Introduction
Currently (as of April 3, 2014)1 there exist more than 72000 (as of April 3, 2014) experimentally determined protein structures complexed with small molecule ligands, providing an extensive data resource on protein binding sites. These binding sites vary in size ranging from six to thirty residues depending upon the size and the nature of the ligand. In most cases, the contribution of the individual amino acids towards the binding of a given ligand is not well understood. A well-established method of demonstrating the importance of a residue at the site is to create point mutants through site-directed mutagenesis2. Efforts towards characterization of entire functional site include tools such as alanine scanning mutagenesis (ASM)3, where each residue is mutated to an alanine and its effect on the function is evaluated. ASM is indeed a well-used technique in experimental biology and has been successfully applied to the problems of protein folding and stability4, protein-protein5,6, and protein-ligand7 interactions. The experimental success of this technique has resulted in further developments, including high-throughput and low-cost variants8, greatly expanding its reach. Yet, given the time, cost and effort required for carrying out experimental biochemistry, a large majority of proteins are yet to be studied through this method.
Due to availability of a variety of structural bioinformatics tools, it is now feasible to carry out alanine scanning mutagenesis computationally9. Spurred by the successes and widespread adoption of the ASM technique, various computational resources now exist for in-silico alanine scanning. Prominent examples include Modeller10 and the Rosetta software suite11. However, most packages are command-line oriented and are out of reach for researchers. Alanine scanning webservers with intuitive user interfaces such as Robetta webserver12, the Rosetta Design web-server13, ROSIE14, FOLDX15, BeATMuSiC16, exist for the problems of protein folding, protein stability and protein-protein interactions. Although, there are workflows to evaluate ligand-binding energetics which require significant computational time and setup through free-energy calculations involving Molecular Mechanics/Generalized Born Surface Area method (MM-GBSA)17,18, there is however, no intuitive web-tool available for analyzing alanine-scanning mutations of small-molecule binding site residues in real time. A common requirement for an experimental biochemist is to identify which amino acids to mutate in the protein to generate loss-of-function mutants. A web-tool to cater to that specific need will therefore be highly useful. The analysis will also provide deep insights into critical residues for interaction, residue pairs or sets that when mutated will abolish ligand binding and provide analytical insights for lead refinement in the process of drug discovery, as well understand drug resistance due to mutations.
We present a computational workflow and webserver, Alanine Binding Site-Scan (ABS-Scan), for automated alanine-scanning mutagenesis of protein-ligand interface residues. The workflow combines the libraries of widely used software packages including Modeller10 for site-specific alanine mutagenesis and Autodock19 for energetic evaluation of protein-ligand complexes.
Workflow
This workflow allows a user to submit a protein-ligand complex of their interest (Figure 1). The user is provided with an option of selecting a distance cut-off to define the binding site around a specific ligand for which, in-silico alanine scanning mutagenesis is carried out. Once the input parameters are obtained, the Modeller library is used to perform site-specific mutagenesis on all selected residues, coupled with steps of energy minimization. Each mutated structure, will then be scored by using Autodock 4.1 force field, to calculate the energetics of a protein-ligand complex. The essentiality of a residue can be determined by difference in interaction score of mutant and wild-type protein (∆∆G value). These results are graphically presented to the user, along with a ranked list of residues in the given site that could be experimentally explored for site-directed mutagenesis. A Jmol applet displays protein-ligand interactions with residues colored according to the computed extents of contribution towards interaction, while a table simultaneously displays inter-molecular energy scores. We also provide a help-section explaining the results along with selected examples.
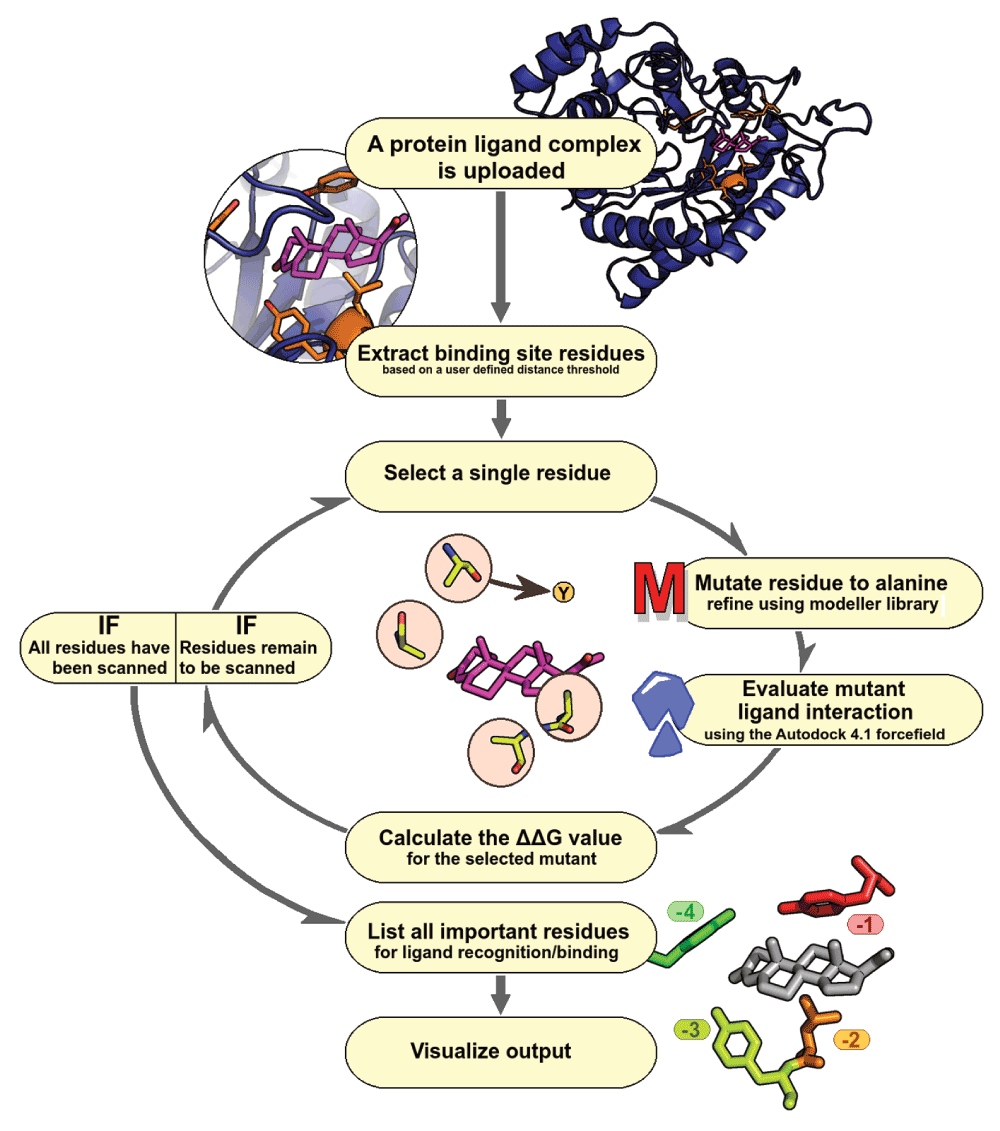
Figure 1. ABS-Scan workflow.
Flowchart depicting various steps involved in ABS-Scan.
Validation
Mainly two types of validation were carried out, first to find a correlation with experimentally determined binding affinities followed by sensitivity evaluation of the predicted ABScan ∆∆G scores. The first exercise involved systematically mining the available experimental information related to alanine-scanning mutagenesis of binding site residues. A methodical search was carried out to mine all the experimental results available in literature on alanine-scanning mutagenesis of residues at the binding site. Advanced search option in PDB was used for this purpose. All the PUBMED extracts were scanned for the term - “alanine scanning”. The results obtained were further filtered to contain only X-Ray experimental data, and abscence of any DNA, RNA or DNA/RNA hybrid in the PDB entity. The results were further restricted to only those entries that had ligands bound to them, and we expected that this would reduce the hits that contain alanine-scanning mutations for evaluating protein-protein interfaces. The above search criteria mentioned yielded 126 structure hits with 56 citations. The list of entries obtained, was further pruned to remove biologically irrelevant ligands, metal ions and modified residues. The list of 79 entries that we finally obtained can be accessed at http://proline.biochem.iisc.ernet.in/abscan/validation. Each of the above experiments involving alanine-scanning mutagenesis reports different mutant evaluation scores. The measures reported to test the fitness of the mutants include various attributes such as Kd, Ka, kcat/KM (for enzymes), specific substrate/product assays etc. These measures cannot be normalized to derive values having uniform units for direct comparison. We picked a few of the examples to see the correlation between experimentally reported mutant evaluation scores and the predicted ∆∆G values. One such example has been described here.
A study on testosterone binding site of rat 3-alpha-hydroxysteroid dehydrogenase (PDBID: 1AFS) by Heredia et al.20 reports that binding site residue in direct contact with the ligand influences the rate determining step of the enzymatic reaction. Alanine scanning mutagenesis was performed on binding site residues of the hydroxysteroid dehydrogenase protein that could interact with the steroid ligand and Kd was experimentally determined for each mutant to prove this. The ABS-Scan analysis performed on this complex with both testosterone and progesterone also confirms this. A good correlation was observed between the reported Kd value and the corresponding ∆∆G score predicted by ABS-Scan (Figure 2). The details of the experimental values, predicted score and the web-server output can be visualized along with other examples at http://proline.biochem.iisc.ernet.in/abscan/validation.
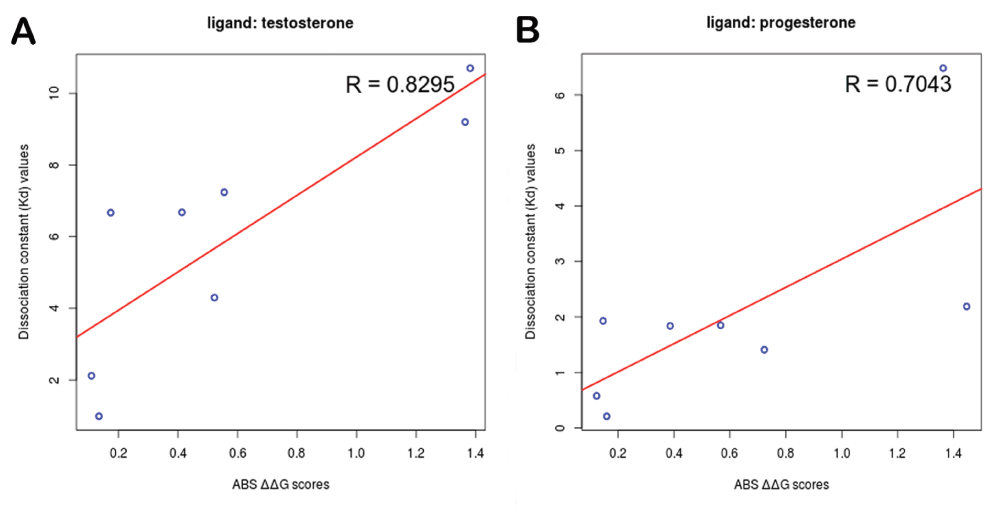
Figure 2. Experimental correlation.
Good agreement is observed between experimental Kd values and predicted ∆∆G values determined for (A) testosterone & (B) progesterone binding site alanine scanning mutagenesis performed on rat 3-alpha-hydroxysteroid dehydrogenase.
In order to determine the sensitivity of ABS-Scan, we compared predictions of essential residues through ABS-Scan in native complexes with corresponding decoy complexes. The complete dataset was obtained from the Community Structure-Activity Resource (CSAR - www.csardock.org/). Decoys in this dataset contain artificial docked complexes of protein with ligands having similar chemical properties to native ligands, but not known to interact with the protein. ABS-Scan is seen to effectively discriminate between the decoy and the native complexes (p-value ~0.004 calculated with Student’s t-test) in ~67% of the cases (∆∆G ≥0.5). This clearly indicates that residues important for ligand interaction can be identified through our approach (Figure 3). The details of validation protocol and results are accessible from the web-resource at http://proline.biochem.iisc.ernet.in/abscan/validation.
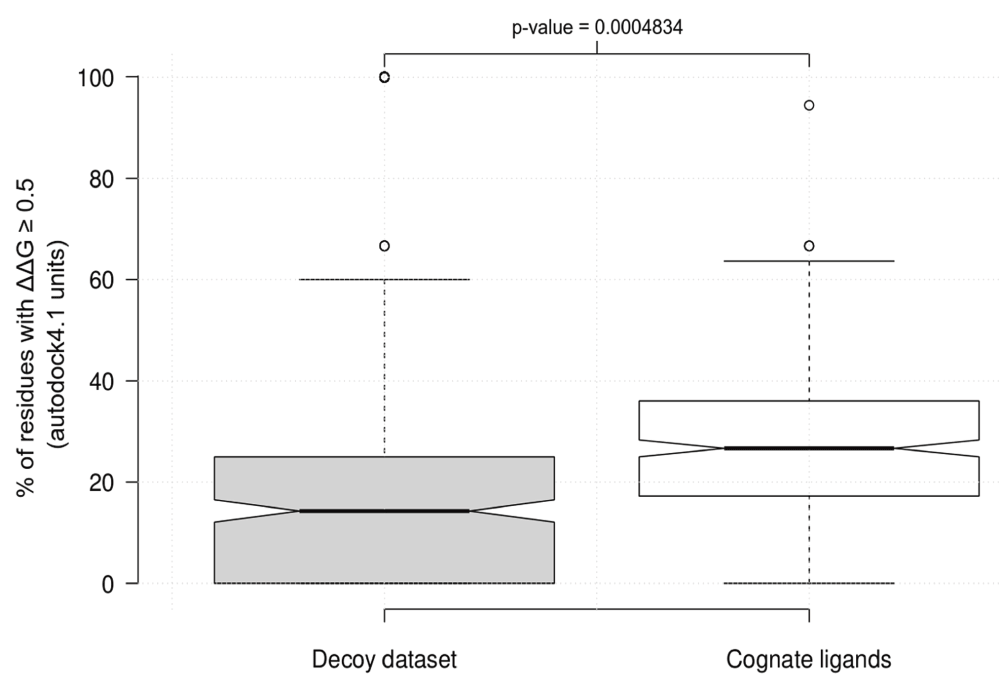
Figure 3. ABS-Scan sensitivity.
Boxplot showing the difference in the % of the residues in the binding site of cognate and decoy dataset having a predicted ∆∆G score ≥0.5.
Implementation
The webserver was implemented using hypertext preprocessor (PHP). Autodock, Modeller and Pymol libraries have been used for modeling the mutation and evaluating the energetics. Integration of these back-end libraries for presentation as a functional and intuitive user interface is accomplished using Shell, Python, Java, HTML and PHP scripts. The web-server is platform independent and will run on any machine having internet access with browser installed. For the advanced users, a command-line interface in the form of a single python script can be accessed from github repository (https://github.com/praveeniisc/ABS-Scan). The script has been tested on Intel 2.83 GHz quad-core system running 32 bit linux OS(Ubuntu 12.04) with Modeller10, MGL AutodockTools19 & Pymol (http://pymol.org) installed. For the web-server d3.js library has been used for displaying the plots. Jmol Applet has been used to visualize the protein-ligand interaction.
Input
The input required for the server is the structure of protein-ligand complex in PDB format. Users can either provide the four-letter PDBID or upload the PDB structure file of the complex. An option is provided to define the cut-off distance and select the ligand to obtain binding site residues which would be mutated to alanine for evaluating the interaction energetics. A default distance cut-off of 4.5 Å is set to select all the residues within this distance from any atom of the ligand. In some the cases, metal ions21 and water molecules are observed to play a crucial role in stabilizing the interactions22. Major problem involved in incorporating the ligand metal ion in ABS-Scan worflow is fixing the charge parameter as metal atoms can have different ionic states (Ex. Fe2+, Fe3+ etc.) which is important for evaluating energetics. Enumerating all important structural water molecules involved in the ligand interaction is also highly dependent on the resolution of the crystal structure. Hence, an advanced option is provided to the user for uploading the PDBQT format of the ligand, to account for cases where the ligand contains unusual atom types, metal ions or uses bridge-water molecules for interaction.
Output
All the results produced by ABScan can be visualized interactively on the web-server. Jmol Applet is used to visualize the contribution of residues towards ligand interaction (Figure 4).
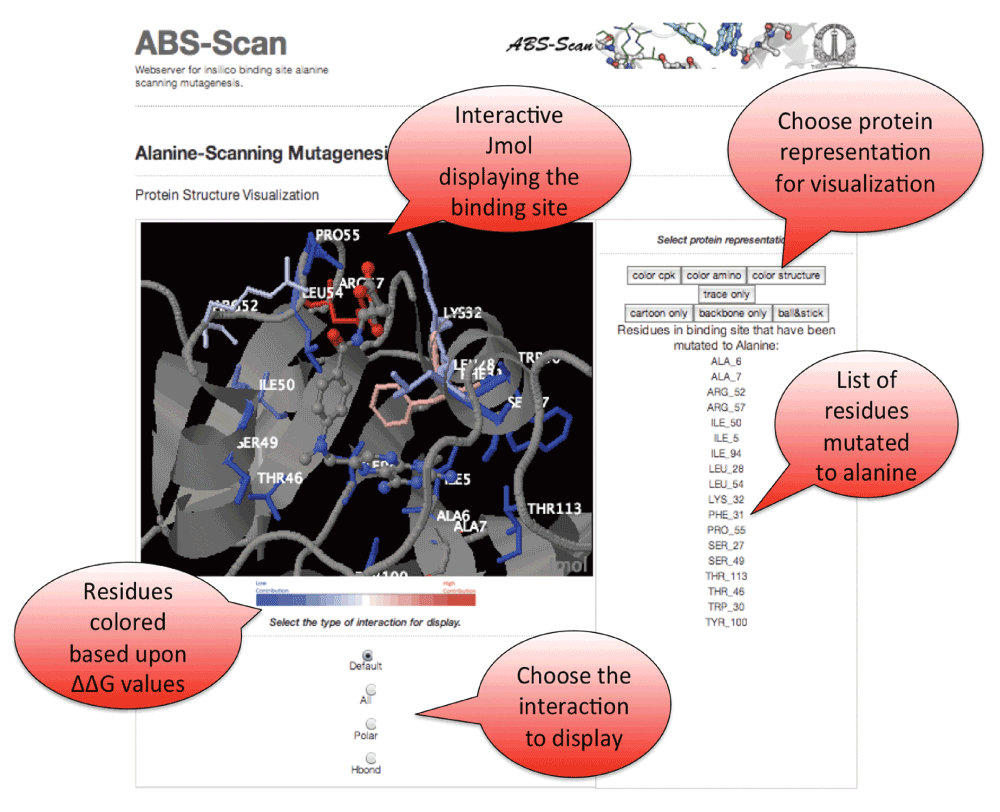
Figure 4. ABS-Scan interactive display.
Snapshot explaining the Jmol applet output on the ABScan server. The individual residues are colored in red to blue gradient depending upon the contribution towards the ligand interaction as predicted by ABScan ∆∆G score. Options to visualize the different kinds of interaction - polar, hbonds etc. is also provided.
d3.js library has been utilized to plot the predicted ∆∆G values and subcomponents of the energetic scores reported by Autodock4 (Figure 5). An option is provided to download publication quality images in SVG/PDF/PNG formats. Twitter bootstrap java library is used for framework development on the webserver. An option is also provided to download the raw files containing individual mutants in PDB format, ∆∆G scores in the raw CSV format along with autodock energy scores.
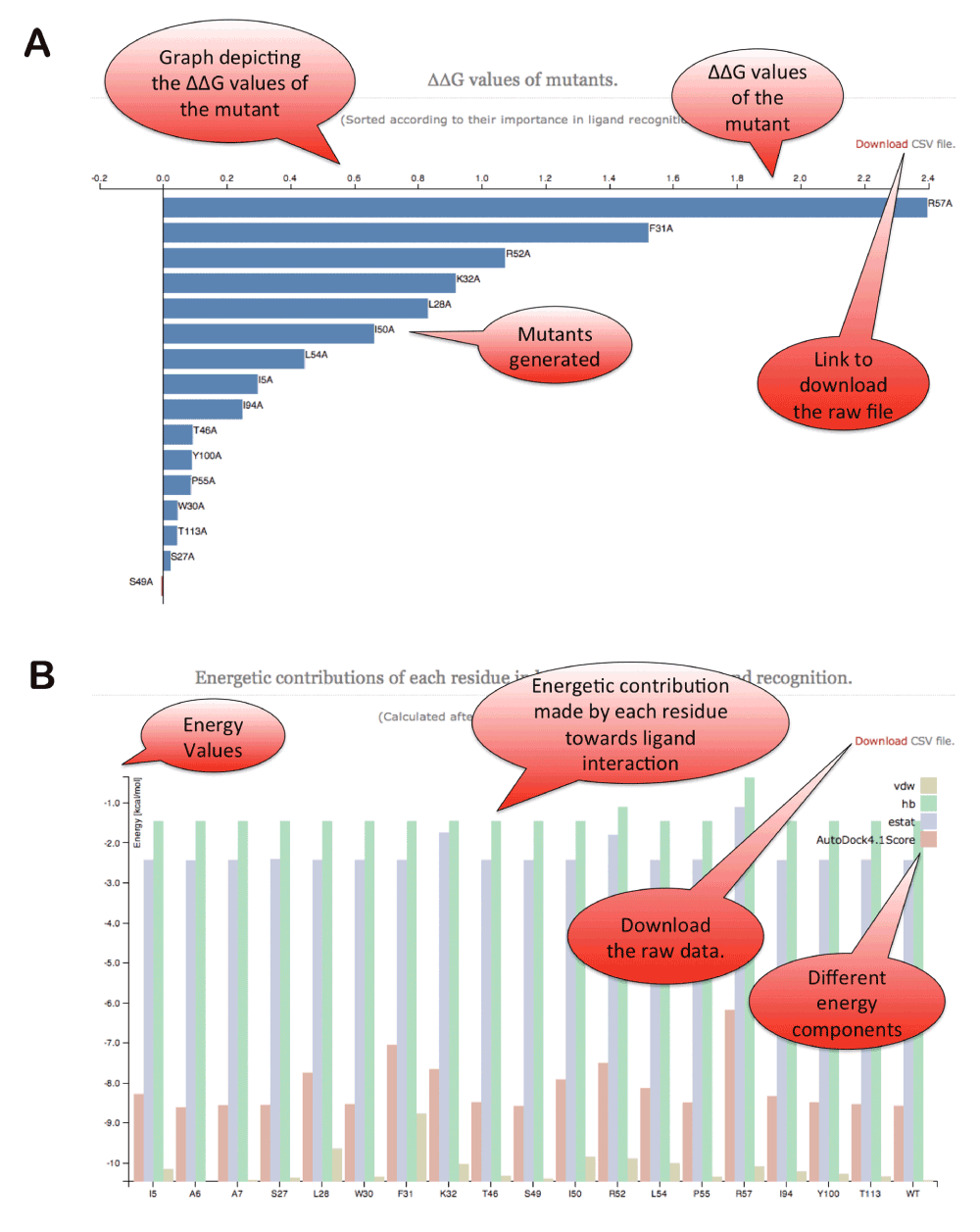
Figure 5. ABS-Scan energy plots.
(A) ∆∆G values reported for each of the alanine mutation performed for the residues present at the binding site. The residues are ordered according to their contribution/∆∆G values. (B) The different energy component of autodock interaction score plotted for each of the alanine mutant produced at the binding site.
Conclusions
ABS-Scan webserver can provide valuable insights on molecular recognition involving protein-ligand interactions. Experimentally determined protein-ligand structures can be studied to understand individual residue contributions towards ligand binding. Modeled complexes can also be submitted to infer the feasibility of the interaction. We believe that ABS-Scan would add one more dimension to the analysis of binding sites in proteins, comparison of various ligand interactions and be of importance to researchers performing ASM studies.
Software availability
Software license
ABS-Scan is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Author contributions
Conceived and designed the experiments: NSC. Performed the experiments: PA,DN,SM. Analyzed the data: PA,DN,SM,NSC. Wrote the paper: PA,DN,NSC. Website design and implementation: PA.
Competing interests
No competing interests were disclosed.
Grant information
The authors(s) declare that no special grants were sanctioned for this project. PA was supported by Bristol-Myers Squibb fellowship while carrying out this work.
Acknowledgements
We acknowledge all the members of the NSC lab for useful suggestions during the development of the web-server and visualization of the results.
Faculty Opinions recommendedReferences
- 1.
Rose PW, Bi C, Bluhm WF, et al.:
The RCSB Protein Data Bank: new resources for research and education.
Nucleic Acids Res.
2013; 41(Database issue): D475–82. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 2.
Morrison KL, Weiss GA:
Combinatorial alanine-scanning.
Curr Opin Chem Biol.
2001; 5(3): 302–7. PubMed Abstract
| Publisher Full Text
- 3.
Weiss GA, Watanabe CK, Zhong A, et al.:
Rapid mapping of protein functional epitopes by combinatorial alanine scanning.
Proc Natl Acad Sci U S A.
2000; 97(16): 8950–4. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 4.
Williams AD, Shivaprasad S, Wetzel R:
Alanine scanning mutagenesis of Abeta(1-40) amyloid fibril stability.
J Mol Biol.
2006; 357(4): 1283–94. PubMed Abstract
| Publisher Full Text
- 5.
Ashkenazi A, Presta LG, Marsters SA, et al.:
Mapping the CD4 binding site for human immunodeficiency virus by alanine-scanning mutagenesis.
Proc Natl Acad Sci U S A.
1990; 87(18): 7150–4. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 6.
Kristensen C, Kjeldsen T, Wiberg FC, et al.:
Alanine scanning mutagenesis of insulin.
J Biol Chem.
1997; 272(20): 12978–83. PubMed Abstract
| Publisher Full Text
- 7.
Tang WJ, Stanzel M, Gilman AG:
Truncation and alanine-scanning mutants of type I adenylyl cyclase.
Biochemistry.
1995; 34(44): 14563–72. PubMed Abstract
| Publisher Full Text
- 8.
Jain PC, Varadarajan R:
A rapid, efficient, and economical inverse polymerase chain reaction-based method for generating a site saturation mutant library.
Anal Biochem.
2014; 449: 90–8. PubMed Abstract
| Publisher Full Text
- 9.
Bromberg Y, Rost B:
Comprehensive in silico mutagenesis highlights functionally important residues in proteins.
Bioinformatics.
2008; 24(16): i207–12. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 10.
Eswar N, Eramian D, Webb B, et al.:
Protein structure modeling with MODELLER.
Methods Mol Biol.
2008; 426: 145–59. PubMed Abstract
| Publisher Full Text
- 11.
Kaufmann KW, Lemmon GH, Deluca SL, et al.:
Practically useful: what the Rosetta protein modeling suite can do for you.
Biochemistry.
2010; 49(14): 2987–98. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 12.
Kim DE, Chivian D, Baker D:
Protein structure prediction and analysis using the Robetta server.
Nucleic Acids Res.
2004; 32(Web Server issue): W526–31. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 13.
Liu Y, Kuhlman B:
RosettaDesign server for protein design.
Nucleic Acids Res.
2006; 34(Web Server issue): W235–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 14.
Lyskov S, Chou FC, Conchúir SÓ, et al.:
Serverification of molecular modeling applications: the Rosetta Online Server that Includes Everyone (ROSIE).
PLoS One.
2013; 8(5): e63906. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 15.
Schymkowitz J, Borg J, Stricher F, et al.:
The FoldX web server: an online force field.
Nucleic Acids Res.
2005; 33(Web Server issue): W382–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 16.
Dehouck Y, Kwasigroch JM, Rooman M, et al.:
BeAtMuSiC: Prediction of changes in protein-protein binding affinity on mutations.
Nucleic Acids Res.
2013; 41(Web Server issue): W333–9. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 17.
Homeyer N, Gohlke H:
FEW: a workflow tool for free energy calculations of ligand binding.
J Comput Chem.
2013; 34(11): 965–73. PubMed Abstract
| Publisher Full Text
- 18.
Greenidge PA, Kramer C, Mozziconacci JC, et al.:
MM/GBSA binding energy prediction on the PDBbind data set: successes, failures, and directions for further improvement.
J Chem Inf Model.
2013; 53(1): 201–9. PubMed Abstract
| Publisher Full Text
- 19.
Trott O, Olson AJ:
AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading.
J Comput Chem.
2010; 31(2): 455–61. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 20.
Bennett MJ, Albert RH, Jez JM, et al.:
Steroid recognition and regulation of hormone action: crystal structure of testosterone and NADP+ bound to 3 alpha-hydroxysteroid/dihydrodiol dehydrogenase.
Structure.
1997; 5(6): 799–812. PubMed Abstract
| Publisher Full Text
- 21.
Andreini C, Bertini I, Cavallaro G, et al.:
Structural analysis of metal sites in proteins: non-heme iron sites as a case study.
J Mol Biol.
2009; 388(2): 356–80. PubMed Abstract
| Publisher Full Text
- 22.
Mobley DL, Dill KA:
Binding of small-molecule ligands to proteins: “what you see” is not always “what you get”.
Structure.
2009; 17(4): 489–98. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 23.
Krüger DM, Gohlke H:
DrugScorePPI webserver: fast and accurate in silico alanine scanning for scoring protein-protein interactions.
Nucleic Acids Res.
2010; 38(Web Server issue): W480–6. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 24.
Anand P, Nagarajan D, Mukherjee S, et al.:
ABS-Scan.
Zenodo.
2014. Data Source





Comments on this article Comments (0)