Keywords
malignant pleural effusion, palliation, pleurodesis
malignant pleural effusion, palliation, pleurodesis
Malignant Pleural Effusion (MPE) is a well described event in the natural history of advanced malignancy. Malignant etiology accounts for 22% of the diagnosed pleural effusions1. Using data from the 2012 Nationwide Inpatient Sample (NIS), Healthcare Cost and Utilization Project (HCUP) and Agency for Healthcare Research and Quality, it is estimated that the aggregate charges (the “national bill”) were 722 million dollars in the USA2.
Palliation with minimal adverse events remains the cornerstone of management3. Talc pleurodesis and Indwelling Pleural Catheters (IPC) are the two most commonly used palliative approaches.
Talc pleurodesis can be achieved either by thoracoscopic instillation i.e.; talc insufflation/poudrage (TTI) or via a bedside chest tube i.e. talc slurry (TS). Existing systematic reviews concluded that thoracoscopic talc insufflation/poudrage was more efficacious in preventing recurrences when compared to bedside chest tube talc slurry4,5. New prospectively designed studies comparing TTI and TS have been published since then6–8. However the best initial approach for talc pleurodesis remains still unclear. To address the need for an update, a systematic review and meta-analysis of studies comparing thoracoscopic talc insufflation/poudrage and talc slurry in terms of patient centered outcomes was performed.
We conducted a systematic review with meta-analysis of studies undertaken between 01/01/1980 and 10/1/2014 using MEDLINE (PubMed, OVID), EBM Reviews (Cochrane database of Systematic Reviews, ACP Journal Club, DARE, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register, Health Technology Assessment and NHS Economic Evaluation Database), EMBASE and Scopus. Unpublished data sets such as conference abstracts and ClinicalTrials.gov were also included in the full review phase to reduce the effect of publication bias9.
The following keywords were used: chemical pleurodesis, pleurodesis, talc pleurodesis, bedside pleurodesis, surgical pleurodesis, medical pleurodesis, thoracoscopic pleurodesis, thoracoscopic talc pleurodesis, thoracoscopic poudrage, thoracoscopic talc poudrage, talc insufflation, thoracoscopic talc insufflation, pleuroscopy, medical thoracoscopy, talc poudrage, talc slurry, tube thoracostomy, chest tube talc slurry and malignant pleural effusion. Both keywords and medical subject headings were used in a Boolean search strategy. An example search strategy can be found in the Appendix 1.
In addition, a pearl growing strategy was employed using frequently cited reviews of malignant pleural effusion treatments. They were included to be analyzed in the full review phase of the study. Approval from the Institutional Review Board was unnecessary because this is a meta-analysis.
Inclusion and exclusion criteria were framed prior to the implementation of the search strategy. To evaluate outcomes in adult malignant pleural effusion patients (18 + years) undergoing talc pleurodesis, we included studies based on the following criteria:
1) A randomized design was used in studying talc pleurodesis in patients with malignant pleural effusion between 01/01/1980 and 10/1/2014.
2) Patients undergoing bedside TS were compared with patients undergoing thoracoscopic talc insufflation/poudrage (TTI) in the above fashion.
3) Sufficient outcomes data were reported [Risk of recurrence of the pleural effusion, respiratory complications and non-respiratory complications].
Non-English publications, case reports and series, pediatric studies, descriptive studies without a control group, retrospective studies and prospective controlled studies without randomization were excluded. Eligible articles were reviewed by two reviewers for inclusion; disagreements were resolved via discussion. An examination of the full-length articles was carried with the intent of eliminating duplicate studies or same patient cohorts.
Two reviewers independently extracted and rated the data from the selected full length articles using a standardized form. From each study, the data abstracted included study name/year, study design (prospective controlled, randomized controlled trial, retrospective etc.), cancer cell type, patient inclusion criteria, sample sizes for the bedside/surgical pleurodesis arms, technique employed in the bedside/surgical arms and the follow-up schedule.
Outcomes data pertaining to the risk of recurrent pleural effusions, respiratory complications, and non-respiratory complications were also extracted.
Talc pleurodesis for recurrent malignant pleural effusion is a palliative procedure and does not aim to have a mortality benefit. Therefore, measuring mortality outcomes was not the focus of the meta-analysis.
Various definitions for recurrent pleural effusions have been used in prior studies6,10. A clinically significant recurrent pleural effusion was defined a priori as accompanied by symptoms or a need for repeat pleural procedures. Where possible, asymptomatic radiological recurrences were not included in the meta-analysis.
Respiratory complications were defined as occurrence of respiratory conditions such as pneumonia, Acute Respiratory Distress Syndrome (ARDS), acute respiratory failure, re-expansion pulmonary edema, bronchospasm, empyema, pulmonary embolism, prolonged air leak, bronchopleural fistula, atelectasis requiring bronchoscopy and subcutaneous emphysema.
Immediate non-respiratory complications were tabulated from the complications mentioned in the full length articles. These included fever, wound infection, chest pain, tumor recurrence at site, myocardial infarction, need for blood transfusions, arrhythmias and immediate post procedural death.
The randomized controlled trials that met inclusion criteria were evaluated for quality using components of the modified Jadad scale11. The presence of the following features was appraised:
A) A description of the study confirming the randomized nature.
B) Method of allocation to the study arms described and whether adequate/inadequate.
C) Description of withdrawals and dropouts.
Due to the nature of the comparison (surgical vs bedside procedure), we felt that other features of the scale (description of a double blind nature) could not be appraised during our quality assessment. Two raters independently determined the quality of the studies included. Disagreements were resolved by discussions and final consensus.
Outcomes data for recurrent pleural effusions, respiratory and non-respiratory complications were summarized using descriptive statistics (simple count, proportion of the study sample). They were visually presented in Forest plots. The Mantel-Haenszel method12 was used to combine data from individual studies and the results were reported as pooled relative risks (RR). Heterogeneity among the studies included was investigated by performing the I2 test13. Meta-analyses were conducted using the fixed effects model when heterogeneity between studies was low (I2 <40%) and the random effects model otherwise9.
To confirm the robust nature of the results, a sensitivity analysis was performed by removing one study at a time and determining the outcome.
Publication bias was examined by visually examining the filled funnel plots using trim and fill method. Other methods (Begg’s correlation14 and Egger’s linear regression intercept)15 were additionally used.
All analyses were performed using a statistical software package (Comprehensive Meta-Analysis, version 2.2.064; Biostat, Englewood, NJ).
Based on initial search, 137 articles were obtained and reviewed independently by two reviewers. Pearl growing strategy was employed to seek additional articles and resulted in five articles. A clinical trial registry (www.ClinicalTrials.gov) was also examined and resulted in one additional article. These 143 articles were reviewed and 115 articles were excluded based on title and abstract. A total of 28 potential studies were thus identified with our search strategy. Twenty-four studies were further excluded, leaving four studies6,10,16,17 for the final analysis. The sequence describing the above process can be found in Figure 1.
None of the studies restricted the study population to a single cancerous cell type.
None of the included studies employed thoracoscopic evacuation of the malignant pleural effusion prior to bedside TS insertion via a chest tube.
Follow-up periods varied through the studies (Range = 30–425 days). Where available, recurrence data for the most distal available time point were selected for the meta-analysis.
All of the studies included in the analysis underwent quality assessment. The average Jadad score11 was 1.5 out of a maximum possible score of 4 (Ranges 1–2). Out of the possible seven ways to assess the quality11, only four questions could be answered due to the nature of the intervention. It was not possible reliably or ethically for the original investigators to have carried out efficient blinding in a surgical versus bedside clinical experiment.
The results of the pooled RR are shown in Figure 2. The four studies included in this analysis enrolled a total of 454 patients with malignant pleural effusion (Table 1). There was no statistically significant difference in the risk of recurrent pleural effusions between the bedside TS (recurrences/patients who underwent pleurodesis, n/N = 51/218 pts) and the TTI groups (recurrences/patients who underwent pleurodesis, n/N = 39/236 pts, pooled RR 0.72; 95% CI 0.50-1.05; Q statistic, 3.58; I2 statistic, 16.27%). There was no evidence of publication bias (P-value = 0.49 for the Begg’s test, P-value = 0.54 for the Egger’s regression intercept). After using the trim and fill methodology (Figure 3), these results did not change (RR- 0.78, 95% CI = 0.54-1.12, Q statistic, 6.8).
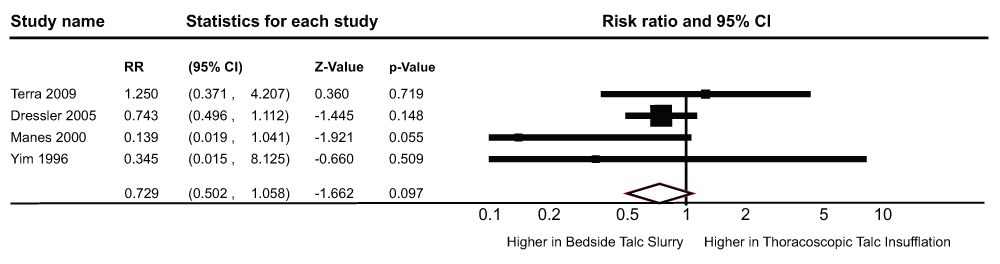
RR, risk ratio, CI, confidence interval.
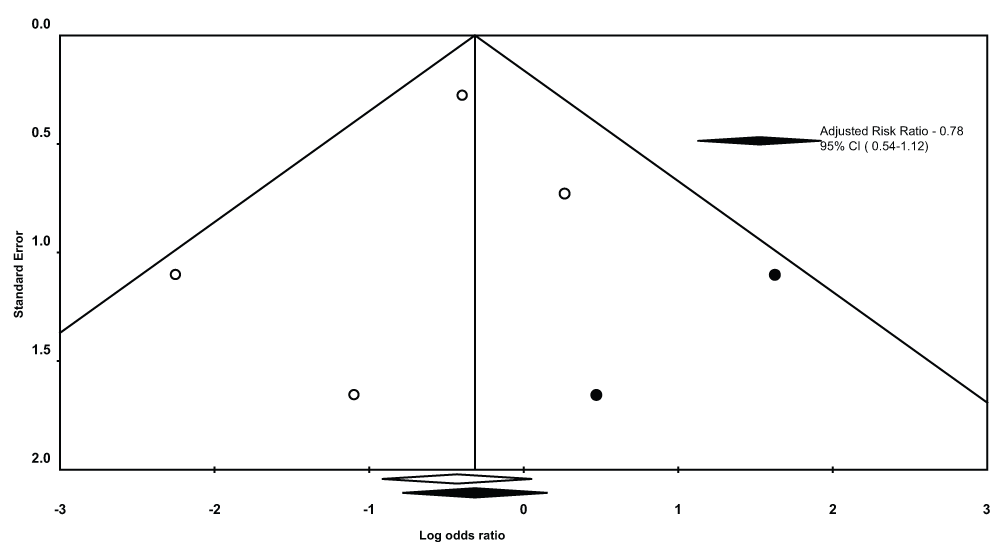
The definitions of a recurrent pleural effusion in the included studies varied. One study defined the recurrence based on radiological data alone6. The remainder of the studies clearly mentioned the number of patients who were symptomatic and required further pleural procedures once a recurrent pleural effusion was diagnosed10,16,17.
A sensitivity analysis pooling data from studies reporting only clinically significant recurrences was performed leaving out one study6. This did not result in a different statistical outcome (pooled RR 0.49; 95% CI 0.11-2.22; Q statistic, 3.52; I2 statistic 43.22%).
As follow-up periods varied widely, a sensitivity analysis pooling the data from studies reporting 30 day outcomes was carried out and did not result in a different statistical outcome.
The results of the pooled RR are shown in Figure 4. The four studies included in this analysis reported outcomes on a total of 591 patients who underwent talc pleurodesis for palliation of malignant pleural effusion (Table 2).
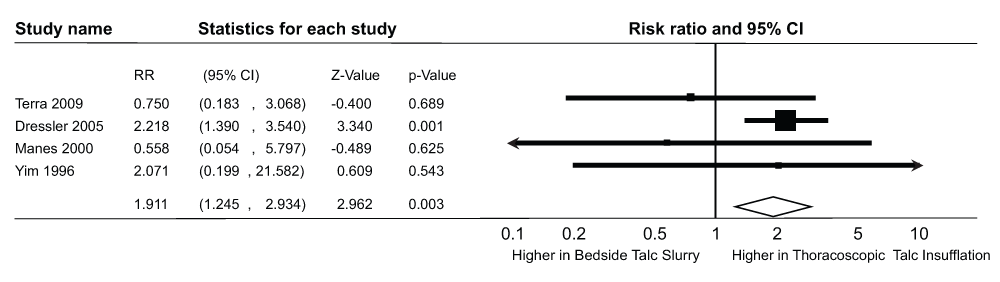
RR, risk ratio, CI, confidence interval.
There was a statistically significant higher risk of post procedural respiratory complications in the TTI group (Incidence of respiratory complications/Pts who underwent pleurodesis, n/N = 59/307 pts) compared to the TS group (Incidence of respiratory complications/Pts who underwent pleurodesis, n/N = 28/284 pts, pooled RR 1.91, 95% CI = 1.24-2.93, Q statistic 3.15, I2 statistic 4.79%). There was no evidence of publication bias (P-value = 1.0 for the Begg’s test, P-value = 0.24 for the Egger’s regression intercept). After using the trim and fill methodology (Figure 5), these results did not change (RR- 1.99, 95% CI = 1.30-3.04, Q statistic, 4.32).
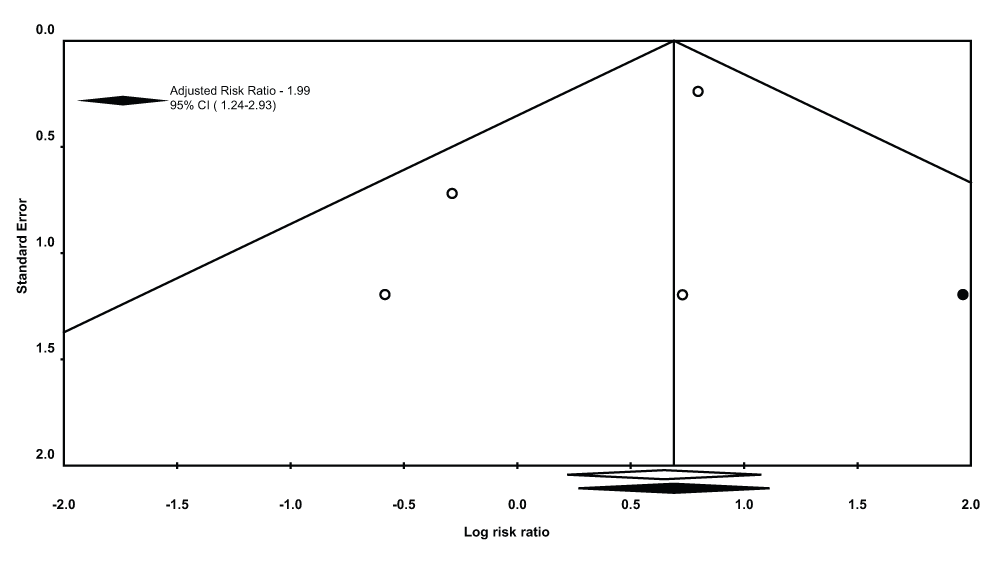
A sensitivity analysis pooling data from studies with ≥ 2 score on the Modified Jadad scale was performed leaving out one study with a score of 117. This did not result in a different statistical outcome (pooled RR 1.99, 95% CI = 1.29-3.08, Q statistic 2.05, I2 statistic 2.49).
The results of the pooled RR are shown in Figure 6. The four studies included in this analysis reported outcomes on a total of 591 patients who underwent talc pleurodesis for palliation of malignant pleural effusion (Table 3).
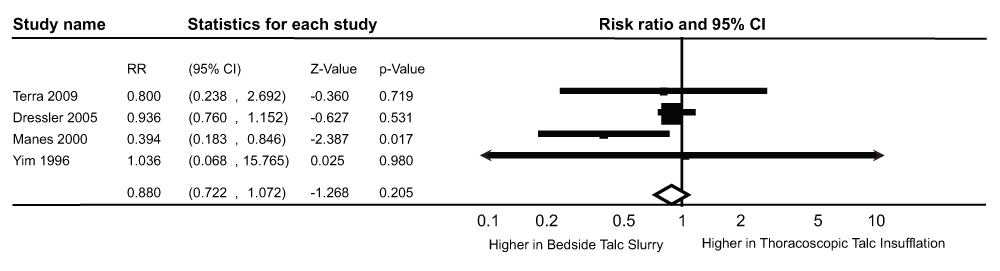
RR, risk ratio, CI, confidence interval.
There was no statistically significant difference in the incidence of non-respiratory complications between the TTI group (Incidence of non-respiratory complications/Pts who underwent pleurodesis, n/N = 110/307 pts) and the TS group (Incidence of non-respiratory complications/Pts who underwent pleurodesis, n/N = 116/284 pts, pooled RR 0.88, 95% CI = 0.72-1.07, Q statistic 4.61, I2 statistic 34.96%). There was no evidence of publication bias (P-value = 1.0 for the Begg’s test, P-value = 0.48 for the Egger’s regression intercept). After using the trim and fill methodology (Figure 7), these results did not change (RR- 0.93, 95% CI = 0.76-1.12, Q statistic, 9.8).
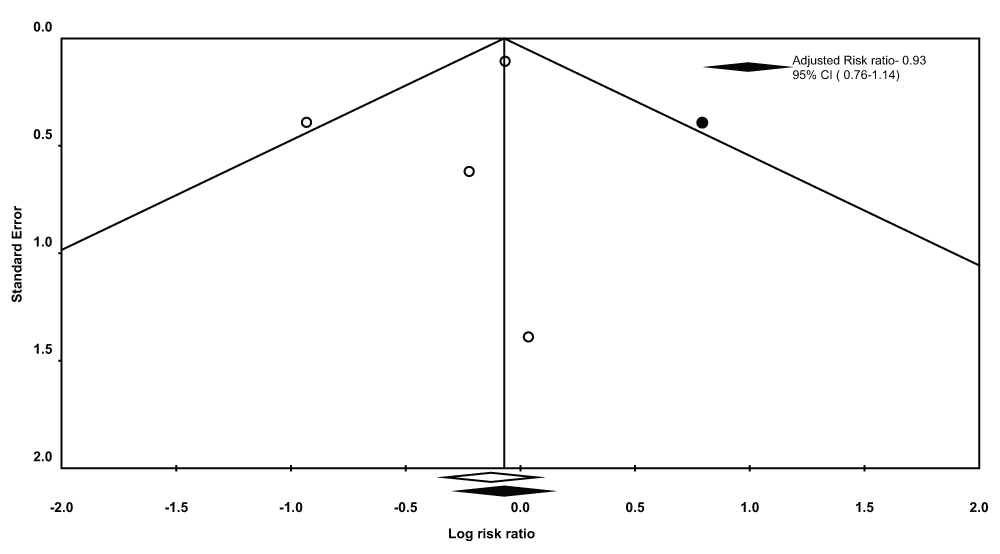
A sensitivity analysis pooling data from studies with ≥2 score on the Modified Jadad scale was performed leaving out one study with a score of 117. This did not result in a different statistical outcome (pooled RR 0.93, 95% CI = 0.76-1.14, Q statistic 0.06, I2 statistic 0.0).
Many experts believe that serial thoracentesis is not an ideal choice for treating the recurrent malignant pleural effusion18,19.
Talc pleurodesis was first performed in 193520 and is still commonly employed in the treatment of malignant pleural effusions. Although studies have shown talc to be the best chemical agent in terms of pleurodesis success and risk of recurrence21,22, the best method of applying talc remains controversial. Our meta-analysis demonstrates that both talc poudrage (TTI) and talc slurry (TS) offer similar protection against clinically significant recurrences. There was no difference in the risk of clinically significant recurrence (i.e., further need for pleural procedures or symptoms). TTI did have a greater risk of respiratory complications. There was, however, no difference in the rate of non-respiratory complications such as fever and need for blood transfusions.
Our results are in contrast to those of previous meta-analyses5, including the recently withdrawn Cochrane analysis which suggested improved success rates of talc pleurodesis utilizing TTI. The conclusion of these analyses was that thoracoscopic pleurodesis with talc was the optimal method for pleurodesis in patients with malignant pleural effusions. However, several newer prospective studies have been published since6,7 and have been incorporated into the present analysis.
Arguments in favor of TTI include the observation that there is more complete lung expansion after the procedure18. This is certainly understandable given that take-down of adhesions is typically performed during the procedure itself as opposed to TS. Interestingly, Terra et al. using CT scanning post-TTI and TS to assess degree of post procedure lung expansion did not find a correlation between clinical outcomes and initial degree of lung expansion7. These authors postulated that factors other than the degree of visceral and parietal pleura apposition were important in determining the success of pleurodesis. Similarly, Mager et al. using 99m Tc-labeled talc showed that rotation protocols did not affect the overall dispersion of talc suspensions after TS23. The degree of dispersion also did not affect pleurodesis success23.
In comparing TTI and TS, several difficulties arise. Pleurodesis success rates vary in the literature, due to the inconsistent definition of pleurodesis success and failure used in different studies. Failure or recurrence has been defined radiologically in some studies6 but it has been argued that patient centered outcomes such as new symptoms and need for further pleural procedures are more pertinent outcomes7. In our meta-analysis, we determined recurrence a priori as the development of symptoms or the further need for pleural procedures and disregarded asymptomatic radiological recurrences where possible.
The technique of both TS and TTI vary significantly between centers and this is evident in the included studies. TS varied in regard to length of chest tube clamping, rotating or not-rotating the patient, size of chest tube, and timing of chest tube removal. With regard to TTI, in three of the four studies TTI was performed under general anesthesia and the ability to tolerate general anesthesia was in fact an entry criteria. Overall, 88% of patients in our analysis underwent general anesthesia for TTI. One could argue that the increased respiratory complications observed with TTI may be related to general anesthesia and single lung ventilation. Despite the concerns of ARDS with the use of ungraded talc, the studies included in our meta-analysis did not report specific cases of ARDS. Non-specific respiratory failure was, however, reported in patients in the study by Dresler6 and Yim et al. reported a case of acute respiratory failure in the TS group16.
With the increasing numbers of interventional pulmonologists performing pleuroscopy (medical thoracoscopy)24 under local and/or moderate sedation, the question of which procedure is the most optimal for talc pleurodesis is increasingly relevant. Whether talc poudrage performed during pleuroscopy with local or moderate sedation and dual lung ventilation is equivalent to surgical thoracoscopy (VATS) in terms of pleurodesis success and complications is unknown. Further studies are needed to compare talc poudrage performed with pleuroscopy versus TS.
One may wonder whether the question of TTI versus TS is still relevant in the era of indwelling pleural catheters (IPCs). Certainly, in the patient with trapped lung, both TS and TTI would likely be ineffective and indeed all of the studies in this meta-analysis excluded patients with possible trapped lung physiology. In the patient with malignant effusion without trapped lung, however, clear superiority of IPCs has not been demonstrated3. In fact, talc pleurodesis may be more economical compared to IPC in patients with good performance status and projected life expectancy of > 6 weeks25,26. The issue of cost is especially relevant in the era of health care reform and accountable care organizations. With the advent of newer molecular/hormonal therapies especially in breast cancer, malignant pleural effusion is increasingly recognized as a non-terminal event27. Perhaps most importantly, patient preference is paramount28 and no study has clearly demonstrated the superiority of IPCs compared to talc pleurodesis.
Our study has several limitations. The results of meta-analyses are dependent on the quality of the studies included. The inclusion of only randomized controlled trials was necessary due to significant bias inherent in non-randomized prospective studies. Despite the lack of heterogeneity between studies, the individual studies varied substantially (technique of talc pleurodesis, varying definitions of recurrence and follow-up schedule). Sensitivity analyses performed leaving out one study at a time did not impact the results, suggesting robust data. Publication bias is an inherent limitation of meta-analyses. It is reassuring to see that accounting for it did not result in a statistically significant departure from the original point estimates.
In conclusion, our meta-analysis demonstrates that there is no difference in malignant pleural effusion recurrence based on patient centered outcomes between talc poudrage and talc slurry. Respiratory complications are more common with talc poudrage via thoracoscopy. Further studies are needed, however, to look at the role of talc pleurodesis via pleuroscopy. The decision of which procedure to perform needs to take into account also the patient preferences.
SM co-conceived the study, participated in the design of the study, search strategy execution, performance of the statistical analysis, and writing of the manuscript.
AK participated in the search strategy execution, performance of the statistical analysis and writing of the manuscript.
PH co-conceived the study, participated in the design of the study, performance of the statistical analysis, and writing of the manuscript.
All authors read and approved the final manuscript.
1. Search “talc poudrage” [MeSH OR tw] – 162 results
2. Search “chemical pleurodesis” [MeSH OR tw] – 266 results
3. Search “pleurodesis” [MeSH OR tw] – 2154 results
4. Search “thoracoscopic pleurodesis” [MeSH OR tw] – 52
5. Search “thoracoscopic talc pleurodesis” [MeSH OR tw] -42
6. Search “thoracoscopic poudrage” [all terms] – 87
7. Search “thoracoscopic talc poudrage” [MeSH OR tw] – 49
8. Search “talc insufflation” [MeSH OR tw] – 57
9. Search “thoracoscopic talc insufflation” [MeSH OR tw] – 19
10. Search “pleuroscopy” and “talc pleurodesis” [All Fields] – 329
11. Search “pleuroscopy” and “talc insufflation” [All Fields] – 69
12. Search “pleuroscopy” and “talc poudrage” [All Fields] – 119
13. Search “talc slurry” [MeSH OR tw] – 95
14. Search 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 10 OR 11 OR 12 OR 13 – 2206 results
15. Search “malignant pleural effusion” [MeSH OR tw] -3426 results
16. Search “malignant pleuritis” [MeSH OR tw] -29
17. Search 14 and 15 – 580 results. Subsequently, the following filters were applied [Clinical trials including Phase I–IV, Controlled Clinical Trials, Randomized Controlled Trials, Reviews, Systematic Reviews, Meta-Analyses, observational studies, abstract availability, publication dates from 01/01/1989 to 12/31/2014, adult human studies, English-language publications] – 71 results.
18. Search 14 and 16. Filters applied [Clinical trials including Phase I–IV, Controlled Clinical Trials, Meta-Analyses, observational studies, randomized controlled trials, systematic reviews, review articles, abstract availability, publication dates from 01/01/1989 to 10/1/2014, adult human studies, English-language publications] – 0 results.
Terms such as thoracoscopic poudrage, chest tube talc slurry, thoracostomy talc slurry, chest tube talc pleurodesis, talc slurry sclerosis did not reveal any results in the [MeSH OR tw] category.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 17 Feb 15 |
read | ||
|
Version 1 27 Oct 14 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)