Introduction
Ribonuclease H1 (RNase H) proteins are well-conserved endonucleases that are found in all domains of life and cleave the RNA strand of an RNA-DNA duplex substrate. The RNase H active site canonically consists of a highly conserved DED(D) motif (Figure 1), three to four carboxylate-containing residues collectively participating in the binding of catalytically required divalent cations, Mg2+ under physiological conditions. This active-site sequence motif and requirement for Mg2+ is widely shared with other nucleases, suggesting a common catalytic mechanism1.
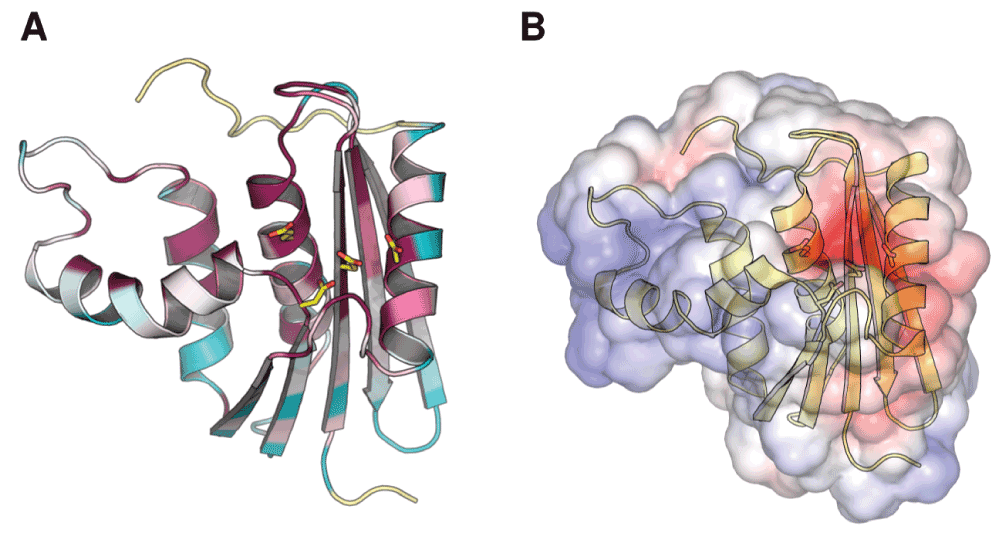
Figure 1. Conservation of the ribonuclease H active site.
(A) Residue conservation among bacterial RNase H homologs mapped onto the structure of ecRNH (PDB ID 2RN2). Highly conserved residues are shown in red, highly variable residues in green, and sites with insufficient data in yellow. Image produced using ConSurf. (B) Electrostatic map of the solvent-accessible surface of ecRNH produced using APBS. Red represents regions of negative charge and blue represents regions of positive charge. The active-site residues are represented as sticks in both cases.
The best-studied member of the RNase H family is the homolog from Escherichia coli (ecRNH), in which this active-site motif is represented as D10, E48, D70, and D1342 (Figure 2A). Measurements of the pKa values of the active-site residues indicate perturbed pKa values for D10 and D70 which normalize upon Mg2+ binding, clearly establishing these residues as critical for interaction with ions3. The pH optimum for the RNase H reaction in vitro is approximately 7.5–8.54, a value at which all active-site residues should be deprotonated3 and therefore accessible for ion binding.
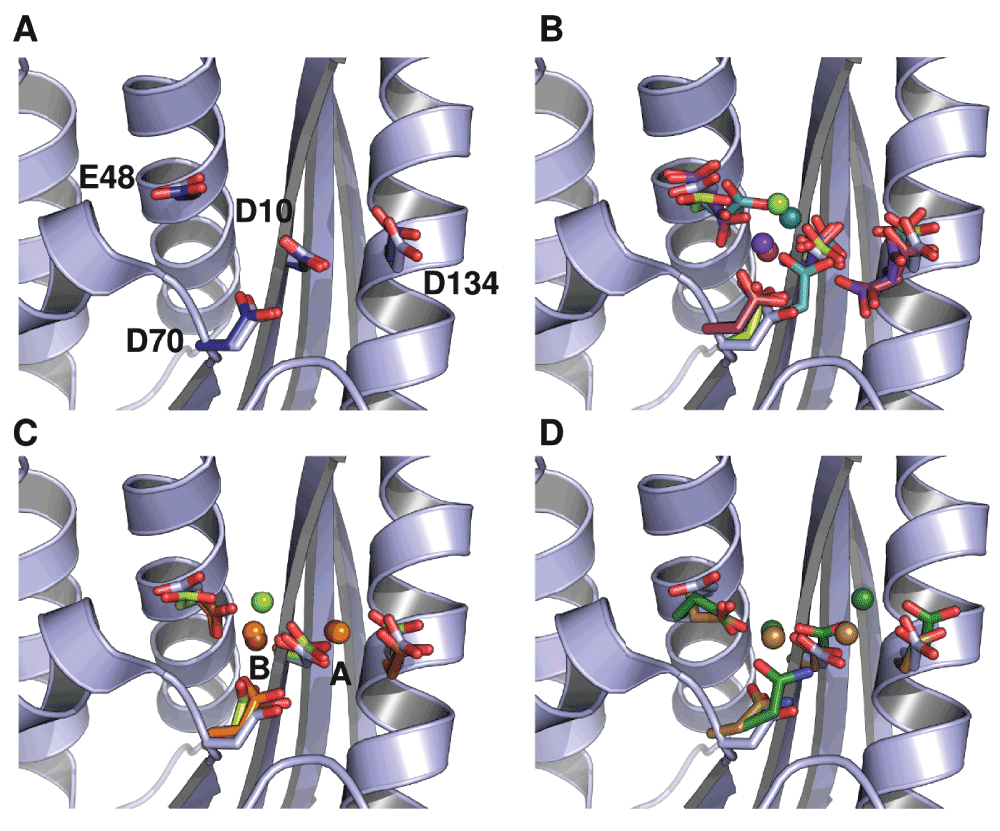
Figure 2. Conformational diversity of metal-ion interactions with ecRNH as determined by crystallography.
In all cases the backbone and active-site sidechains from ecRNH in the absence of ion (PDB ID 2RN2) are shown in light blue for comparison. (A) Structural superposition of the four active-site residues in two structures of ecRNH in the absence of metal ions: 2RN2 (light blue) and 1RNH (dark blue). (B) Structural diversity of RNases H in complex with a single Mg2+ ion: ecRNH (1RDD), green; XMRV WT (4E89), dark cyan; XMRV ΔC (3P1G), maroon; MoMLV ΔC (2HB5), purple. The two deletion mutants (indicated as ΔC) bind Mg2+ in slightly different positions than do their corresponding full-length proteins and both contain two alternate conformations for E48 and D134. (C) Comparison of Mg2+ and Mn2+ complexes: ecRNH with Mg2+ (1RDD), green; ecRNH with Mn2+ (1G15), orange; HIV RNase H domain with ecRNH helix C insertion with Mn2+ (3HYF), brown. (D) Structural diversity of RNases H in complex with substrate: Homo sapiens RNase H with Ca2+ ions (2QKK), brown; Bacillus halodurans RNase H with Mn2+ ions (1ZBI), dark green.
Despite extensive study, the interaction of metal ions with the ecRNH active site is poorly understood. Activity has been reported in the presence of Mn2+ as well as the physiologically relevant Mg2+5. Significant differences have been observed between the protein’s interactions with Mg2+ and Mn2+. Co-crystallization studies of ecRNH with high concentrations of Mg2+ find a single bound metal ion6 (Figure 2B). By contrast, co-crystallization with Mn2+ reveals two bound ions, one associated with residues D10 and D134 (denoted the A site), and one associated with D10, E48, and D70 (denoted the B site)7 (Figure 2C); the B site is similar but not identical to the previously identified Mg2+ site. Single Mn2+ sites have been identified in the presence of mutations of E48 and/or D1348, both of which are dispensable for Mn2+-dependent activity9. Crystallographic studies of related RNases H from the archaeal extremophile Bacillus halodurans10 and from Homo sapiens11 in complex with substrate find two bound ions in the active site (Figure 2D).
Experimental evidence from nuclear magnetic resonance (NMR) studies locates the area surrounding the active site as the region most susceptible to perturbation upon interaction with ions (Figure 3). Titration of Mg2+ with ecRNH, monitored independently by 1H and 25Mg2+ NMR, suggests that only a single ion binds to the protein in the absence of substrate5. The identified binding site has relatively weak affinity; Kd has been reported in the micromolar5 to low millimolar range12. The second site may be occupied only upon binding of substrate8, possibly due to the presence of high local concentration in the ion cloud of the highly negatively charged nucleic acid molecule. Conformational changes in the active site upon binding the first ion have been suggested as well, with the second site proposed as being responsible for the attenuation of activity at high ion concentrations13. Collectively, these results have been used to propose both a one-metal7,8,14 and a two-metal10,15,16 catalytic mechanism. Computational work using the quantum mechanics/molecular mechanics (QM/MM) method applied to the Bacillus halodurans17–19 and Homo sapiens20 complexes generally supports the two-metal mechanism.
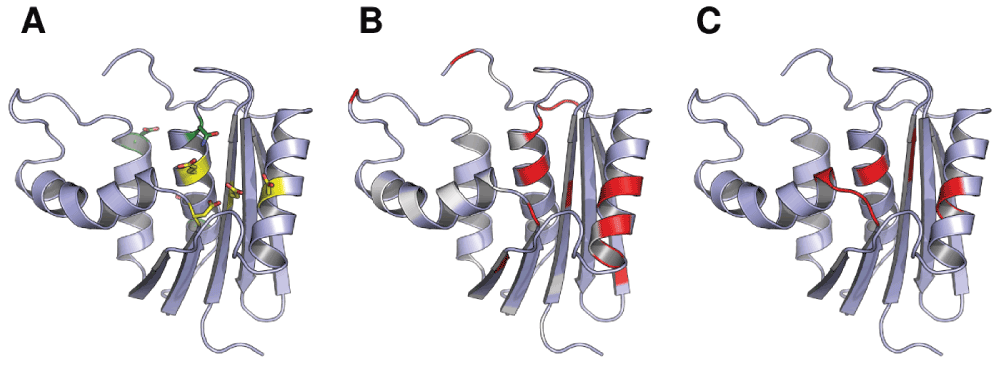
Figure 3. Experimental NMR measurements of the effects of Mg2+ binding mapped onto the structure of ecRNH.
(A) Active site residues (yellow) and other DENQ residues (green) that experience perturbation upon Mg2+ binding24. (B) Chemical shift perturbation values for sidechain Cγδ reflecting the effects of Mg2+ binding24. White corresponds to no chemical shift change, red corresponds to a large change, and non-DENQ residues are shown in light blue. (C) Residues previously shown to experience backbone 15N or 1H chemical shift changes upon binding Mg2+25.
RNase H domains are widely distributed in cellular organisms, but also occur as a component of the reverse transcriptase protein found in retroviruses, in which they are required for viral proliferation21. For this reason, inhibitors of retroviral RNase H domains, particularly that of HIV, have been widely reported15,22, although none to date have reached clinical use. Most such inhibitors interact with the active site in the metal-bound state22 and therefore must be selective for retroviral RNase H domains to find clinical utility. The HIV RNase H domain (hivRNH) has been reported to bind two metal ions even in the absence of substrate23, although the reason for this difference in behavior between hivRNH and ecRNH is not clear. The Homo sapiens RNase H domain (hsRNH) has not been structurally characterized in the absence of substrate and its binding behavior is less well understood. However, it has higher sequence identity and is more structurally similar to ecRNH than hivRNH.
Combined nuclear magnetic resonance (NMR) and molecular dynamics (MD) studies of the behavior of the ecRNH active site residues suggest that the residues of the ecRNH active site are preorganized in the apo state for the binding of a single Mg2+ ion24. However, experimental constraints prevent the detailed observation of the protein’s dynamic behavior in the presence of a bound ion at ps-ns timescale. The present work aims to more fully understand the dynamics of ecRNH in the Mg2+-bound state through molecular dynamics simulations of ecRNH in the presence of single Mg2+ ions in various positions in the active site as suggested by crystallographic studies. In addition, the dynamic behavior of the active site in the apo state is compared with homologs from other organisms.
Methods
For each initial protein structure, protonation states for titratable residues were assigned either by experimental measurement (for ecRNH3) or by prediction using the H++26 pKa predictor. Unless otherwise specified, all simulations were performed at a pH of 5.5 to recapitulate the conditions used in prior NMR experiments on ecRNH27,28. It should be noted that the experimental pH optima for the RNase H reaction in vitro are approximately 7.5–8.5 for ecRNH and 8.5–9.5 for ttRNH4; however, NMR data are not available at these pH ranges due to sample precipitation. The active site residue D10 has a pKa of 6.1 in the absence of Mg2+; however, its pKa normalizes in the presence of Mg2+ 3 and therefore is not expected to be protonated at either the experimental or optimal pH. Crystallographic water molecules were removed from all structures prior to solvation using Schrodinger’s Maestro tool, version 8.5 or 9.1, as distributed in the Desmond software package. Simulations were performed as described24,29 using Desmond academic release 3 or source release 2.4.2.130. Proteins were described with the Amber99SB force field31, solvated with TIP3P water32 in a cubic box with a 10Å buffer region from solute to box boundary, and neutralized with Cl− ions. Bonds to hydrogen atoms were constrained using the M-SHAKE algorithm33. Simulations containing Mg2+ ions used the Aqvist parameter set34. Electrostatics were calculated with the PME method using a 9Å cutoff. All simulations used a 2.5fs inner timestep on a 1-1-3 RESPA cycle and were performed in the NVT ensemble using a Nosé-Hoover thermostat after equilibration to constant box volume for 5ns in the NPT ensemble. All simulations described in this work were run for 100ns unless otherwise noted. These simulation conditions applied to the apo state of RNase H homologs have previously been shown to reproduce NMR data well35.
Order parameters were calculated by the equation36:
in which µi and µj represent the x, y, and z components of a unit vector in the direction of a given chemical bond. This represents the long-time limit of the angular reorientational correlation function for a given bond vector.
Protein Data Bank (PDB, RRID:nif-0000-00135) structures used for initiating trajectories are listed, along with their resolutions and any system-specific preparation steps, in Table 1.
Table 1. Crystal structures used to initiate molecular dynamics simulations of RNases H.
| PDB ID | Protein | Source organism | Resolution (Å) | Comments |
|---|
| 2RN237 | ecRNH | Escherichia coli | 1.48 | — |
| 1RDD6 | ecRNH | Escherichia coli | 2.80 | E. coli WT with single bound Mg2+ ion |
| 1RIL38 | ttRNH | Thermus
thermophilus | 2.80 | — |
| 2E4L39 | soRNH | Shewanella
oneidensis | 2.00 | — |
| 3H0840 | ctRNH | Chlorobium
tepidum | 1.60 | Missing residues in handle and active-site loop
modeled in from 1RIL |
| 2QK911 | hsRNH | Homo sapiens | 2.55 | Substrate removed and catalytically inactivating D210N
mutation reversed in Maestro 8.5 |
| 3K2P41 | hivRNH | HIV | 2.04 | Inhibitor and bound metal ions removed in Maestro 9.1;
chosen as the HIV structure with lowest RMSD to the
unbound state (PDB ID 1HRH) with the active-site loop
resolved |
| 3V1O42 | xmrvRNH | XMRV | 1.88 | Full length XMRV RNase H domain with no bound ion |
| 3P1G43 | xmrvRNH ΔC | XMRV | 1.60 | Helix C and handle region deletion mutant of XMRV
RNase H domain with single bound Mg2+ ion |
Results and discussion
The crystallographic Mg2+ site is unstable in simulation
A simulation was initiated from the crystal structure of ecRNH in the Mg2+-bound state (PDB ID 1RDD)6. However, the position of the ion identified in this structure is not stable in simulation and exits the binding site immediately upon initiation of the trajectory. The ion transiently interacts with the protein at a variety of sites on the protein surface over the course of the 89ns trajectory but never returns to its original position in the active site (Figure 4A).
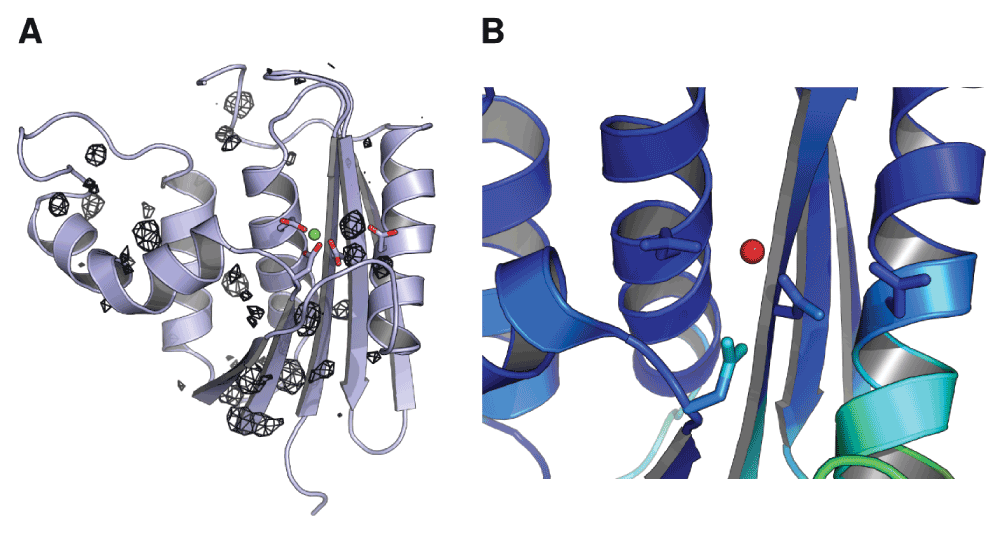
Figure 4. Mg2+ ion dynamics in a simulation initiated from the ecRNH Mg2+-bound crystal structure.
(A) Occupancy map from an 89ns simulation initiated from the ecRNH structure solved in the presence of Mg2+ (PDB ID 1RDD), contoured to 0.05% occupancy (corresponding to at least 45ps total residence time). The ion exits the active site and interacts with a variety of regions on the protein surface. (B) The active-site region of the 1RDD structure, colored by atomic B-factor. The B-factor of the ion is substantially larger than the surrounding residues, and is in fact larger than the B-factor of any other atom in the structure save crystallographic waters.
Historically, simulation of the behavior of multivalent ions using standard molecular mechanics force fields has been a long-standing challenge44. It is therefore possible that the instability of this position in simulation is an artifact of force field errors. However, given that ions in this position are not observed in the substrate-bound structures of RNase H homologs (Figure 2D), and that the B-factor of the Mg2+ ion in the 1RDD structure is much higher than those of the surrounding residues (Figure 4B), it is likely that this position does not reflect the most stable conformation of the protein-ion complex in solution.
Both the crystallographic A and B sites are stable in simulation
Additional simulations were carried out under the same conditions for single Mg2+ ions in each of the two Mn2+ binding sites identified for ecRNH. Because the crystal structure of ecRNH solved in the presence of Mn2+ (PDB ID 1G15) exhibits disorder in both the active-site and handle loops7, the ion positions were instead modeled into the apo ecRNH structure (PDB ID 2RN2) by superposition. For the model of the B-site Mg2+ ion, the rotamer of E48 was also corrected to match the orientation observed in the 1G15 structure. For comparison to an alternative homolog, the Mg2+ ion in the B site was also modeled into the RNase H structure from the thermophilic bacterium Thermus thermophilus (ttRNH, PDB ID 1RIL), whose structure was also solved in the absence of divalent ions38.
Mg2+ ions were found to be stably associated with the ecRNH active site in both simulations, despite the fact that the ions were modeled into a structure that did not originally contain them (Figure 5). This observation clearly supports the hypothesis that conformational preorganization in the active site promotes ion binding. It is possible that the effectiveness of this modeling procedure was facilitated by a well-documented feature of crystal packing in ecRNH, in which the amino group of a lysine sidechain in a neighboring molecule inserts into the negatively charged active site in a position approximating the B site8. However, a short simulation of ttRNH, whose structure does not contain this contact, with Mg2+ modeled into the B site was also stable, suggesting that crystal contacts in ecRNH are not responsible for the observation of preorganization in its active site.
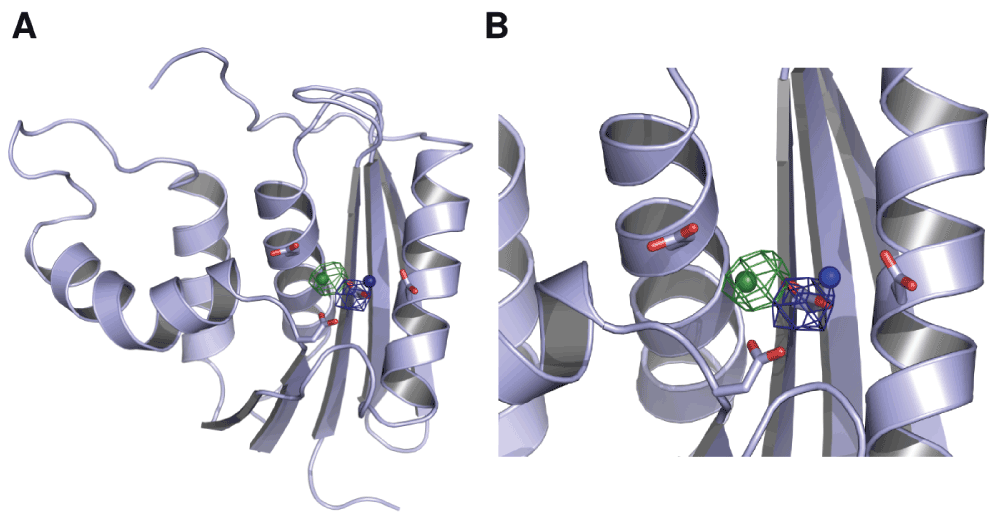
Figure 5. Mg2+ ion dynamics in an ecRNH simulation initiated with single ions in the A and B sites.
(A) Occupancy maps contoured at 10% occupancy for the A site (blue) and B site (green). Neither ion leaves the active site over the 100ns trajectory length. (B) Closer view of the active site in which the four active-site residues in apo ecRNH and the positions of the two Mn2+ from which the trajectories were initiated (derived from PDB ID 1G15) are shown for comparison.
The active site is conformationally preorganized for ion binding in the B site
The presence of Mg2+ located in either the A or the B site did not substantially affect the dynamics of the active-site residues as determined by S2. All four residues remain highly rigid in the presence of a Mg2+ ion in either position (Figure 6). The major difference between the unbound, A site, and B site trajectories’ sidechain dynamics was observed in a short loop between helix D and β-sheet 5. Experiments have demonstrated dynamics in this region on the ps-ns timescale, suggesting that the loop is simply incompletely sampled in 100ns simulations rather than significantly perturbed by ion binding. No significant differences in the behavior of these residues upon introduction of ions are observed experimentally24.
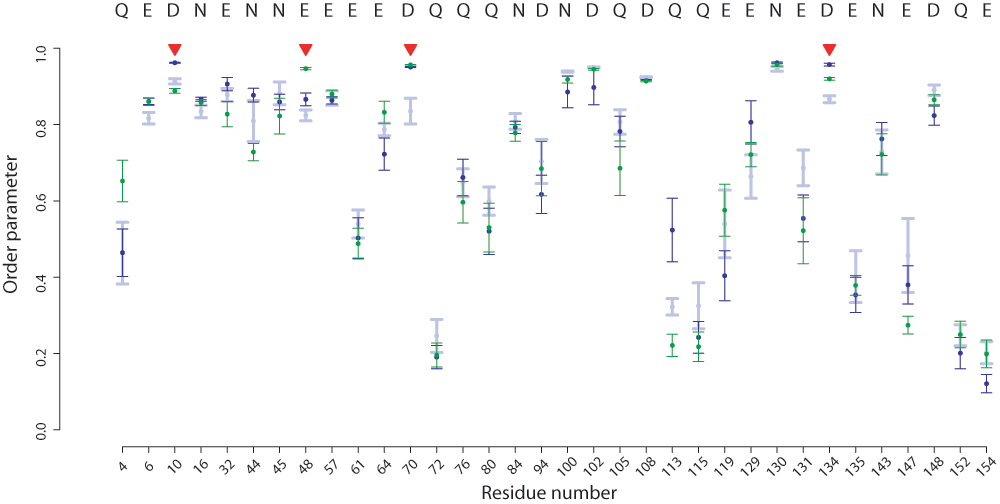
Figure 6. Differences in DENQ sidechain S2 values in the presence of Mg2+ ions in ecRNH.
Calculated S2 values are shown for various simulation conditions with standard errors of the mean: ecRNH apo (light blue), A site (dark blue), B site (green). The four active-site residues are indicated with red triangles. In no case does the difference between any two simulations reach statistical significance.
Of the four conserved catalytic residues, D134 is known to be somewhat dispensable; catalytic activity is retained, though reduced, by substitutions with N or H, which also increase thermostability45. In conjunction with crystallographic evidence, this suggests that the B site is occupied in the absence of substrate. Because measurements of the sidechain 13Cγδ resonances by NMR could not clearly distinguish the behavior of D134 (the unique participant in the A site) from E48 (the unique participant in the B site)24, comparisons of the two trajectories provide an additional opportunity to distinguish between these two sites.
Although single metal ions in both sites were stably bound to the protein, the RMSD over the course of each 100ns trajectory was larger for the ion in the A site (1.2Å) compared to the B site (0.6Å), which in turn is similar to the RMSD of a 30ns control simulation of ttRNH with an ion modeled into the B site (0.6Å). Additionally, a small amount of motion in the direction of the B site was observed for the ion in the A site; the initial and final positions differ by 1.7Å (Figure 5). (By comparison, the A and B sites are about 4Å apart.)
Distinct conformations were also observed for several neighboring residues, reflecting reorganization of local hydrogen bonding networks to accommodate ion binding in each of the two sites. N45 does not differ significantly in sidechain rigidity between the two trajectories, but it does differ in conformation: in the A site trajectory, it is oriented away from the substrate-binding site and participates in a network of interactions that also includes the conserved site T43, while in the B site trajectory N45 is primarily oriented into solvent and occupies the rotamer found in the hsRNH-substrate complex.
The hydrogen-bonding network surrounding D134 unsurprisingly differs considerably between the A and B site trajectories. Occupancy of inter-sidechain hydrogen bonds in this region is summarized in Table 2. H124, which interacts with substrate in the hsRNH complex and is known to be associated with product release, forms hydrogen bonds with D134 in the B site trajectory, partially displacing one of the hydrogen bonds formed between D134 and R138 in the apo trajectory. By contrast, H124 interacts primarily with E131 in the A site trajectory, while D134 coordinates Mg2+ in a monodentate manner, partially displacing the R138-D134 interaction. This conformation too is at odds with experimental evidence, since E131 experiences minimal chemical shift perturbation upon Mg2+ binding24.
Table 2. Hydrogen bond occupancy in the network surrounding D134 in ecRNH.
| H-bond | Apo | A Site | B Site |
|---|
| H124-E131 | 2.2% | 47.0% | 16.7% |
| H124-D134 | 0.8% | 1.5% | 23.8% |
| H124-E135 | 0% | 0% | 0.3% |
| R138-E131 | 0% | 11.3% | 0% |
| R138-D134 | 74.1% | 48.4% | 65.5% |
| R138-E135 | 43.4% | 29.8% | 50.1% |
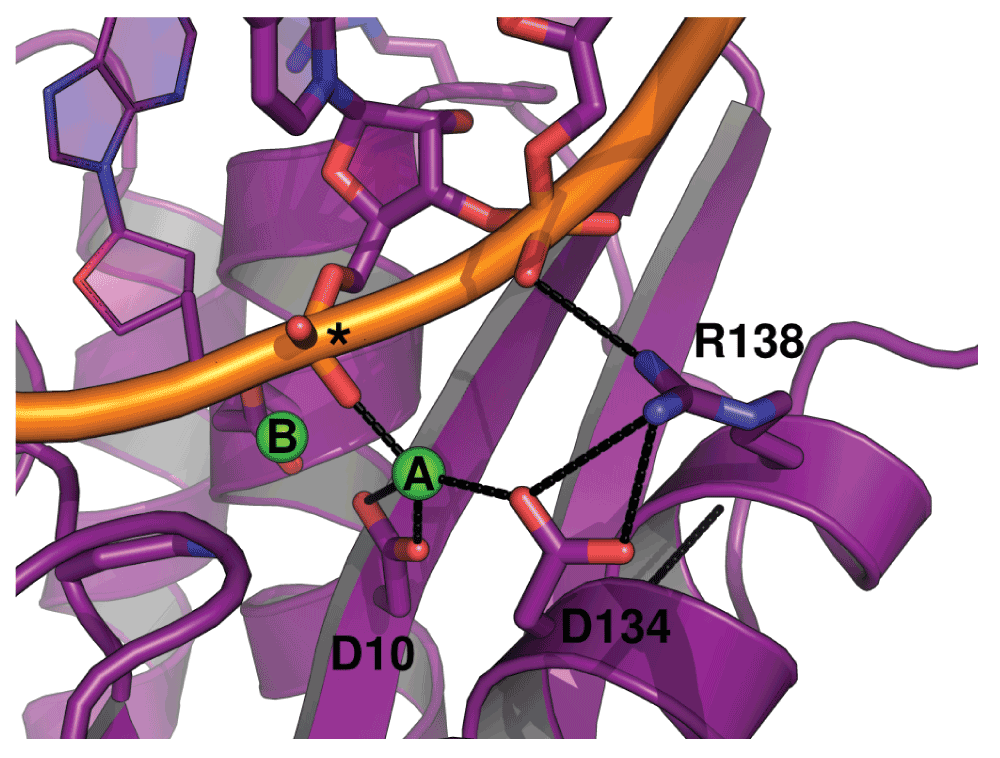
Figure 7. Hydrogen-bonding environment of the A site of hsRNH in complex with substrate.
Interactions between D134, R138, metal ion A, and the phosphate backbone of the RNA strand are shown (PDB ID 2QKK). An asterisk indicates the scissile phosphate. The preorganization of the R138-D134 salt bridge in the apo state of ecRNH likely minimizes the entropic cost of forming this interaction upon substrate binding.
These results collectively add to prior experimental evidence that the B site is the primary site for metal ion binding in the absence of substrate. Furthermore, the presence of a metal ion in the B site may induce reorganization of the surrounding sidechains into conformations conducive to subsequent substrate binding.
Rigidity of active-site sidechains is conserved within the RNase H family
Given that all known RNase H homologs have extremely similar active-site structures, it is likely that measurements made on the ecRNH protein can be generalized to other RNase H homologs. S2MD values were therefore calculated from previous simulations of the four handle-region-containing bacterial RNase H homologs of known structure, as well as for hsRNH in the absence of substrate29.
As might be expected from the high level of structural conservation in the active-site region, the five handle-region-containing RNase H homologs compared differ very little in the dynamics of their active site residues (Figure 9). Notably, the trajectory initiated from the hsRNH structure, which was solved in the presence of substrate and which contained a Na+ ion in a position similar to the B site in ecRNH, differs very little from trajectories initiated from any other RNase H structure lacking these additional components. This observation provides strong support for the interpretation that the rigid active-site residues are conformationally preorganized for metal-ion interactions even in the unbound state.
In order to better understand the relationships between dynamic processes in RNase H domains of retroviral origin compared to those from cellular organisms, additional simulations in the absence of divalent ions were performed on a set of retroviral RNase H homologs, whose sequences are shown in Figure 8. In brief, no significant differences are observed between simulations initiated from the XMRV full-length structure compared to its ΔC mutant (in which helix C and the handle region are removed), between the XMRV ΔC mutant compared to the HIV homolog (which naturally lacks the handle sequence), or between any of the retroviral domains compared to ecRNH (Figure 9). This result suggests that the preorganization of the active site on the ps-ns timescale is not significantly altered by differences in amino acid sequence among the subset of family members examined.
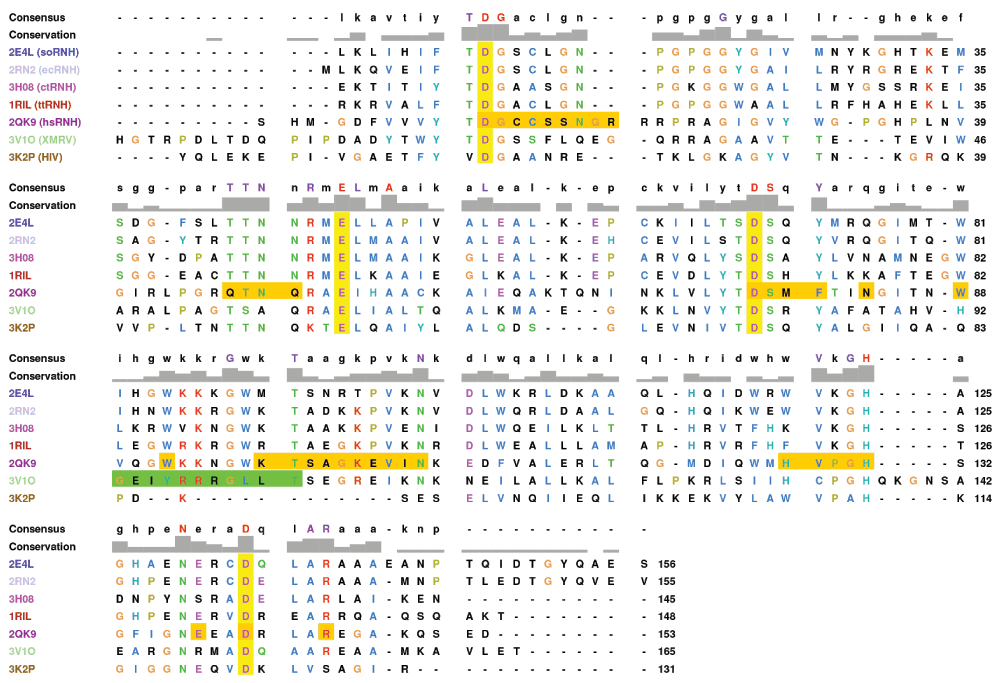
Figure 8. Multiple sequence alignment of RNase H homologs.
The Promals3D46 structural alignment of the PDB structures used to initiate simulations (see Table 1) is shown with active-site residues highlighted in yellow. Residues that form contacts with substrate in the hsRNH complex (PDB ID 2QK9) are highlighted in orange. The ΔC deletion of XMRV is shown in green. The catalytically inactivating D70N mutation in 2QK9 has been reversed and missing residues from the ctRNH structure (PDB ID 3H08) have been filled in for clarity. Residues are numbered relative to ecRNH. Figure prepared with UCSF Chimera47.
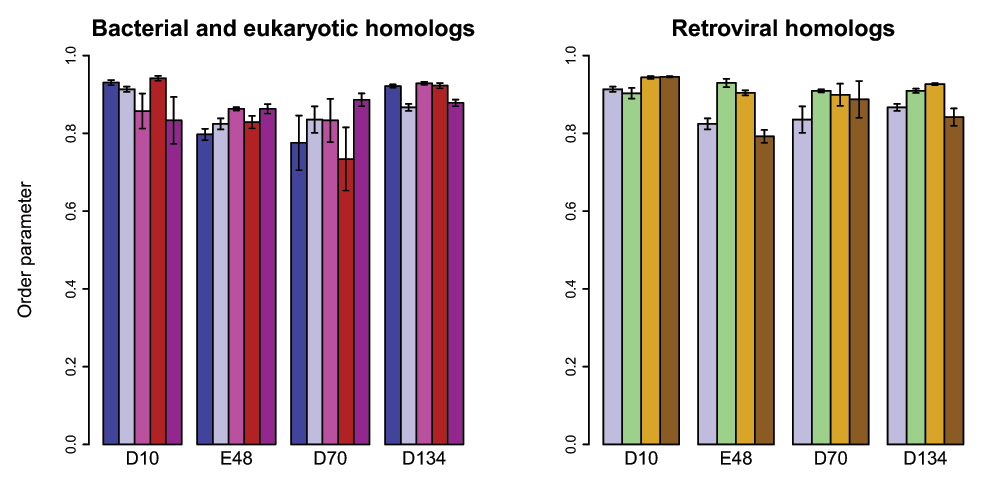
Figure 9. Active-site dynamics in RNase H homologs.
Calculated S2 values are shown for the four active-site residues in RNase H homologs. Left: soRNH (dark blue), ecRNH (light blue), ctRNH (magenta), ttRNH (red), hsRNH (purple). Right: ecRNH (light blue), XMRV WT (green), XMRV ΔC (yellow), HIV (brown). All simulations were carried out at 300K in the AMBER99SB force field with TIP3P water with structures protonated to reflect a pH of 5.5.
Conclusions
In this work we aimed to use molecular dynamics simulations to understand the dynamic behavior of the RNase H family in complex with catalytically required Mg2+ ions. We observe that the well-studied RNase H homolog from E. coli contains a conformationally preorganized active site that is highly rigid on the ps-ns timescale in the presence of a single Mg2+ ion, which is likely located at the B site crystallographically identified by examining the Mn2+ complex. Additionally, we examined the apo state dynamics of the active site—previously validated by comparison to NMR data in the case of the E. coli homolog24—and found that similar patterns of active-site rigidity are present in all homologs examined, including representatives of bacterial, eukaryotic, and retroviral RNases H. This result suggests that the dynamics of the active site residues are only subtly modulated by the amino acid sequence variations present among structurally characterized family members. Instead, we hypothesize that this behavior is imposed by the overall protein fold, the topology of which serves to force the negatively charged active-site residues into proximity with one another. Although it has long been recognized that RNases H share similar topologies and active-site conformations with other endonucleases48, the present work extends this observation from static crystal structures to dynamics on the ps-ns timescale.
Simulations of the Homo sapiens RNase H homolog and the Thermus thermophilus argonaute protein (a distant RNase H homolog with similar active-site architecture) in complex with two Mg2+ ions and substrate analogs also observe high active-site rigidity49; this is consistent with the present data and implies that active-site preorganization is a general property of this larger family of nucleases. Recent analysis of the larger RNase H-like superfamily identifies a common catalytic core in which the relative orientation of the active-site residues correlates with function, distinguishing endonucleases such as RNase H1 from exonuclease family members50. Although selective inhibitors of the HIV RNase H domain have been developed based on the hypothesis that the metal ion dependence of the HIV domain’s catalytic mechanism differs from that of the human homolog15, it is likely that the physical origin of this selectivity is not dependent on the active-site conformations sampled on the ps-ns timescale.
Data availability
ZENODO: Molecular dynamics derived side chain order parameters for Asp, Glu, Asn, and Gln residues in ribonucleases H, and molecular dynamics trajectories for E. coli ribonuclease H, doi:10.5281/zenodo.843151
Author contributions
KAS: Conceived and designed experiments, performed experiments, analyzed data, wrote the paper. AGP: Conceived and designed experiments, wrote the paper.
Competing interests
No competing interests were disclosed.
Grant information
This work was funded by an NSF graduate research fellowship (KAS) and NIH grant GM50291 (AGP).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgements
We thank the Center for Computational Biology and Bioinformatics (C2B2) for computational resources. We thank Jae-hyun Cho and Paul Robustelli for helpful discussions.
Faculty Opinions recommendedReferences
- 1.
Tadokoro T, Kanaya S:
Ribonuclease H: molecular diversities, substrate binding domains, and catalytic mechanism of the prokaryotic enzymes.
FEBS J.
2009; 276(6): 1482–1493. PubMed Abstract
| Publisher Full Text
- 2.
Kanaya S, Kohara A, Miura Y, et al.:
Identification of the amino acid residues involved in an active site of Escherichia coli ribonuclease H by site-directed mutagenesis.
J Biol Chem.
1990; 265(8): 4615–4621. PubMed Abstract
- 3.
Oda Y, Yamazaki T, Nagayama K, et al.:
Individual ionization constants of all the carboxyl groups in ribonuclease HI from Escherichia coli determined by NMR.
Biochemistry.
1994; 33(17): 5275–5284. PubMed Abstract
| Publisher Full Text
- 4.
Kanaya S, Itaya M:
Expression, purification, and characterization of a recombinant ribonuclease H from Thermus thermophilus HB8.
J Biol Chem.
1992; 267(14): 10184–10192. PubMed Abstract
- 5.
Huang HW, Cowan JA:
Metallobiochemistry of the magnesium ion. Characterization of the essential metal-binding site in Escherichia coli ribonuclease H.
Eur J Biochem.
1994; 219(1–2): 253–260. PubMed Abstract
| Publisher Full Text
- 6.
Katayanagi K, Okumura M, Morikawa K:
Crystal structure of Escherichia coli RNase HI in complex with Mg2+ at 2.8 A resolution: proof for a single Mg(2+)-binding site.
Proteins.
1993; 17(4): 337–346. PubMed Abstract
| Publisher Full Text
- 7.
Goedken ER, Marqusee S:
Co-crystal of Escherichia coli RNase HI with Mn2+ ions reveals two divalent metals bound in the active site.
J Biol Chem.
2001; 276(10): 7266–7271. PubMed Abstract
| Publisher Full Text
- 8.
Tsunaka Y, Takano K, Matsumura H, et al.:
Identification of single Mn(2+) binding sites required for activation of the mutant proteins of E. coli RNase HI at Glu48 and/or Asp134 by X-ray crystallography.
J Mol Biol.
2005; 345(5): 1171–1183. PubMed Abstract
| Publisher Full Text
- 9.
Tsunaka Y, Haruki M, Morikawa M, et al.:
Dispensability of glutamic acid 48 and aspartic acid 134 for Mn2+-dependent activity of Escherichia coli ribonuclease HI.
Biochemistry.
2003; 42(11): 3366–3374. PubMed Abstract
| Publisher Full Text
- 10.
Nowotny M, Gaidamakov SA, Crouch RJ, et al.:
Crystal structures of RNase H bound to an RNA/DNA hybrid: Substrate specificity and metal-dependent catalysis.
Cell.
2005; 121(7): 1005–1016. PubMed Abstract
| Publisher Full Text
- 11.
Nowotny M, Gaidamakov SA, Ghirlando R, et al.:
Structure of human RNase H1 complexed with an RNA/DNA hybrid: insight into HIV reverse transcription.
Mol Cell.
2007; 28(2): 264–276. PubMed Abstract
| Publisher Full Text
- 12.
Oda Y, Nakamura H, Kanaya S, et al.:
Binding of metal ions to E. coli RNase HI observed by 1H–15N heteronuclear 2D NMR.
J Biomol NMR.
1991; 1(3): 247–255. PubMed Abstract
| Publisher Full Text
- 13.
Keck JL, Goedken ER, Marqusee S:
Activation/attenuation model for RNase H: A one-metal mechanism with second-metal inhibition.
J Biol Chem.
1998; 273(51): 34128–34133. PubMed Abstract
| Publisher Full Text
- 14.
Oda Y, Yoshida M, Kanaya S:
Role of histidine 124 in the catalytic function of ribonuclease HI from Escherichia coli.
J Biol Chem.
1993; 268(1): 88–92. PubMed Abstract
- 15.
Klumpp K, Hang JQ, Rajendran S, et al.:
Two-metal ion mechanism of RNA cleavage by HIV RNase H and mechanism-based design of selective HIV RNase H inhibitors.
Nucleic Acids Res.
2003; 31(23): 6852–6859. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 16.
Nowotny M, Yang W:
Stepwise analyses of metal ions in RNase H catalysis from substrate destabilization to product release.
EMBO J.
2006; 25(9): 1924–1933. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 17.
De Vivo M, Dal Peraro M, Klein ML:
Phosphodiester cleavage in ribonuclease H occurs via an associative two-metal-aided catalytic mechanism.
J Am Chem Soc.
2008; 130(33): 10955–10962. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 18.
Rosta E, Woodcock HL, Brooks BR, et al.:
Artificial reaction coordinate “tunneling” in free-energy calculations: the catalytic reaction of RNase H.
J Comput Chem.
2009; 30(11): 1634–1641. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 19.
Rosta E, Nowotny M, Yang W, et al.:
Catalytic mechanism of RNA backbone cleavage by ribonuclease H from quantum mechanics/molecular mechanics simulations.
J Am Chem Soc.
2011; 133(23): 8934–8941. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 20.
Elsässer B, Fels G:
Atomistic details of the associative phosphodiester cleavage in human ribonuclease H.
Phys Chem Chem Phys.
2010; 12(36): 11081–8. PubMed Abstract
| Publisher Full Text
- 21.
Hostomsky Z, Hostomska Z, Matthews DA:
Ribonucleases H. In Stuart M. Linn and Richard J. Roberts, editors, Nucleases. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY 2nd edition. 1993; 341–376. Reference Source
- 22.
Ilina T, LaBarge K, Sarafianos SG, et al.:
Inhibitors of HIV-1 Reverse Transcriptase-Associated Ribonuclease H Activity.
Biology.
2012; 1(3): 521–541. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 23.
Davies JF 2nd, Hostomska Z, Hostomsky Z, et al.:
Crystal structure of the ribonuclease H domain of HIV-1 reverse transcriptase.
Science.
1991; 252(5002): 88–95. PubMed Abstract
| Publisher Full Text
- 24.
Stafford KA, Ferrage F, Cho JH, et al.:
Side chain dynamics of carboxyl and carbonyl groups in the catalytic function of Escherichia coli ribonuclease H.
J Am Chem Soc.
2013; 135(48): 18024–18027. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 25.
Oda Y, Iwa S, Ohtsuka E, et al.:
Binding of nucleic acids to E. coli RNase HI observed by NMR and CD spectroscopy.
Nucleic Acids Res.
1993; 21(20): 4690–4695. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 26.
Anandakrishnan R, Aguilar B, Onufriev AV:
H++ 3.0: automating pK prediction and the preparation of biomolecular structures for atomistic molecular modeling and simulations.
Nucleic Acids Res.
2012; 40(Web Server issue): W537–W541. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 27.
Butterwick JA, Patrick Loria J, Astrof NS, et al.:
Multiple time scale backbone dynamics of homologous thermophilic and mesophilic ribonuclease HI enzymes.
J Mol Biol.
2004; 339(4): 855–871. PubMed Abstract
| Publisher Full Text
- 28.
Butterwick JA, Palmer AG:
An inserted Gly residue fine tunes dynamics between mesophilic and thermophilic ribonucleases H.
Protein Sci.
2006; 15(12): 2697–2707. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 29.
Stafford KA, Robustelli P, Palmer AG 3rd:
Thermal adaptation of conformational dynamics in ribonuclease H.
PLoS Comput Biol.
2013; 9(10): e1003218. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 30.
Bowers KJ, Chow E, Xu H, et al.:
Scalable algorithms for molecular dynamics simulations on commodity clusters. In Proceedings of the 2006 ACM/IEEE conference on Supercomputing, Tampa, Florida, ACM. 2006; 84. Publisher Full Text
- 31.
Hornak V, Abel R, Okur A, et al.:
Comparison of multiple Amber force fields and development of improved protein backbone parameters.
Proteins.
2006; 65(3): 712–725. PubMed Abstract
| Publisher Full Text
- 32.
Jorgensen WL, Chandrasekhar J, Madura JD, et al.:
Comparison of simple potential functions for simulating liquid water.
J Chem Phys.
1983; 79(2): 926–935. Publisher Full Text
- 33.
Kräutler V, van Gunsteren WF, Hünenberger PH:
A fast SHAKE algorithm to solve distance constraint equations for small molecules in molecular dynamics simulations.
J Comput Chem.
2001; 22(5): 501–508. Publisher Full Text
- 34.
Aaqvist J:
Ion-water interaction potentials derived from free energy perturbation simulations.
J Phys Chem.
1990; 94(21): 8021–8024. Publisher Full Text
- 35.
Robustelli P, Stafford KA, Palmer AG 3rd:
Interpreting protein structural dynamics from NMR chemical shifts.
J Am Chem Soc.
2012; 134(14): 6365–6374. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 36.
Chandrasekhar I, Clore GM, Szabo A, et al.:
A 500 ps molecular dynamics simulation study of interleukin-1 beta in water. Correlation with nuclear magnetic resonance spectroscopy and crystallography.
J Mol Biol.
1992; 226(1): 239–250. PubMed Abstract
| Publisher Full Text
- 37.
Katayanagi K, Miyagawa M, Matsushima M, et al.:
Structural details of ribonuclease H from Escherichia coli as refined to an atomic resolution.
J Mol Biol.
1992; 223(4): 1029–1052. PubMed Abstract
| Publisher Full Text
- 38.
Ishikawa K, Okumura M, Katayanagi K, et al.:
Crystal structure of ribonuclease H from Thermus thermophilus HB8 refined at 2.8 A resolution.
J Mol Biol.
1993; 230(2): 529–542. PubMed Abstract
| Publisher Full Text
- 39.
Tadokoro T, You DJ, Abe Y, et al.:
Structural, thermodynamic, and mutational analyses of a psychrotrophic RNase HI.
Biochemistry.
2007; 46(25): 7460–7468. PubMed Abstract
| Publisher Full Text
- 40.
Ratcliff K, Corn J, Marqusee S:
Structure, stability, and folding of ribonuclease H1 from the moderately thermophilic Chlorobium tepidum: comparison with thermophilic and mesophilic homologues.
Biochemistry.
2009; 48(25): 5890–5898. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 41.
Himmel DM, Maegley KA, Pauly TA, et al.:
Structure of HIV-1 reverse transcriptase with the inhibitor beta-Thujaplicinol bound at the RNase H active site.
Structure.
2009; 17(12): 1625–1635. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 42.
Zhou D, Chung S, Miller M, et al.:
Crystal structures of the reverse transcriptase-associated ribonuclease H domain of xenotropic murine leukemia-virus related virus.
J Struct Biol.
2012; 177(3): 638–645. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 43.
Kirby KA, Marchand B, Ong YT, et al.:
Structural and inhibition studies of the RNase H function of xenotropic murine leukemia virus-related virus reverse transcriptase.
Antimicrob Agents Chemother.
2012; 56(4): 2048–2061. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 44.
Mamatkulov S, Fyta M, Netz RR:
Force fields for divalent cations based on single-ion and ion-pair properties.
J Chem Phys.
2013; 138(2): 024505. PubMed Abstract
| Publisher Full Text
- 45.
Haruki M, Noguchi E, Nakai C, et al.:
Investigating the role of conserved residue Asp134 in Escherichia coli ribonuclease HI by site-directed random mutagenesis.
Eur J Biochem.
1994; 220(2): 623–631. PubMed Abstract
| Publisher Full Text
- 46.
Pei J, Kim BH, Grishin NV:
PROMALS3D: a tool for multiple protein sequence and structure alignments.
Nucleic Acids Res.
2008; 36(7): 2295–2300. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 47.
Pettersen EF, Goddard TD, Huang CC, et al.:
UCSF chimera--a visualization system for exploratory research and analysis.
J Comput Chem.
2004; 25(13): 1605–1612. PubMed Abstract
| Publisher Full Text
- 48.
Yang W, Steitz TA:
Recombining the structures of HIV integrase, RuvC and RNase H.
Structure.
1995; 3(2): 131–134. PubMed Abstract
| Publisher Full Text
- 49.
Maláč K, Barvík I:
Complex between human RNase HI and the phosphonate-DNA/RNA duplex: molecular dynamics study.
J Mol Graph Model.
2013; 44: 81–90. PubMed Abstract
| Publisher Full Text
- 50.
Majorek KA, Dunin-Horkawicz S, Steczkiewicz K, et al.:
The RNase H-like superfamily: new members, comparative structural analysis and evolutionary classification.
Nucleic Acids Res.
2014; 42(7): 4160–79. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 51.
Stafford KA, Palmer AG III:
Molecular dynamics derived side chain order parameters for Asp, Glu, Asn, and Gln residues in ribonucleases H, and molecular dynamics trajectories for E. coli ribonuclease H.
ZENODO.
2014. Data Source









Comments on this article Comments (0)