Keywords
medium pH, Pseudotsuga menziesii, Douglas-fir, micropropagation, 2-(N-morpholino)ethanesulfonic acid, MES
medium pH, Pseudotsuga menziesii, Douglas-fir, micropropagation, 2-(N-morpholino)ethanesulfonic acid, MES
The Christmas tree industry plays an important role within Pennsylvania agriculture as well as across the nation. The goal of this micropropagation project was to develop a true-to-type clonal propagation system to alleviate the cost of tree-to-tree variation by conventional seedling propagation. Understanding plant materials and their growing conditions may provide better assistance for later developmental stages in tissue culture.
The medium pH of plant tissue culture has been shown to be very important to many aspects of explant development and growth. Sensitivity or tolerance to medium pH change in vitro varies accordingly to specific requirements of individual species. Similar to soil pH, medium pH level may influence nutrient uptake (Ramage & Williams, 2002), cellular pH adjustment (Ballarin-Denti & Antoniotti, 1991), rooting and cellular growth (Leifert et al., 1992; de Klerk et al., 2008), plant gene expression and transcriptional pH responses in roots (Lager et al., 2010), and the efficiency of Agrobacterium-mediated transformation (Ogaki et al., 2008; Rai et al., 2012). Medium pH also can act to facilitate or inhibit nutrient availability in the medium such as ammonium uptake in vitro can be facilitated with a stable pH of 5.5 (Thorpe et al., 2008).
Medium pH fluctuations may be attributed to medium components, autoclaving, ion exchange, and environmental conditions. Medium components may modify pH prior to and after autoclaving (Skirvin et al., 1986; Owen et al., 1991). Organic, inorganic salts, amino acids, vitamins, sucrose, gelling agents, and plant growth regulators are the common components added to tissue culture medium. Williams et al. (1990) reported adding agar significantly elevated medium pH prior to autoclaving in group of pre-adjusted pH from 3.5 to 5.5 of MS medium (Murashige & Skoog, 1962) but less increment was found in group of pre-adjusted medium pH from 5.5 to 7.0, or decrease in group of pre-adjusted medium pH from 7.0 to 8.0. In contrast, post-autoclaving medium pH increased in group of pre-adjusted pH of 3.5–4.5 but had more significant medium pH decrease in group of pre-adjusted pH of 5–8. Additions of synthetic or natural organic acids generally increase medium buffering ability (Thorpe et al., 2008). Organic compounds such as 2-(N-morpholino)ethanesulfonic acid (MES) could especially help to maintain suitable medium pH range for explant development (Parfitt et al., 1988; de Klerk et al., 2008; Yuan et al., 2012). MES and vitamin additions were also found to enhance embryo growth during the initiation stage of Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) (Franco 1950) somatic embryogenesis (Pullman et al., 2005).
After placing explants on the medium, medium pH fluctuations were also observed for various species. Medium pH of loblolly pine (Pinus taeda) grown in liquid suspension medium showed subsequent pH decrease from 5.5 to 4.6 within 5 days of incubation followed by pH increase to 6.0 in 19 days of incubation (Pullman et al., 2005). Significant medium pH decrease was also found after placing mulla mullas (Ptilotus exaltatus) shoots on MS medium for 4 weeks (Williams et al., 1990) and an U shaped curve of medium pH fluctuation was found in shoot tip culture of crabapple (Malus sp. cv. Almey) and pear (Pyrus communis L. cv. Seckel) on MS medium (Singha et al., 1987). According to Skirvin et al. (1986) and Thorpe et al. (2008), explant nutrient absorption in vitro is a function of ion exchange where deposition of free hydrogen ions (H+) and hydroxyl ion (OH-) in the medium may contribute to acidic or alkaline medium pH. In contrast, photooxidation induced chelating event bound free iron to reduce iron availability may also influence medium pH (Hangarter & Stasinopoulos, 1991). Secretion of plant secondary metabolites into culture medium is common in vitro (Dörnenburg & Knorr, 1995) but its role and function in altering medium pH and nutrient absorption are not clear.
Medium pH fluctuations can involve many factors, and could eventually become problematic if lacking of care attentions. Douglas-fir shoot culture can be grown in a modified version (mDCR) of Douglas-fir cotyledon revised medium (DCR, Gupta & Durzan, 1985; Gupta & Durzan, 1987a). However, the interactions between the species and its growing medium are not well understood. To ensure an optimal shoot culture development and provide high quality shoots for later development of rooting protocol, medium pH can be a key indicator in determine subculture time. It may also be further utilized as a diagnostic tool for some abnormal growth symptoms such as necrosis, caused by low pH induced nutrient deficiency. The experimental system may be applied to studies of explant development and nutrient relationship in vitro (Singha et al., 1990). Hence, the objectives of this study are to 1) determine medium pH change over various times under storage conditions and in the presence of explants, 2) evaluate the effects of prolonged culturing on medium pH and explant growth performance, and 3) assess the effects of addition of a pH stabilizer, MES to Douglas-fir micropropagation medium.
Spring buds from juvenile (HF205 and HF210) and mature (PS-2) donor trees collected in 2006 were utilized for this study. These bud samples were collected prior to breaking dormancy. To classify juvenility, Douglas-fir trees of Lincoln seed source planted 10 years ago and not yet producing cones at the Penn State Horticulture Research Farm of Russell E. Larson Agricultural Research and Education Center at Rock Springs were the selected donors. The selected mature donor tree, as a result from a previous genetics study conducted by Gerhold (1984) was over 40 years old at the Penn State golf course. These bud samples were stored in a 4ºC cold room until further culture initiation. Preparation, sterilization, and dissection of collected bud sample were followed as per Traore et al. (2005).
This study consisted of a four-factor factorial design with three replications for each factor combination. Two juvenile genotypes, HF205 and HF210, and one mature genotype, PS-2 were entered for evaluations. Two types of media were used including mDCR only and mDCR with 2 g/L of MES (mDCR+MES) (M3671, Sigma-Aldrich, St. Louis, MO, USA). Levels of media pH were pre-adjusted to 3.6, 5.1, 5.7, 6.3, and 7.8 before adding 7 g of agar, MES, and autoclaving. Five dissected vegetative buds from each genotype were placed on each treatment combination. Controls consisted of medium without the presence of explants, at each pH level. They were placed in full dark versus light conditions in 25°C growth chambers. For treatments, explants were dissected and placed into mDCR versus mDCR+MES media for incubation for 1, 3, 5, 7, 14, 21, 28, and 35 days.
During the dissection process, measurements of samples were taken for initial bud weight (mg) and petri dish (PD) weight (mg) with solidified media. After each treatment and incubation time, final media pH, final PD weight (mg), and explant final weight (mg) were recorded. Explant weight change (mg) was obtained by subtracting the initial bud weights from final explant weights. Medium pH was measured at five positions in the plates, between the explants, using a Thermo Orion PerpHecT pH meter (Thermo Fisher Scientific Inc., Waltham, MA, USA). Data analyses were performed using Minitab (Minitab Inc., State College, PA, USA), and graphs were generated by SigmaPlot (Systat Software Inc., Chicago, IL, USA), including ANOVA General Linear Model (GLM), Tukey Honestly Significant Difference test (HSD), and linear regression with significance set at p<0.05.
After autoclaving, media pH shifts were found according to each pre-adjusted media pH. From 100 samples, mDCR medium showed a greater extend of pH fluctuations than mDCR+MES post-autoclaving. Media initially set at pH 3.6, 5.1, and 5.7 were increased for both media type (pH changes of 0.83, 0.58, 0.17 and 0.76, 0.22, 0.11 for mDCR and mDCR+MES, respectively) whereas medium initially at pH 7.8 were decreased (-0.66 and -0.59 for mDCR and mDCR+MES, respectively). Medium of pre-adjusted pH 6.3 showed decrease in mDCR (-0.11) but increase for mDCR+MES (0.05). In general, dark storage was better to maintain stable media pH than storage in light. Overall, media pH was 5.8 (n=400) when kept in the dark condition compared to pH 5.4 (n=400) in the light (P=0.000). Over the incubation times tested, media pH was maintained at fairly stable condition with a slight decreasing trend in the dark storage condition. In contrast, media pH had a stronger decreasing trend when incubated in the light. The mDCR+MES media maintained media with less pH change over the incubation times when compared to mDCR media in the light (Figure 1).
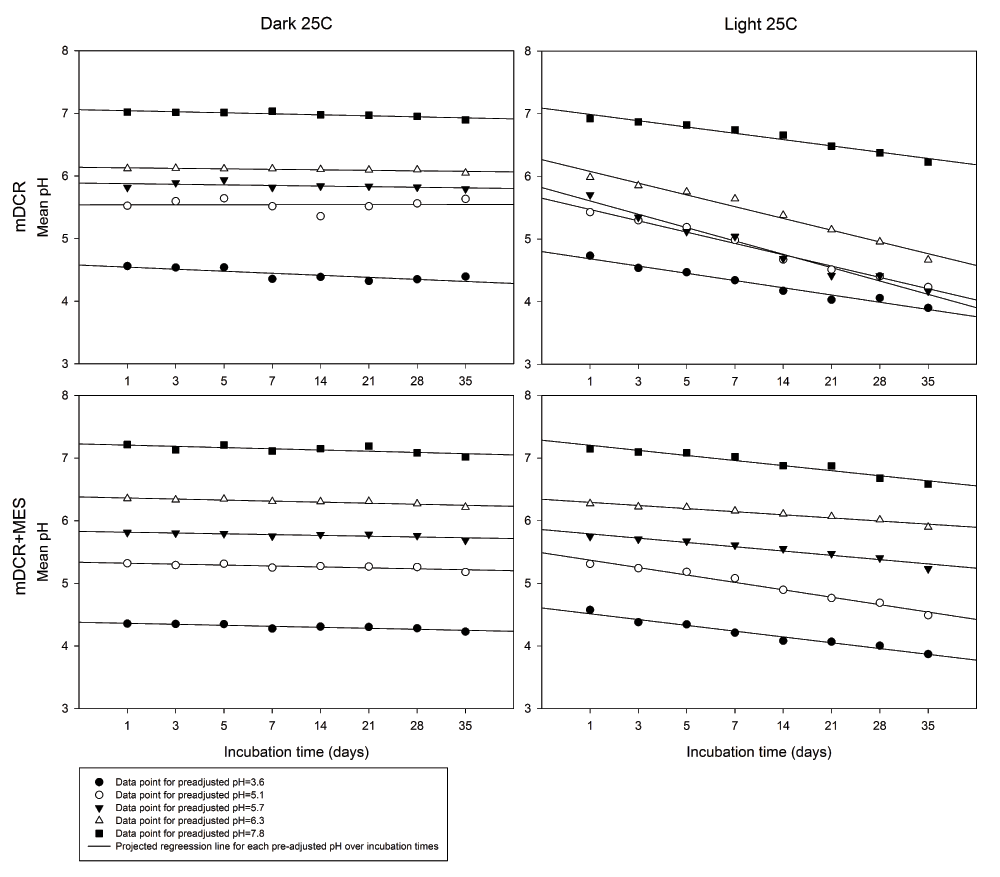
Medium pH was pre-adjusted to 3.6, 5.1, 5.7, 6.3, and 7.8 prior to adding 7 g/L of agar, MES and autoclave. Medium was incubated in a growth chamber with full light or darkness at 25ºC until it reached incubation requirement. Post-autoclaving pH was recorded using a pH meter at each incubation time. Datapoints represent mean pH (n=5 for each datapoint), and were fitted with linear regression lines. Please see Dataset 1 for the raw data.
After placing explants into the media, the pH of the medium was significantly influenced by all factors, genotype, media type, initial pH level, and incubation time (all P=0.000). Overall medium pH was 5.45 from medium incubated with PS-2 (n=1,355), which significantly greater than 5.41 and 5.19 from those incubated with HF210 (n=1,384) and HF205 (n=950), respectively. The medium pH from mDCR+MES (n=1,805) was significantly greater than the pH of mDCR only medium (n=1,884) (5.45 vs. 5.28, respectively) (P=0.000). Decreasing media pH over incubation time and variation among genotypes were both observed. Media with addition of MES was better able to maintain stable media pH up to 21 days of incubation from each of the 5 different initial pH levels (Figure 2). Regardless of initial pH level, incubation time had a strong significant effect on media pH (P=0.000). Combined two types of media, mean medium pH showed the lowest value of 5.04 (n=435) at 21-day of incubation. In contrast, the highest mean medium pH 5.88 was recorded at the 42-day of incubation (n=125). Overall medium pH at each initial pH (3.6, 5.1, 5.7, 6.3, and 7.8) showed significant differences between each other including 4.90 (n=744), 5.08 (n=740), 5.30 (n=745), 5.57 (n=740), and 5.99 (n=720), respectively. The media pH stabilizer MES demonstrated its ability to prevent media pH from dropping at the higher or lower ends of initial pH levels. Within individual genotype, both media type, and incubation time showed significant effects on media pH for all genotypes (P=0.000).
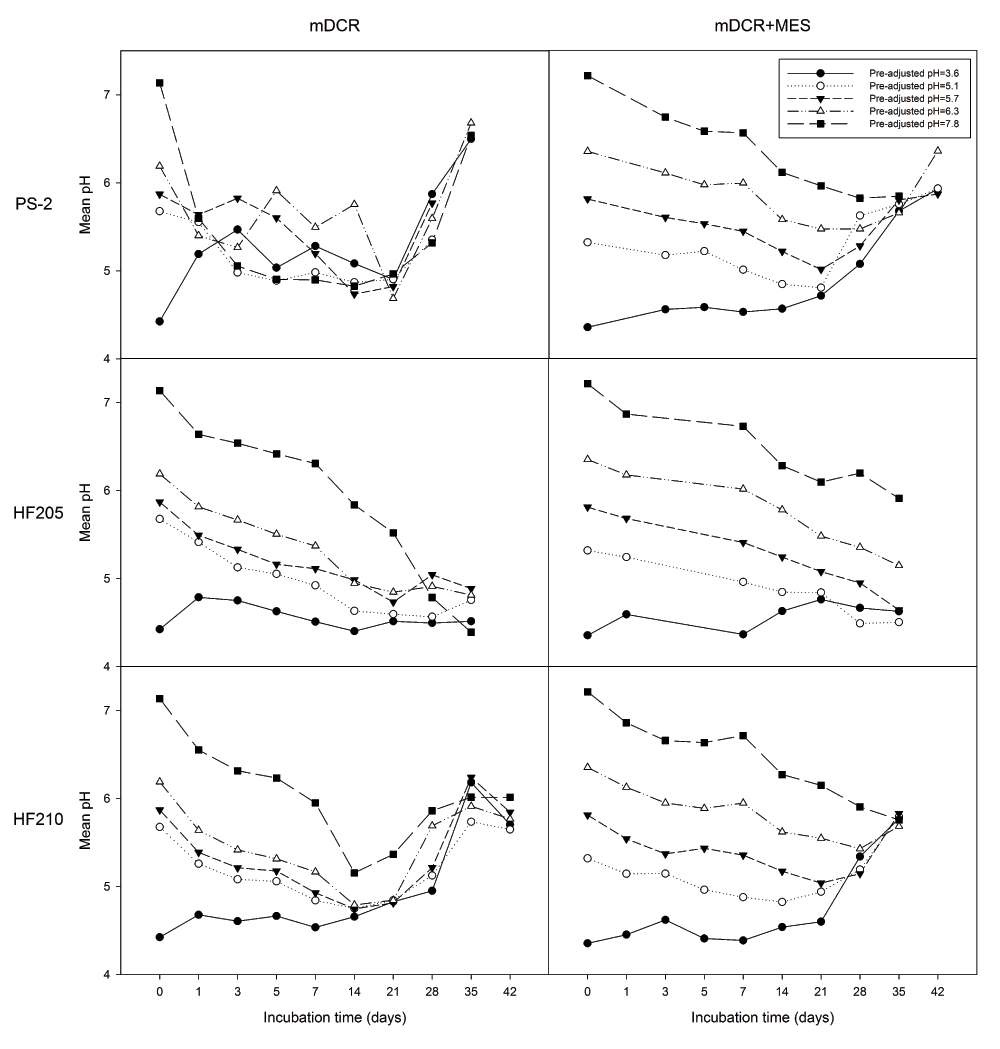
Genotypes included one mature genotype, PS-2 and two juvenile genotypes, HF205 and HF210. After surface sterilization and dissection, inner vegetative buds from above three genotypes were placed on either mDCR or mDCR+MES medium for incubation up to 42 days. These buds were incubated in a growth chamber at 25ºC with light regime adjusted to 16-hour light followed by 8-hour darkness each day. Medium pH was recorded using a pH meter when sample reached each incubation requirement where five pH values were recorded within each petri dish. Datapoints represent mean pH. Please see Dataset 2 and 3 for the raw data.
For explants growth response, genotype (P=0.000), incubation time (P=0.000), and initial pH (P=0.012) showed significant effects on explant weight increment (mg). The addition of MES into the media did not show a significant effect on explant weight increment (P=0.281) (Figure 3). Overall, HF210 (30.86 mg, n=264) and PS-2 (22.50 mg, n=190) had a significant weight increment greater than HF205 (13.83 mg, n=205) (P=0.000). Explant weight increment of HF210 did not show a significant difference when compared with PS-2 (P=0.2903). However, a distinct trend in explant weight decrease was observed after 28 days of incubation in mDCR only medium for HF210. Initial medium pH of 3.6 had a significantly greater bud weight change (P=0.005) than medium pH 7.8 (25.89 vs. 19.23 mg, n=134 vs. 130, respectively). An increasing trend of bud weight change was observed but bud weight did not show significant increase during the first week of incubation.
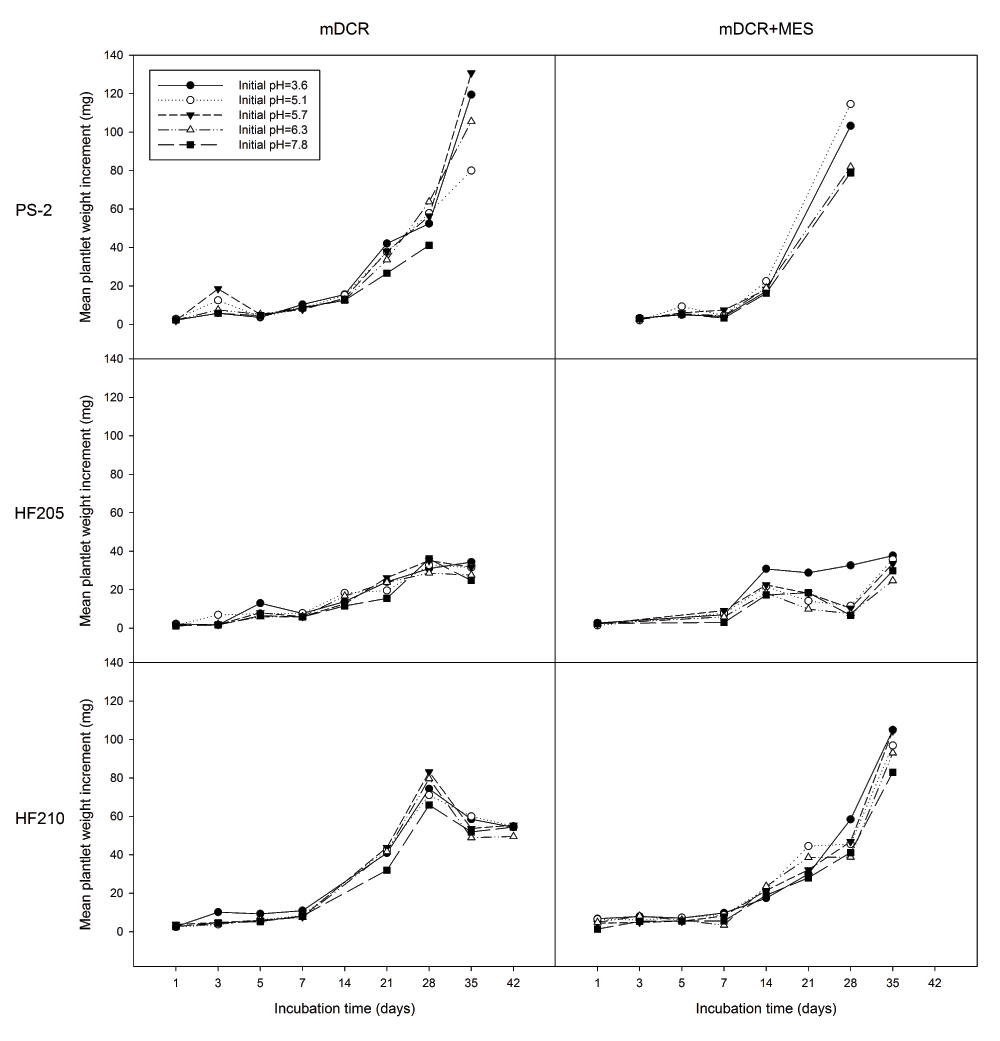
Genotypes included one mature genotype, PS-2 and two juvenile genotypes, HF205 and HF210. After surface sterilization and dissection, inner vegetative buds from above three genotypes were placed on either mDCR or mDCR+MES medium at each initial pH level (3.6, 5.1, 5.7, 6.3, and 7.8) for incubation up to 42 days. These buds were incubated in a growth chamber at 25ºC with light regime adjusted to 16-hour light followed by 8-hour darkness each day. Bud weight change was recorded using an electronic scale when sample reached each incubation requirement. Datapoints represent mean weight change (mg). Please see Dataset 4 for the raw data.
Comparing individual genotypes, medium type exhibited non-significant effect on explant weight increment of all three genotypes (P>0.05). For HF205, the weight differences were observed at the higher and lower ends of the given initial pH levels (Figure 4). Incubation time showed significant effect on explant weight increment for all three genotypes (P=0.000). For all three genotypes, explant weight did not show any significant differences for the first 7 days of incubation. However afterwards, explant weight growth dramatically increased for PS-2 and HF210. HF205 showed much less weight increment than the other two genotypes (Figure 5).
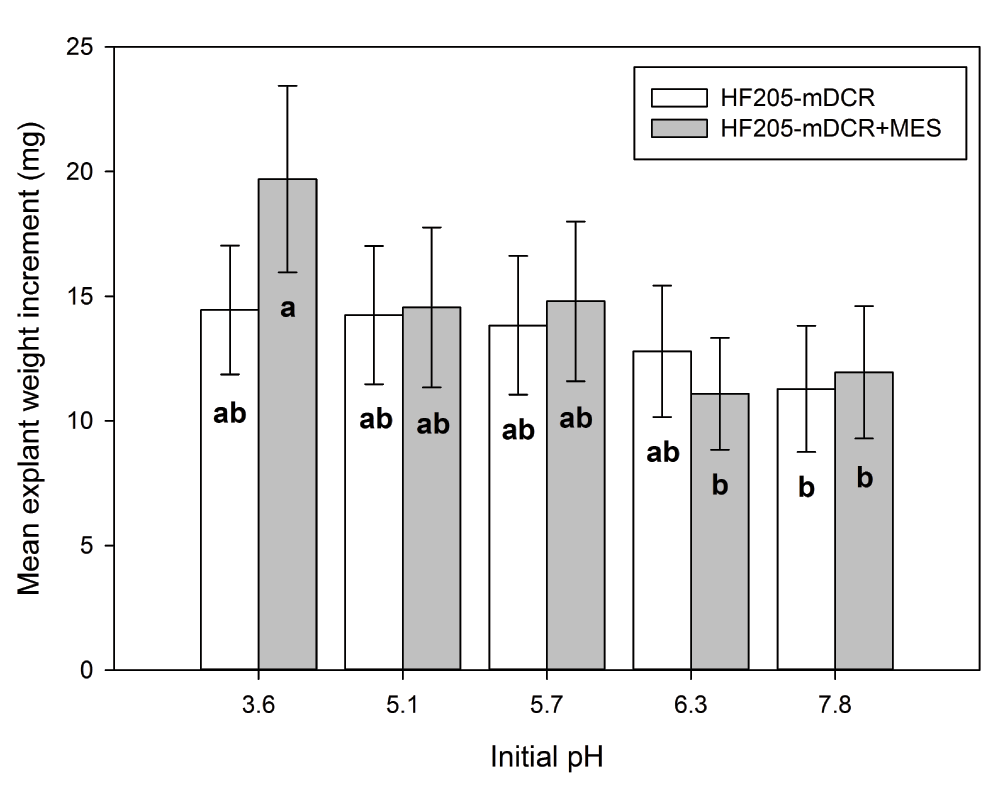
After surface sterilization and dissection, inner vegetative buds from HF205 were placed on either mDCR or mDCR+MES medium at each initial pH level (3.6, 5.1, 5.7, 6.3, and 7.8) for incubation up to 42 days. These buds were incubated in a growth chamber at 25ºC with light regime adjusted to 16-hour light followed by 8-hour darkness each day. Bud weight change was recorded using an electronic scale when sample reached each incubation requirement. Vertical bars represent mean weight change (mg)±S.E. Means sharing the same letter indicate non-significant difference between means (P>0.05). Tukey’s (HSD) multiple comparison was used.
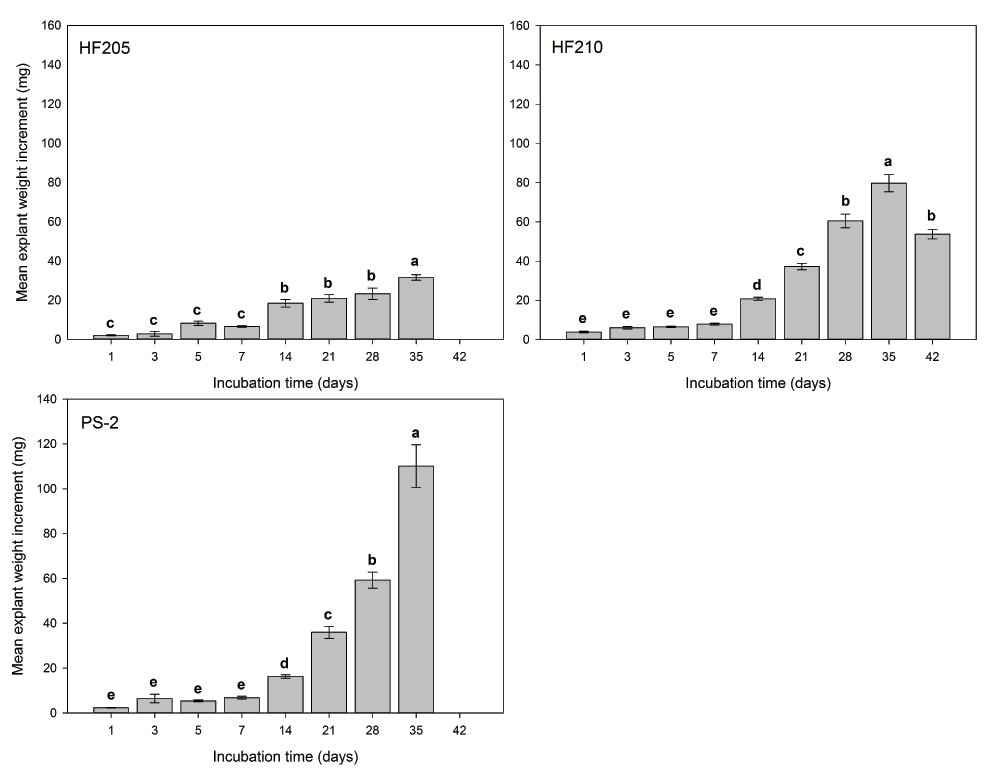
After surface sterilization and dissection, inner vegetative buds from three genotypes were placed on either mDCR or mDCR+MES medium at each initial pH level (3.6, 5.1, 5.7, 6.3, and 7.8) for incubation up to 42 days. These buds were incubated in a growth chamber at 25ºC with light regime adjusted to 16-hour light followed by 8-hour darkness each day. Bud weight change was recorded using an electronic scale when sample reached each incubation requirement. Vertical bars represent mean weight change (mg)±S.E. Sample sizes according to the incubation time (1–42 days) were for HF205, n=45, 15, 15, 30, 30, 20, 20, and 30, for HF210, n=30, 30, 30, 30, 30, 31, 30, 38, and 15, and for PS-2, n=15, 30, 30, 30, 30, 16, 33, and 6. Means sharing the same letter indicate non-significant difference between means (P>0.05). Tukey’s (HSD) multiple comparison was used. Please see Dataset 4 for the raw data.
After growing in medium for 28-days or more without being subcultured, explants showed various growth deformities such as chlorosis, delayed needle expansion, tip browning, browning of the bottom of explants and surrounding medium, vitrification, and even death. These symptoms occurred especially at the lower and higher ends of the initial pH levels, after prolonged culturing. Regardless of the given initial pH levels, explant growth did not show obvious delay at the early culture stages. The mentioned problems were only found at the later times of culturing. Moisture condensation was a common problem in the plate-based tissue culture system. Some of the deformities observed may have been associated with excessive amount of water droplets falling onto the medium surface or coming into contact with the explants.
In general, mDCR medium with MES provided more stability of the pre-adjusted pH values after autoclaving in both the absence and presence of explants in the medium. We observed that after autoclaving, medium pH changed, but mDCR medium with MES showed less medium pH fluctuation than without MES. Storage of medium in the dark resulted in less medium pH fluctuation than storage under light. For mDCR medium incubated with explants, pH showed a gradual decrease that was followed by a sharp increase over the incubation time. However, mDCR+MES medium exhibited a slower decrease in pH or was followed by a convergent medium pH change for all pre-adjusted pH levels. Explant weight gain over time showed an inverse relationship with medium pH change, but also differed between juvenile and mature genotypes. The addition of MES did not show significant influence on explant weight growth. However, a distinct decrease in explant weight growth was observed after 28 days of incubation in the mDCR only medium.
Medium storage is a common practice in tissue culture. The use of premade medium serves two main purposes. One is to hold the medium for a period of time to observe whether any contamination occurs in the medium. This ensures maximum explant growth performance achieved when antibiotics are not present in the medium. The other purpose is to facilitate timely arrangements of routine culture initiations and transfers. Owen et al. (1991) demonstrated that light affects post-autoclave medium storage, resulting in reduced medium pH over storage time. Our data confirmed their findings. Medium pH was more stable in the dark storage condition regardless of the presence of MES. The addition of MES could stabilize medium pH under the light condition, in both the presence and absence of explants.
Fluctuation of medium pH can be influenced by many factors. Hydrolysis, enzymatic break down, photooxidation and photolysis on light-sensitive medium components may all contribute to the fluctuation of medium pH. Sucrose hydrolysis often requires splitting of water molecules and breaking glycosidic bonds of the disaccharides. Once the breakage of glycosidic bonds has occurred, hydrogen ions from the splitting of water molecules bind with glucose, whereas hydroxyl groups bind with fructose. Since tissue culture medium often is adjusted to slight acidic conditions (pH 5.2–5.8), autoclaving provides a suitable temperature for catalyzing sucrose hydrolysis. Acid facilitated autocatalyzed sucrose hydrolysis was reported as being both pH and temperature dependant, where lower pH at a given temperature promotes more sucrose hydrolysis (Heidt et al., 1952; Wann et al., 1997). The availability of hydrogen ions in the acidic medium solution also depends on the buffering ability of the nutrient components (Thorpe et al., 2008). Furthermore, carbon sources, the amount of carbohydrates, and gelling agents act together to determine the amount of sucrose hydrolysis and the medium pH after autoclaving. As a result, medium with lower original pH may become higher while medium with higher original pH may become lower to reach equilibrium of the solution.
After explants are introduced into the medium vessels, the sucrose is further converted into monosaccharides inter- and intra-cellularly by invertase or other plant enzymes (Thorpe et al., 2008). Egger & Hampp (1993) found the optimum activity of soluble acid invertase was at pH 4.1 in developing spruce (Picea abies (L.) Karst.) needles. They also reported that other sucrose synthesis enzymes, sucrose phosphate synthase, and sucrose synthase were pH dependant for their optimal activities (pH 7.7 and 6.7, respectively). Hence, as explants host numerous biological activities they must balance pH levels accordingly for each of the biochemical reactions. Ion uptake and release become the mechanism by which cells adjust for pH requirements. Dodds & Roberts (1995) attributed the fluctuation of medium pH after autoclaving may be a result of imbalance of anion and cation uptake.
Photochemistry may further induce degradation of photo-sensitive compounds in the medium, and trigger pH fluctuation. Photolysis is an event introduced by photons. For example, when a water molecule receives energy from photons during the photosynthesis process, photolysis occurs to generate electrons, hydrogen ions, and oxygen. If the free hydrogen ions are not bound by other substrates and excreted into the medium, they may cause medium pH fluctuation. Hangarter & Stasinopoulos (1991) reported a light induced Fe-catalyzed photooxidation of EDTA, which caused reduced root growth of the Arabidopsis thaliana ecotype Columbia. EDTA, an ion chelator, is considered to be a buffering agent in the tissue culture medium. After photooxidation occurred, formaldehyde and glyoxylic acid are produced, which can be toxic to explants, concomitant with increased chelated ferric oxide that explants can not readily use. This light induced change of buffering ability could definitely alter nutrient availability in the medium, and further affect the fluctuation of medium pH.
MES can be utilized to stabilize medium pH for Douglas-fir micropropagation. MES has been employed in various tissue culture systems to maintain stable medium pH over extended cultural times. Park & Son (1992) reported the addition of MES alone in the medium, or together with Dithiothreitol, increased protoplast yield and viability from hybrid poplar protoplast culture system. Similarly, MES and arabinogalactan-protein, alone or combined, were found to maintain suitable medium pH and to enhance embryogenesis in white cabbage (Brassica oleracea var. capitata) microspore culture system (Yuan et al., 2012). For conifer species, MES was also utilized as pH stabilizer for silver fir (Abies alba L.) (Hartmann et al., 1992) and European larch (Larix decidua Mill.) (Korlach & Zoglauer, 1995) protoplast culture systems. Protoplasts, being a single cell without cell wall, are probably extremely sensitive to rapid pH changes. The effects of pH changes could be as important for shoot culture or other tissue culture prospects for explant productivity. Especially, MES may help to maintain stable medium pH for bulk medium preparation in large scale aspects of a propagation project.
Our data suggested a 21-day subculture practice may be suitable for maintaining medium freshness, medium pH level, and desirable explant growth for Douglas-fir shoot culture. Although there might be specific pH requirements for individual species, explants of Douglas-fir genotypes showed various responses or adaptations to medium pH changes. Some genotypes may be able to tolerate or adapt better to fluctuations in medium pH, and to show continuous growth in a wide range of pH levels. The effects of MES and nutrient acquisition by explants in culture may require further investigations on specific aspects of nutrient dynamics regarding the effects of both medium and explants in vitro.
Figshare: http://dx.doi.org/10.6084/m9.figshare.1257689 (Chen et al., 2014).
CCC performed the experiments, analyzed the data, and wrote the first draft of this manuscript. All authors contributed equally in data interpretations as well as writing and further refinement of the manuscript.
This work was supported by the Pennsylvania Department of Agriculture (grant number PDA ME 446711 to JEC) and the Schatz Center for Tree Molecular Genetics, the Pennsylvania State University.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors would like to thank Eric Dice and all the members of tissue culture group from JEC lab for their assistance in tissue culture works. Thanks also go to Drs. Henry Gerhold, Larry Kuhns, Haiying Liang, James Sellmer, and Abdoulaye Traore for their valuable comments and suggestions, and for providing plant materials.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 08 May 15 |
read | read |
|
Version 1 08 Dec 14 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)