontaneous synaptic currents, indicating active synaptic connections (Figure 4A). No synaptic activity was found in any tested cell at day 23 (n=19). However, ~50% of neurons at day 30 and 37 showed spontaneous synaptic activity, which increased from day 30 to day 37 in both frequency and current amplitudes (number of neurons recorded was 18 and 23 respectively) (Figure 4B). No attempt was made to distinguish between excitatory and inhibitory synaptic currents in the present study. At day 37, spontaneous synaptic currents had a mean rise time of 1.84±0.16 msec, and their mean decay time was 3.25±0.71 msec. These results suggest that the capability of in-vitro hESCs-derived neurons to develop synaptic connections can arise at a relatively early time-point during IVND, and is concomitant with the developmental timing of burst firing. Furthermore, these results indicate that electrical maturation involves the development of intrinsic properties in individual neurons, in parallel with the development of network-activity.
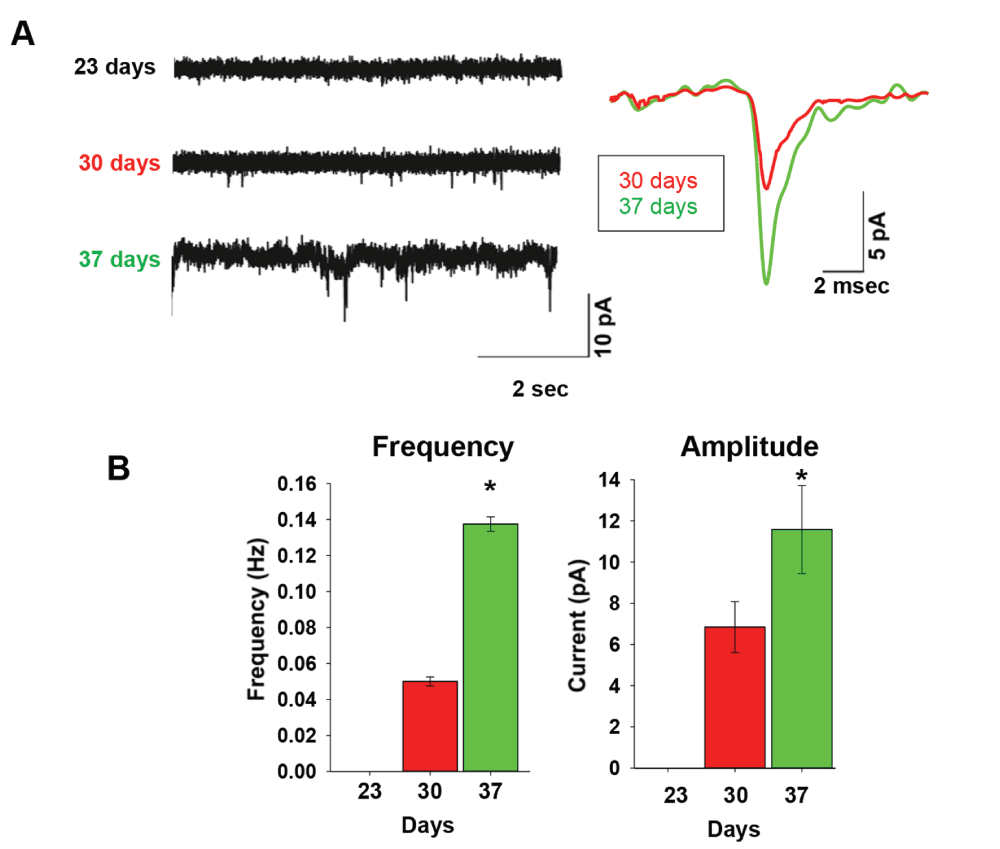
(A) Representative traces for spontaneous synaptic currents recorded in voltage clamp mode at -60 mV for days 23, 30 and 37. (B) Analysis of spontaneous synaptic currents frequency (Hz) and amplitude (pA). Sample size: n=19 at day 23; n=18 at day 30; n=23 at day 37. Values are mean ± SEM. *P<0.05, ANOVA.
Currently, there is no standard protocol to analyze the developmental stage in which all the required electrophysiological properties for proper function are already present in neurons derived by IVND of hPSCs. Several studies provide electrophysiological data on hPSCs-derived neurons but they do so only for a single specific end-point of the process, in order to compare control neurons to diseased ones7–9,19–26. However, the dynamic of electrical maturation at several time-points along the process has not been extensively investigated, and currently there are only a few studies which addressed this question, with inconsistent results10–13,27–29. Here, we have analyzed systematically the electrical maturation of human neurons derived from hESCs. We show that, by applying the dual SMAD inhibition in the IVND protocol on hESCs, early embryonic neurons are generated demonstrating electrical maturation already by day 37, including firing of spike bursts with increased amplitude and reduced duration. Our results further show that this electrical maturation is accompanied by a steady increase in K+ currents, which enabled faster and more reliable repolarization. Moreover, a steady and gradual increase in spontaneous synaptic activity is observed at the same three time-points, suggesting that electrical maturation occurs not in individual neuron, but also in the developing neuronal networks. Nevertheless, no spontaneous action potential discharges could be detected, indicating that the network is still not fully functional.
As shown here, the neurons we derived could fire trains of APs not before day 37 (~5 weeks) of IVND. Indeed, a study in which the same dual SMAD inhibition protocol was used to generate neurons, spike bursts were measured already by week 4, but this was observed only in ~40% of the hESCs-derived neurons12, as compared to our results demonstrating spike bursts in 100% of the neurons at the same time. In comparison, other studies have shown that when dopaminergic neurons or GABAergic interneurons were derived from hPSCs, a similar phenomenon of time-dependent electrical maturation was observed, but only following >8 weeks of IVND11,13,28. Furthermore, other IVND protocols applied on hESCs, showed no incidence of burst firing and no significant differences between time-points in APs parameters10,27. Our results indicate that measurement of spike frequency, amplitude, duration, threshold and after hyperpolarization can serve as predictive parameters for electrical maturity. Interestingly, spike duration was found to be the most reliable predictor parameter, and its measurement at each time-point tested. Similar to APs, K+ currents reflect the process of electrical maturation in a time-dependent manner. In addition, we have established that the slope of IK and IA steadily increases with time. Indeed, in the study of Takazawa et al., 2012, hESCs-derived spinal motor neurons demonstrating bursts of multiple APs at day 36, also showed a time-dependent maturation in the transient and in the sustained K+ currents (IA and IK, respectively,29). Furthermore, when neural differentiation through dual SMAD inhibition was performed to produce forebrain neurons, as we have done in this study, a time-dependent increase in the amplitude of both IA and IK was indeed shown during the first 4 weeks12. In contrast, Nicholas et al., 2013 showed in hPSCs-derived GABAergic interneurons a steady increase of an unspecified K+ current, reaching a maximal peak of ~1.5 nA only after 30 weeks of IVND13, but Hartfield et al., 2014 showed no significant changes between relevant time-points in the average peak amplitude of K+ currents in hPSCs-derived dopaminergic neurons27. These observations indicate that development of K+ currents takes place during the earliest stages of electrical maturation. However, more research is needed to understand how and when these K+ channels are expressed on the membranes of in-vitro developing neurons, and whether the increase in their current is caused by an increase in their density throughout the membrane or by maturation of their intrinsic activation properties.
We have shown here time-dependent development of spontaneous synaptic activity, at a relatively early time-point during IVND, and concomitant with the developmental timing of burst firing, indicating that electrical maturation involves the development of intrinsic properties in individual neurons, in parallel with the development of network-activity. Other studies however, have shown that although action potential can be produced at earlier stages of differentiation, the generation of neuronal networks as evidenced by spontaneous synaptic activity, is observed only at later stages of IVND, (at least 8 weeks of differentiation into GABAergic forebrain interneurons13,28, or 10 weeks of differentiation into dopaminergic midbrain neurons11). It has been suggested that IVND of hESCs produces immature embryonic-like neuronal cells, which take several months to develop the characteristic genetic and electrophysiological properties of mature adult-like neurons30,31. Others have proposed that IVND of hPSCs mimic the real time-frame of in-vivo human embryonic neurogenesis, due to an “intrinsic clock-like mechanisms”32. It is commonly accepted that synaptogenesis in humans starts only by the end of fetal life and during the first months of postnatal life6,33. Nevertheless, here we show significant spontaneous synaptic activity already by day 37, suggesting that in-vitro conditions results in an accelerated rate of maturation and development. The different timing of neuronal functional maturation observed in the different studies could be explained by high variability in cultures due to the different hPSCs used, different IVND protocols, different seeding density of cells, and other factors related to the IVND protocol used.
In conclusion, our results shed light on the dynamic development of the electrophysiological properties of individual neurons as well as in-vitro neuronal networks. Furthermore, these findings suggest critical electrophysiological parameters that can be used to predict the precise timing in which neuronal functionality is acquired by human cells developing in-vitro. Therefore, the results of the present study provide a valuable tool for the direct measurement of electrical maturity, which can be implemented when studying neurodevelopmental and neurodegenerative diseases. Timing of electrical maturation can greatly vary among different cell lines of hESCs and hiPSCs, as well as between different protocols for IVND. Therefore, analysis of the parameters proposed in this study, which are universal for neuronal electrical activity and easy to reproduce, could serve to calibrate and adjust the time-course of electrical maturation in every cell line and for every protocol.
figshare: Data files electrical maturation of neurons derived from human embryonic stem cells doi: 10.6084/m9.figshare.113247534
M.S. and D.B.Y. designed the study, wrote the manuscript and approved it. M.T. performed the experiments, collected and analyzed the data and wrote the manuscript.
M.T. was supported by the TEVA National Network of Excellency scholarship in neuroscience.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Ms. Efrat Biton (Weizmann Inst.) for technical assistance and Dr. Michael Boland (Scripps Ins.) for valuable advice in differentiation procedures.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 01 Oct 14 |
||
|
Version 1 19 Aug 14 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)