Keywords
creatine transporter, mouse model, creatine deficiency syndrome 1
creatine transporter, mouse model, creatine deficiency syndrome 1
We are glad to present a revised version of our work “A novel mouse model of creatine transporter deficiency”.
We would like to bring to your attention that we extended the measurement of creatine concentration also to body fluids, more specifically serum and urine. Thus, Table 1 and its relative legend have been modified in order to show also these new data.
In addition, we provided the information on GAA content in all organs and body fluids for which we measured creatine concentration. To show these data, we added a Table 2 with the relative legend. Figures have not been modified.
We also revised the text according to reviewers suggestions, in particular extending the discussion about the effects of the mutant genotype on motor behavior and their relationship with animals’ cognitive performance.
See the authors' detailed response to the review by Andreas Schulze
See the authors' detailed response to the review by Luis M. Valor
See the authors' detailed response to the review by Benedetto Sacchetti
The creatine (Cr) transporter (CrT, alias CRTR, MGC87396, CT1, SLC6A8, OMIM 300036) deficiency (CCDS1, OMIM #300352) is an X-linked inherited metabolic disorder characterized by cerebral Cr deficiency which results in intellectual disability, language and speech impairment, seizures and movement and behavioral disturbances, and affects about 1% of males with non-syndromic mental disability (van de Kamp et al., 2014). CrT loss of function is mostly caused by missense mutations and small deletions which are concentrated in the transmembrane domains 7 and 8 of the protein (van de Kamp et al., 2014). In physiological conditions, about half of our normal Cr requirement is satisfied by the diet. De novo endogenous synthesis of Cr takes place mainly in the kidney, liver and pancreas and involves the enzymes l-arginine: glycineamidinotransferase (AGAT) and S-adenosyl-l-methionine:N-guanidinoacetatemethyltransferase (GAMT) (Wyss & Kaddurah-Daouk, 2000). Cr is a polar hydrophilic molecule unable to cross the lipidic membranes, which uses a Na+- and Cl−- dependent plasma membrane CrT to enter the cells (Nash et al., 1994). CrT is widely expressed in the brain tissue with a prominent presence in the cortical and subcortical regions involved in motor and sensory processing, learning and memory, and regulation of emotion-related behavior (Lowe et al., 2014; Mak et al., 2009).
Patients affected by cerebral creatine deficiency syndrome-1 (CCDS1) share depletion of brain Cr and the clinical phenotype with patients carrying the other two defects of Cr metabolism which involve mutations of genes encoding the biosynthesizing enzymes AGAT and GAMT (Item et al., 2001; Stockler et al., 1994). Replenishment of the brain Cr pool is the only effective therapy for Cr deficiency diseases (Battini et al., 2002; Schulze et al., 2001; Stockler et al., 1996). Unfortunately, in CCDS1 patients even very high doses of Cr, alone or combined with the Cr precursors arginine and glycine to stimulate endogenous Cr synthesis, fail to restore the Cr content in brain (Chilosi et al., 2008; Valayannopoulos et al., 2012). There have been attempts to normalize the levels of Cr in the brain with Cr-lipophilic analogs, but these compounds have proven ineffective when administered to patients (Fons et al., 2010). Thus, CCDS1 is still missing an effective treatment.
Preclinical animal models are crucial tools to dissect disease pathogenic mechanisms and develop new therapeutic strategies. Only two murine models of CCDS1 are available so far, and they have only been analyzed at the behavioral and neurochemical level. An ubiquitous CrT knockout mouse model has been generated by deletion of 2–4 exons in the Slc6a8 gene. Learning and memory deficits, impaired motor activity and Cr depletion in brain and muscles have been reported in animals at three-four months of age (Skelton et al., 2011). Another murine model is based on the use of the CaMKII promoter to drive Cre-recombinase expression, achieving a CrT deletion only in postnatal forebrain excitatory neurons. This strategy was successful in avoiding the peripheral Cr depletion and the motor deficits shown by germline CrT knockout mouse. Behavioral analysis in mice at 12 months of age revealed learning and memory impairments that could be ameliorated by supplementation of cyclocreatine, a Cr analog (Kurosawa et al., 2012).
For translational studies, the phenotype variations observed in different mouse models, carrying similar mutations and the effects of genetic backgrounds highlight the importance of validating phenotypes and therapeutic efficacy in multiple models and in different laboratories (Katz et al., 2012). Such validation will hopefully increase the relevance of preclinical studies to the human disease. To increase the number of CCDS1 models, we generated a novel murine model of CCDS1 obtained by ubiquitous deletion of 5–7 exons in the Slc6a8 gene. These mice presented a remarkable Cr depletion in the brain tissue and displayed cognitive defects resembling the key features of human CCDS1, and providing a new promising CCDS1 animal model.
A Cre-conditional allele of Slc6a8 has been produced by introducing the loxP sites flanking exon 5–7 of the gene in embryonic stem (ES) cells via homologous recombination (vector PRPGS00081_A_A09 obtained from the NIH Knock-out Mouse Program, KOMP). The presence of lox sites has been checked by sequencing (sequencing service by MWG, Germany). The plasmid was linearized with NruI before electroporation into ES cells (129/Sv x C57BL/6N, clone A8, gift of A. Wutz, Wellcome Trust Centre for Stem Cell Research, Stem Cell Institute, University of Cambridge). G418-resistant clones were identified and screened by long-range PCR (Applied Biosystems Gene AMP PCR system 2700). Hybridization with a specific probe for the 5′ and 3′ arms was used to confirm the PCR results. Two independent positive ES cell clones were injected into C57BL/6N host embryos using a piezo-drill assisted 8-cell stage injection procedure developed at EMBL, Monterotondo Italy. Four out of five offspring (all >95% ES cell derived) provided germline transmission. Germline transmission of the allele was confirmed by long-range PCR and the neomycin selection cassette was removed by crossing with FLP recombinase expressing mice (Farley et al., 2000). Germline knockout mice were produced by crossing the constitutive allele to the HPLRT::Cre recombinase deleter mouse (Tang et al., 2002; Figure 1).
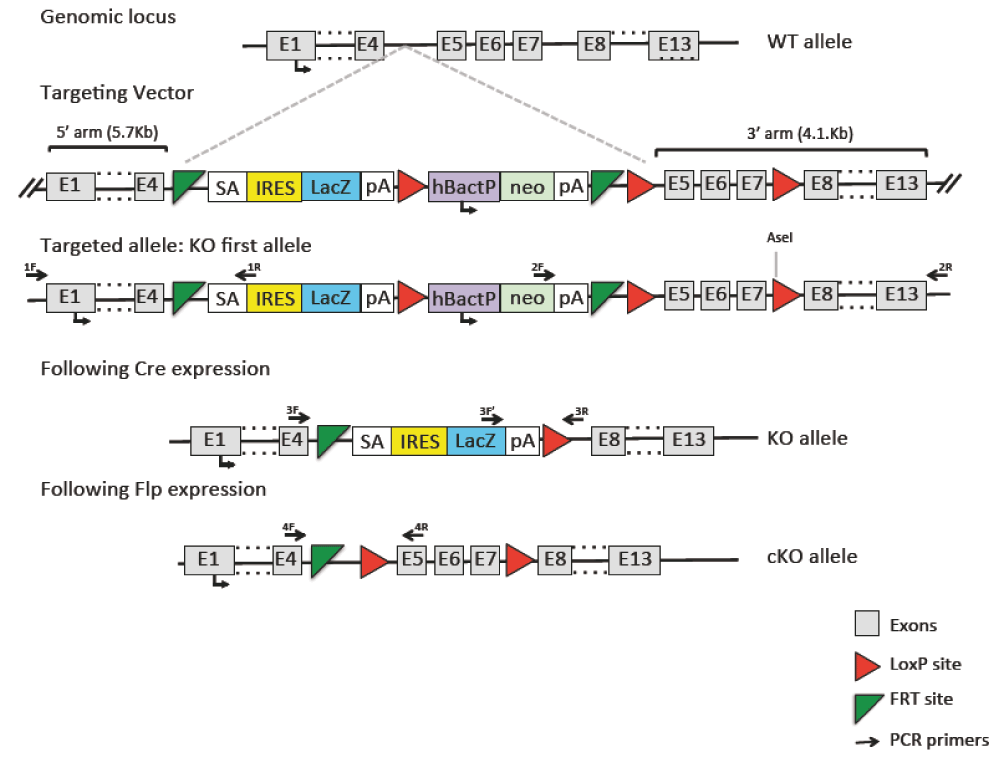
A targeting vector was obtained from KOMP to generate mice carrying a floxed allele. Crossing these mice with a Flp deleter mouse line produced a conditional KO mouse line (cKO allele). Crossing this line with a line expressing Cre-recombinase in the germline produced the Slc6a8 null mouse used in this study (KO allele). 1F, 1R, 2F, 2R, 3F, 3F’, 3R, 4F, 4R report the sites targeted by the PCR primers to assess allele presence.
Animals were maintained at 22°C under a 12-h light–dark cycle. Food (4RF25 GLP Certificate, Mucedola) and water were available ad libitum. The chow was not added with creatine (personal communication of the manufacturer). All experiments were carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and were approved by the Italian Ministry of Health (authorization number 147/2014-B). All necessary efforts were made to minimize both stress and the number of animals used. As CrT deficiency is an X-linked pathology and only males are consistently affected, we focused our study on male animals. Young adult males (postnatal day P40 at the beginning of testing) of each genotype (CrT–/y mutants and CrT+/y wild-type littermates) were used in behavioral experiments, while a separate group of animals (P30) was assigned to Cr level assay.
Genomic DNA was isolated from mouse tail using a kit, and the protocol suggested by the manufacturer (DNeasy Blood & Tissue Kit, Qiagen, USA). DNA was amplified for mutant and wild-type (WT) allele using a standard PCR protocol with the following primers: F:AGGTTTCCTCAGGTTATAGAGA; R:CCCTAGGTGTATCTAACATCT; R1: TCGTGGTATCGTTATGCGCC. For PCR amplification we used 300 ng of DNA in a 25 μL reaction volume containing 0.2 mM of each dNTP, 2 μM of F primer, 1 μM of R, 1 μM of R1 primer and 0.5 U/μL Red Taq DNA polymerase (Sigma-Aldrich, Italy). The PCR conditions were as follows: 94°C for 4 min followed by 37 cycles at 94°C for 30 s, 58°C for 30 s, 72°C for 40 s and a final extension at 72°C for 7 min. Amplicons were separated using 2% agarose gel and visualized under UV light after staining with Green Gel Plus (Fisher Molecular Biology, Rome, Italy). Amplicon sizes were: WT allele = 462 bp; mutant allele = 371 bp.
For Cr and GAA assay, mouse tissues, immediately frozen on dry ice and stored at -80°C until the analysis, were homogenized in 0.7 ml PBS buffer (Sigma-Aldrich, Italy) at 4°C using a ultrasonic disruptor (Microson Heat System, NY, USA) for brain or a glass manual homogenizer (VWR, Italy) for kidney, heart and muscle. After centrifugation (600 × g for 10 min at 4°C) an aliquot of the homogenate (50 µl) was assayed for protein content (Lowry et al., 1951), and the supernatant used for Cr assay as previously described (Alessandri et al., 2005). Briefly, 50 µl of saturated sodium hydrogen carbonate and 50 µl of a mixture containing 2- phenylbutyric acid (I.S.) in toluene (6.09 mmol/l; Sigma-Aldrich, Italy) were added to 200 µl of homogenate or to 100 µl of serum and urine, respectively. After adding 1 ml of toluene and 50 µl of hexafluoro-2,4-pentanedione (Sigma-Aldrich, Italy) to form bis-trifluoromethyl- pyrimidine derivatives, the mixture was stirred overnight at 80°C. The organic layer was centrifuged, dried under nitrogen and 2 µl of the residue derivatized at room temperature with 100 µl of BSTFA+TMCS (Sigma-Aldrich, Italy) injected into the Gas Chromatography/Mass Spectrometry (GC/MS) instrument. GC analyses were performed using an Agilent 6890N GC equipped with an HP5MS capillary column (0.25 mm × 30 m, film thickness 0.25 ìm) and an Agilent mass spectrometer 5973N (Agilent Technologies, Italy). The mass spectrometer was set in EI- single ion monitoring mode (SIM). The ions with m/z of 192 for I.S., 258 for Cr and 225 for guanidinoacetic acid (GAA) were used for calculation of the metabolites, using standard curves ranging 5–90 µmol/L and 0.30–6 µmol/L for Cr and GAA, respectively. Data were processed by the G1701DA MSD ChemStation software. All the aqueous solutions were prepared using ultrapure water produced by a Millipore system. Creatinine in urine was measured using an enzymatic colorimetric assay (Sentinel Diagnostics, Italy) and the UV spectrophotometer Cary50 (Agilent Technologies, Italy) set at 546 nm.
The testing order consisted of: open field (1 day duration), object recognition test (ORT) at 24h (3 days), Y maze (1 day), Morris water maze (MWM) with hidden platform (7 days), and locomotor activity (1 day). The mice were tested on one task at a time with the next behavioral test starting at least 2 days after the completion of the previous one. In order to reduce the circadian effects, all behavioral tests were performed during the same time interval each day (1400–1800h; light phase). All behavioral tests were conducted in blind with respect to the genotype of animals. Mice were weighed at the end of experiments (P60).
We followed the protocol reported in Lonetti et al., 2010. Briefly, the apparatus consisted of a square arena (60 × 60 × 30 cm) constructed in poly(vinyl chloride) with black walls and a white floor. The mice received two sessions of 10-min duration in the empty arena on two consecutive days to habituate them to the apparatus and test room. Animal position was continuously recorded by a video tracking system (Noldus Ethovision XT). In the recording software an area corresponding to the center of the arena (a central square 30 × 30 cm), and a peripheral region (corresponding to the remaining portion of the arena) were defined. The total movement of the animal and the time spent in the center or in the periphery area were automatically computed. The mice activity during the first day of habituation was analyzed for evaluating the behavior in the open field arena. The ORT consisted of two phases: sample and testing phase. During the sample phase, two identical objects were placed in diagonally opposite corners of the arena, approximately 6 cm from the walls, and mice were allowed 10 min to explore the objects, then they were returned to their cage. The objects to be discriminated were made of plastic, metal, or glass material and were too heavy to be displaced by the mice. Arena and objects were cleaned with 10% ethanol between trials to stop the build-up of olfactory cues. The testing phase was performed 24h after the sample phase. One of the two familiar objects was replaced with a new one, while the other object was replaced by an identical copy. The objects were placed in the same locations as the previous ones. The mice were allowed to explore objects for 5 min. To avoid possible preferences for one of two objects, the choice of the new and old object and the position of the new one were randomized among animals. The amount of time spent exploring each object (nose sniffing and head orientation within <1.0 cm) was recorded and evaluated by the experimenter blind to the mouse genotype. Mice exploring the two objects for less than 10 s during the sample phase were excluded from testing. A discrimination index was computed as DI = (Tnew - Told)/(Tnew + Told), where Tnew is the time spent exploring the new object, and Told is the time spent exploring the old one.
Spontaneous alternation was measured using the Y-maze, as described in Begenisic et al., 2014. We used a Y-shaped maze with three symmetrical grey solid plastic arms at a 120-degree angle (26 cm length, 10 cm width, and 15 cm height). Mice were placed in the center of the maze and allowed to freely explore the maze for 8 minutes. The apparatus was cleaned with 10% ethanol between trials to avoid the build-up of odor traces. All sessions were video-recorded for offline blind analysis. The arm entry was defined as all four limbs within the arm. A triad was defined as a set of three arm entries, when each entry was to a different arm of the maze. The number of arm entries and the number of triads were recorded in order to calculate the alternation percentage (generated by dividing the number of triads by the number of possible alternations and then multiplying by 100).
Mice were trained for four trials per day and for a total of 7 days in a circular water tank, made from grey polypropylene (diameter, 120 cm; height, 40 cm), filled to a depth of 25 cm with water (23°C) rendered opaque by the addition of a small amount of a non-toxic white paint. Four positions around the edge of the tank were arbitrarily designated North (N), South (S), East (E), and West (W), which provided four alternative start positions and also defined the division of the tank into four quadrants, i.e., NE, SE, SW, and NW. A square clear Perspex escape platform (11 × 11 cm) was submerged 0.5 cm below the water surface and placed at the midpoint of one of the four quadrants. The hidden platform remained in the same quadrant during training, while the start positions (N, S, E, or W) were randomized across trials. Mice were allowed up to 60 s to locate the escape platform, and their swimming paths were automatically recorded by the Noldus Ethovision system. On the last trial of the last training day, mice received a probe trial, during which the escape platform was removed from the tank and the swimming paths were recorded over 60 s while mice searched for the missing platform. The swimming paths were recorded and analyzed with the Noldus Ethovision system.
OptoM3 multi-channel activity monitors (Columbus Instruments, OH, USA) were used to quantify spontaneous horizontal activity of animals. Monitors were placed in the colony area and testing was conducted in the same conditions of animal facility housing. All measurements were performed from 6:00 P.M. to 6:00 A.M. (dark phase) and to 6:00 A.M. to 6:00 P.M. (light phase), using animals maintained on a 12 hr light/dark cycle from 6:00 A.M. to 6:00 P.M. Individual mice were placed in 33 × 15 × 13-cm (length × width × height) clear plastic cages for 24h and total distance travelled was calculated from infrared beam breaks by determining activity at 1-min intervals. Horizontal activity was measured by the sequential breaking of infrared beams, 2.54 cm on center, in the horizontal plane of the x axis.
All statistical analyses were performed using SigmaStat Software. Differences between two groups were assessed with a two-tailed t test. The significance of factorial effects and differences among more than two groups were evaluated with ANOVA/RM ANOVA followed by Holm-Sidak test. Rank transformation was exploited for data not normally distributed. The level of significance was p < 0.05.
In order to determine the effectiveness of our approach for targeting CrT gene, the Cr levels were measured by GC/MS in various tissues. We observed a significant reduction of Cr in the brain (both cerebral cortex and hippocampus; Two Way ANOVA on rank transformed data, post hoc Holm-Sidak method, p < 0.001), muscle (p < 0.001), heart (p < 0.001), kidney (p < 0.05) and serum (p < 0.001) of CrT−/y mice with respect to wild-type (WT) littermates (n = 4/tissue for each group; Table 1). In contrast, an increase of Cr levels (n = 5 for CrT−/y mice, n = 4 for WT mice; Two Way ANOVA on rank transformed data, post hoc Holm-Sidak method, p < 0.05) and creatine/creatinine ratio was present in the urine of mutant (16.95 ± 1.46) with respect to WT animals (1.20 ± 0.17; t test, p < 0.001). To ensure that kidney Cr reduction was not due to impaired Cr biosynthesis, we measured kidney production of guanidinoacetic acid (GAA). No difference was observed between CrT–/y (9.76 ± 0.71 nmol/mg of protein) and CrT+/y mice (10.70 ± 0.63 nmol/mg of protein; t test, p = 0.359). Intriguingly, a moderate change in GAA levels was observed in some tissues (Table 2) suggesting that Cr biosynthesis is affected by the dramatic Cr decrease caused by CrT deletion.
Cr levels (mean ± SEM) in CrT–/y and CrT+/y animals (n = 4 per tissue for both groups, except n = 5 for urine measurement in CrT−/y mice). Cr levels have been measured by GC/MS. A reduction of Cr content was evident in the brain, muscle, heart, kidney and serum of mutant animals, while an increase of Cr levels was reported in urine (Two Way ANOVA on rank transformed data, post hoc Holm-Sidak method). * p < 0.05; *** p < 0.001.
GAA levels (mean ± SEM) in CrT–/y and CrT+/y animals (n = 4 per tissue for both groups, except n = 5 for urine measurement in CrT−/y mice). An increase of GAA levels was present in the brain, muscle and heart of mutant animals, while no difference was detected in kidney, serum and urine (Two Way ANOVA on rank transformed data, post hoc Holm-Sidak method). * p < 0.05; ** p < 0.01; *** p < 0.001.
The general appearance of CrT–/y mice was normal and no particular problems of breeding were observed. To evaluate the effects of CrT deletion on body weight, the mice with targeted disruption of CrT gene were weighed at P60, and compared with WT littermates. CrT−/y animals (n = 9) showed a significantly reduced body weight compared to CrT+/y animals (n = 9; t test, p < 0.01; Figure 2).
We first analyzed the general motor activity and anxiety-related behavior of CrT−/y (n = 9) and CrT+/y mice (n = 9) in the open field arena. Even though both groups of animals tended to avoid the center of the arena, remaining in the peripheral region for a significantly longer duration (Two Way ANOVA, post hoc Holm-Sidak method), the time spent by CrT−/y mutant mice in both the central and peripheral portion of the apparatus was not different from that recorded for WT animals (Two Way ANOVA, post hoc Holm-Sidak method, p = 0.725 and p = 0.922 respectively; Figure 3a, b, e). No difference between CrT−/y and CrT+/y animals was present even in motion speed and total distance moved (t test, p = 0.807 and p = 0.736 respectively; Figure 3c, d).

(a, b) CrT–/y (n = 9) and CrT+/y mice (n = 9) spent a comparable amount of time in the center (CrT–/y: 75.16 ± 10.82 s, CrT+/y: 67.60 ± 18.11 s; a) and in the peripheral region (CrT–/y: 524.41 ± 10.87 s, CrT+/y: 526.52 ± 18.45 s; b) of the open field arena. A Two-Way ANOVA analysis shows no significant effect of genotype for both comparisons (p = 0.725 and p = 0.922, respectively). (c) Walking speed of animals during the exploration of open field arena. We found no significant difference (CrT–/y: 7.85 ± 0.43 cm/s, CrT+/y: 7.65 ± 0.71 cm/s; t test, p = 0.807). (d) The total distance moved in the open field arena did not differ between CrT mutants (4706.34 ± 258.75 cm) and WT animals (4535.28 ± 427.11 cm; t test, p = 0.736). (e) Representative examples of movement path during the open field session for a CrT–/y (left) and a CrT+/y mouse (right). Error bars, s.e.m.
We assessed declarative memory abilities in the object recognition test (ORT) evaluating animal ability to discriminate a new versus a familiar object. During the sample phase (Figure 4a), all experimental groups equally explored the objects, with total exploration time of mutant mice (n = 8) very close to that recorded for the control group (n = 6; t test, p = 0.358). After a delay of 24h, the testing phase revealed that while CrT+/y mice displayed a clear preference toward the novel object spending a significantly longer time exploring it, an impaired performance was found in CrT–/y animals, which exhibited a significantly lower discrimination index than control animals (t test, p < 0.05, Figure 4b).

(a) On the left, a schematic representation of the sample condition in object recognition task. Histograms depict the performance of CrT–/y and CrT+y during the sample phase: no difference in the total exploration time of objects was detected between the experimental groups (CrT–/y: n = 8, exploration time = 56.91 ± 5.40 s; CrT+/y: n = 6, exploration time = 67.20 ± 10.23 s; t test, p = 0.358). (b) On the left, a schematic diagram of the test condition. Histograms display object discrimination indexes of CrT–/y and CrT+y during the testing phase: a significantly lower discrimination index was found in CrT–/y mice (0.261 ± 0.053) compared to CrT+y animals (0.448 ± 0.059; t test, p < 0.05). *, statistical significance. Error bars, s.e.m.
To evaluate whether CrT deletion may affect spatial working memory, we used the analysis of spontaneous alternation in the Y maze (Figure 5a). Animals of both groups equally explored all the three arms of the maze. Indeed, no effect of genotype was detected for either the number of entries in the single arms of the maze (designated A, B, C) or the total number of arm entries, indicating that the exploratory disposition of mutant animals (n = 9) was not altered compared to WT littermates (n = 9; Two-Way ANOVA, post hoc Holm-Sidak method, p = 0.640, p = 0.966, p = 0.252, p = 0.523 respectively, Figure 5b). In contrast, CrT−/y mice showed a significantly smaller rate of spontaneous alternation with respect to WT controls (t test, p < 0.05, Figure 5c).
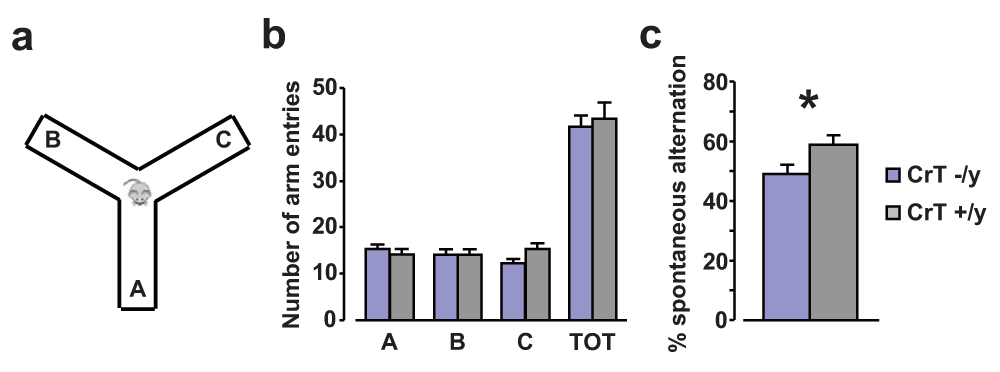
(a) Schematic diagram of the Y maze apparatus. (b) Histograms depict the mean number of entries in the single arms of the maze (A, B, C) and the total number of arm entries for the different experimental groups: animals of both groups equally explored all the three arms of the maze and general exploratory behavior of CrT–/y animals (n = 9; A: 15.22 ± 1.12, B: 14.22 ± 1.08, C: 12.22 ± 1.05, TOT: 41.67 ± 2.41) was totally comparable to that exhibited by WT littermates (n = 9; A: 14.00 ± 1.26, B: 14.11 ± 1.29, C: 15.22 ± 1.27, TOT: 43.33 ± 3.58; Two-Way ANOVA, post hoc Holm-Sidak method, p = 0.640, p = 0.966, p = 0.252, p = 0.523 respectively). (c) Alternation rate in the Y maze was significantly lower in CrT–/y mice (49.24 ± 3.20%) compared to that recorded for CrT+/y littermates (58.91 ± 2.99%; t test, p < 0.05). *, statistical significance. Error bars, s.e.m.
We further assessed spatial memory abilities in the Morris water maze (MWM) task, a cognitive paradigm which allows testing both spatial learning and memory. Since a main effect of genotype was found on mean swimming speed recorded all along the training phase (t test, p < 0.05; Figure 6a), we analyzed path length, which is a quantity independent of swimming velocity. We found that the mean distance covered to locate the submerged platform on the last three days of training was longer in CrT–/y mice (n = 9) compared to CrT+/y littermates (n = 5; t test, p < 0.05; Figure 6b, c). To measure the strength of spatial learning and to discriminate between spatial and non-spatial memory strategies we performed a probe trial in which the hidden platform was removed and the amount of time spent in the former region of the platform was measured. The probe test confirmed the spatial memory impairment of CrT–/y mice: CrT+/y animals spent significantly longer time in the quadrant where the platform was located during the previous learning days (NE*; Two-Way RM ANOVA, post hoc Holm-Sidak method, p < 0.05 for all comparisons); in contrast, CrT–/y mice showed no preference for the target quadrant, indicating that they did not remember the location of the hidden platform (Two-Way RM ANOVA, post hoc Holm-Sidak method; Figure 6d). A statistically significant effect of genotype was detected in the time spent exploring the target quadrant (Two-Way RM ANOVA, post hoc Holm-Sidak method, p < 0.05; Figure 6d).
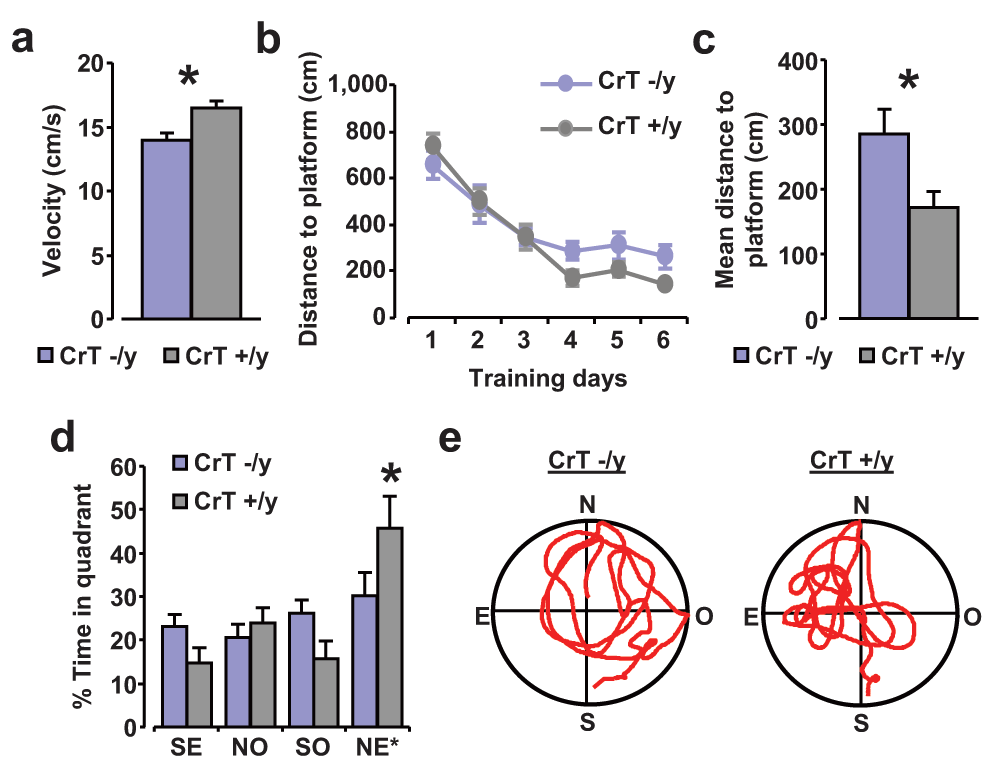
(a) Mean swimming speed measured all along the training phase for CrT–/y and CrT+/y animals: mutant mice (14.00 ± 0.53 cm/s) resulted to be slower swimmers with respect to control littermates (16.44 ± 0.60 cm/s; t test, p < 0.05). (b, c) Learning curves for CrT–/y (n = 9; blue) and CrT+/y mice (n = 5; grey) during the training phase. The histogram shows the mean swimming path covered to locate the submerged platform on the last three day of training for the two groups. A t-test analysis showed a statistical difference between CrT–/y (285.24 ± 37.53 cm) and CrT+/y animals (171.58 ± 23.80 cm; p < 0.05). (d) Probe trial. A Two-Way RM ANOVA followed by Holm-Sidak multiple comparison revealed that while CrT+/y spent significantly more time in the NE quadrant than in the other ones, CrT–/y did not show any preference for the target quadrant. In addition, the percentage of time spent in the target quadrant was shorter in CrT–/y mice (30.31 ± 5.33%) than in the other group (45.73 ± 7.35%). (e) Representative examples of swimming path during the probe session for a CrT–/y (left) and a CrT+/y mouse (right). *, statistical significance. Error bars, s.e.m.
To investigate the presence of movement impairments in CrT−/y mice in a non-aversive environment, we investigated home-cage-locomotor activity. We found that CrT–/y mice (n = 9) are significantly less active than the CrT+/y group (n = 8, Two-Way ANOVA, post hoc Holm-Sidak method, p < 0.001). More specifically, CrT−/y mice showed decreased horizontal activity during the night period (Two-Way ANOVA, post hoc Holm-Sidak method, p < 0.001), while no effect of genotype was observed for exploration during daytime (p = 0.535; Figure 7a, b).
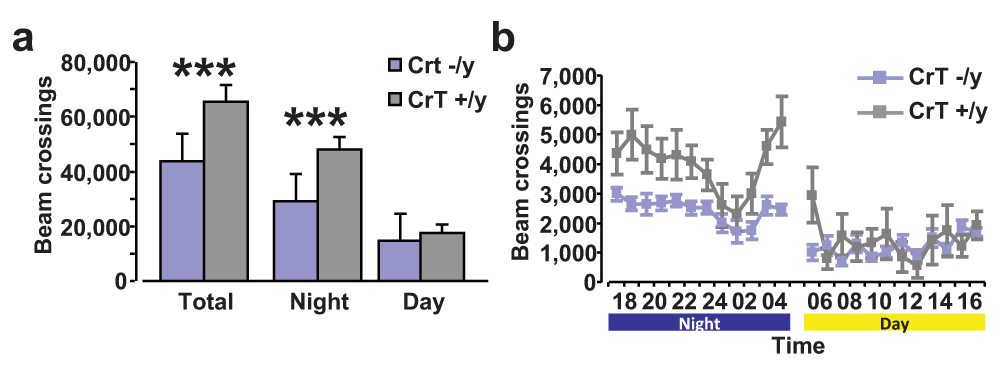
(a) Total horizontal distance travelled throughout 24h (left), and over the dark (middle) or light phase (right). CrT–/y mice had a significant decrease in motor activity in comparison to control animals during the whole period of testing (CrT–/y: 43,594.22 ± 2,639.39 beam crossings, CrT+/y: 65,587.63 ± 5,831.19 beam crossings) and the night phase (CrT–/y: 29,109.67 ± 1,695.35 beam crossings, CrT+/y: 48,094.13 ± 4,843.56 beam crossings; Two-Way ANOVA, post hoc Holm-Sidak method, p < 0.001 for both comparisons), while the motor behavior of the two groups was similar in the day-time (CrT–/y: 14,484.56 ± 1,458.08 beam crossings, CrT+/y: 17,493.50 ± 2,957.57 beam crossings; p = 0.535). (b) Time course of horizontal activity of CrT–/y (blue) and CrT+/y (grey) animals during 24h. Data are plotted as total number of beam crossings ± SEM in each time block of 60 min. Dark and light phases are indicated. *, statistical significance. Error bars, s.e.m.
We have generated a new murine model of human CrT deficiency carrying a loss of function deletion of 5–7 exons in the murine orthologous of Slc68a gene. Given that most disease-underlying mutations in human CCDS1 lead to loss of CrT function (van de Kamp et al., 2014), our model has a good degree of construct validity. Beyond the genetic deletion, neurochemical abnormalities found in CrT−/y mice, reproducing the reduced levels of Cr that characterize the brain of CCDS1 patients (van de Kamp et al., 2012), are also helpful to confirm the successful disruption of CrT gene and the construct robustness of this model. Importantly, Cr deficiency is apparent in both the cerebral cortex and hippocampus, i.e., two brain regions crucially involved in the patient cognitive defects. These results seem to support the hypothesis that, despite AGAT and GAMT expression (Carducci et al., 2012; Schmidt et al., 2004; Tachikawa et al., 2004), in CrT deficiency conditions endogenous synthesis does not compensate for the loss of Cr uptake in the mouse (Skelton et al., 2011) as well as in the human brain (Cecil et al., 2001). In contrast to the preservation of Cr levels in skeletal muscle of CCDS1 patients (deGrauw et al., 2003), we observed that mutant mice exhibit Cr reductions in muscle and other peripheral tissues and body fluids. This observation, which is in agreement with data from a different CrT knockout mouse (Russell et al., 2014; Skelton et al., 2011), further confirmed that the recombination resulted in a ubiquitous disruption of the CrT gene. In particular, the reduction of serum Cr level may be explained by defective gut absorption from the diet (Garcia-Miranda et al., 2009; Skelton et al., 2011). The only body fluid in which Cr levels resulted to be increased is urine; it is likely that the lack of a functional transporter impairs the creatine salvage normally operated by the kidney (van de Kamp et al., 2014). Consistently, we found an elevated creatine/creatinine (Cr/Crn) ratio in the urine of mutant mice, probably due to a combination of reduced renal reabsorption of creatine and decreased creatinine excretion.
Our behavioral investigation highlighted that CrT−/y mice carrying a different deletion than previously reported (Skelton et al., 2011) exhibit a broad spectrum of phenotypes establishing the validity of this model and corroborating its utility in translational studies. Mutant mice, indeed, show cognitive impairments in a battery of learning and memory tests aimed at assessing both explicit and implicit memories such as object-recognition task, Y maze and Morris water maze. The memory deficiency assessed across a variety of behavioral tasks indicates that CrT−/y animals have a general cognitive impairment, which is a key clinical feature in CCDS1 patients.
While the motor development is only mildly delayed in CCDS1 patients (van de Kamp et al., 2013) and myopathic symptoms have been rarely described (Anselm et al., 2006; van de Kamp et al., 2013), mostly as late onset deficits (deGrauw et al., 2002; Hahn et al., 2002; Kleefstra et al., 2005), we found that reduced muscle levels of Cr measured in mutant animals were accompanied by alterations of motor behavior. CrT−/y mice, indeed, showed significantly decreased home-cage-locomotor activity (particularly evident during the night period) and they were slower swimmers than CrT+/y mice. In contrast, we found that vulnerability to stress and anxiety responses are not sensitive to CrT deletion. The lack of a genotype effect for the motor behavior in the open field test may be due to the aversive nature of the arena, which may affect the explorative aptitude of both wild-type and mutant mice, thus masking the difference in motor activity between the two groups. It is worth noting that our data allow to exclude the possibility that an impaired motor activity can interfere with the animals’ cognitive performance. Indeed, in the ORT sample phase and the Y maze test the total exploration of objects and/or arena was equal for mutant and control mice, while for the Morris water maze we analyzed the path length covered to locate the submerged platform avoiding the confounding effects of the reduced swimming speed of mutant mice. Future studies using conditional mouse models with a disruption of CrT allele only in the brain tissue will be useful to dissect the role of peripheral Cr in the development of cognitive deficits. It has been reported that a CrT deletion exclusively restricted to forebrain excitatory neurons during late postnatal development induces selective learning and memory deficits without affecting motor behavior (Kurosawa et al., 2012).
Because of the importance of Cr in normal retinal function and development (Acosta et al., 2005), it has been suggested that an alteration of visual capabilities might play a role in the cognitive deficits displayed by CrT−/y animals. We reported that during the ORT sample phase all experimental groups equally explored and observed the objects, with the total exploration time of mutant mice very close to that recorded for the control group, suggesting that no major impairment is present in the visual system of CrT–/y animals. In addition, to avoid possible confounding effects due to reduced visual acuity, the tank used in the Morris water maze task was surrounded by a set of extra-maze cues in a visual discrimination range detectable even by partially-sighted animals.
In conclusion, this CrT−/y murine model will provide a new tool for improving preclinical evaluation of potential CCDS1 intervention treatments. The results confirm previous data suggesting that CCDS1 can be well modeled in mice (Kurosawa et al., 2012; Skelton et al., 2011). Null mice display an impairment of motor behavior rarely present in human patients; however, the use of conditional mice will avoid this problem. Since CCDS1 is still an untreatable pathology, there is a compelling need for developing effective therapeutic strategies. The availability of murine models that reliably reproduce the human condition will fuel and support the research in this field. To assess the reproducibility and the predictive validity of promising treatments for CCDS1 as well as for other disorders, the validation of findings in more than one animal model is strongly desirable prior to launching later-stage translational or clinical projects (Katz et al., 2012). Since another invalidating hallmark of CCDS1 is the frequent occurrence of seizures, additional studies in CrT−/y mice analyzing the behavioral response to kainic-acid injection will be required to provide useful information about seizure susceptibility in this model.
F1000Research: Dataset 1. Complete data for neurochemical and behavioral assessment in a mouse model of creatine transporter deficiency., 10.5256/f1000research.5369.d42180 (Baroncelli et al., 2015).
GC, VL and TP conceived the study. TP and LB designed the experiments. LB, MGA, JT, EP and MM carried out the research. EA, FZ and CG produced and provided the mouse model. LB and TP wrote the manuscript. All authors were involved in the revision of the draft manuscript and have agreed to the final content.
Work was supported by the International Foundation for CDKL5 Research (E.A.), and EMBL (E.A., F.Z., C.G.). Production and housing of transgenic mice was supported by the EMBL Transgenic Services and Mouse Facilities under the guidance of Pedro Moreira and Maria Kamber, respectively.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 22 Jan 15 |
read | read | read |
|
Version 1 29 Sep 14 |
read | read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)