Introduction
Insects have positive and negative impacts on humans, in terms of health, economy, and food stores. Insects pollinate plants to increase global food production, with 35% of global production of crops depending on animal pollinators1,2. Insects also cause significant destruction of crops and food stores3–5. Insects can also transmit fatal diseases such as dengue fever6, malaria7, yellow fever and epidemic typhus8. Insects use olfaction to sense their surroundings and to guide important activities, including feeding, mating and oviposition. This makes the insect olfactory system receptors an attractive target for the chemical control of deleterious insect species.
Insects use odorant receptors (ORs) to recognize and distinguish a diverse range of odorants9,10. Each OR is composed of two functionally essential parts: a highly conserved co-receptor subunit (Orco) and one of a large number of variable odorant-binding (or “tuning”) subunits11–17. These subunits associate in an unknown stoichiometry to form an odorant-gated ion channel18,19. ORs have also been proposed to initiate, or be modified by, second messenger cascades13,18. While the odorant-binding subunit is responsible for interacting with odorants9,20,21, both the odorant-binding subunits and Orco are involved in forming the ion channel pore21,22. Insect ORs are not related to the receptors and channels of humans and other tetrapods15, suggesting that control of detrimental insect activity may be possible through the development of insect OR selective compounds. A current approach to developing these compounds is to identify the particular odorant binding subunits that recognize behaviorally important odorants10,23–26 and then conduct large scale ligand screens27,28, but high diversity among the odorant binding subunit repertoires of different species makes this approach exceptionally labor intensive29,30.
The recent identification of the synthetic compound VUAA1 as a novel OR agonist that acts directly on Orco27, suggests that manipulation of insect behavior might be achieved by targeting Orco. Based on the VUAA1 structure, several additional synthetic Orco agonists and a larger, more diverse series of synthetic Orco antagonists have been identified31–33. Importantly, several of these Orco antagonists were shown to inhibit odorant activation of ORs through a non-competitive mechanism31–33. These findings suggest that Orco antagonists might be useful in altering insect behavior.
Orco subunits are highly conserved across insect species, suggesting that Orco serves an essential function common to all insect ORs15,34. This high conservation underlies observations that Orco subunits from different species are functionally interchangeable; an Orco subunit from one species can form functional ORs with an odorant-binding subunit from a different species21,22. As the “pharmacology” of synthetic Orco agonists and antagonists has expanded, it has also become clear that Orco subunits from disparate insect species have very similar sensitivities to known Orco ligands27,31–33,35. This suggested to us that the binding site for Orco ligands may serve as a modulatory site for compounds endogenous to the insects or may be a target of exogenous compounds, such as those generated by plants. Insects use a variety of amines as neurotransmitters and neuromodulators36–39. Plants also generate a variety of amines that may play a role in resistance to insect herbivores40–42. For these reasons, we screened a panel of biogenic and trace amines for agonist and antagonist activity at insect Orco subunits. We found tryptamine to be a potent Orco antagonist with broad activity at Orco subunits from different species. Tyramine and phenethylamine also function as Orco antagonists, but were substantially less potent than tryptamine. Importantly, we found that tryptamine, acting through Orco, could inhibit odorant activation of a wide range of ORs from a variety of insect species. Our findings suggest a role for Orco as a modulatory site common to all insect ORs and support the development of Orco-directed compounds that can be used to manipulate insect behavior.
Methods
Materials
Xenopus laevis frogs were purchased from Nasco (Fort Atkinson, WI). The care and use of Xenopus laevis frogs in this study were approved by the University of Miami Animal Research Committee (Animal Welfare Assurance #A-3224-01, Protocol #13-056) and meet the guidelines of the US National Institutes of Health. All experimentation was conducted on cultured oocytes after surgical removal from the frogs (see below). The amines screened in this study (Figure 1), odorants (L-fenchone, acetophenone, geranyl acetate, 6-methyl-5-hepten-2-one, 2-nonanone and eugenol), OLC12 and other chemicals were from Sigma-Aldrich. Cqui\Orco (from Culex quinquefasciatus), Onub\Or6, Onub\Orco (from Ostrinia nubilalis), Mcar\Or5 and Mcar\Orco (from Megacyllene caryae) were cloned and inserted into the pGEMHE vector43 as previously described23,24,44,45. Dmel\Or35a and Dmel\Orco (from Drosophila melanogaster) were generously provided by J. Carlson and L. Vosshall, respectively. Agam\Or27, Agam\Or28, Agam\Or31, Agam\Or39, Agam\Or48, Agam\Or65 and Agam\Orco (from Anopheles gambiae) were generously provided by L. Zweibel.
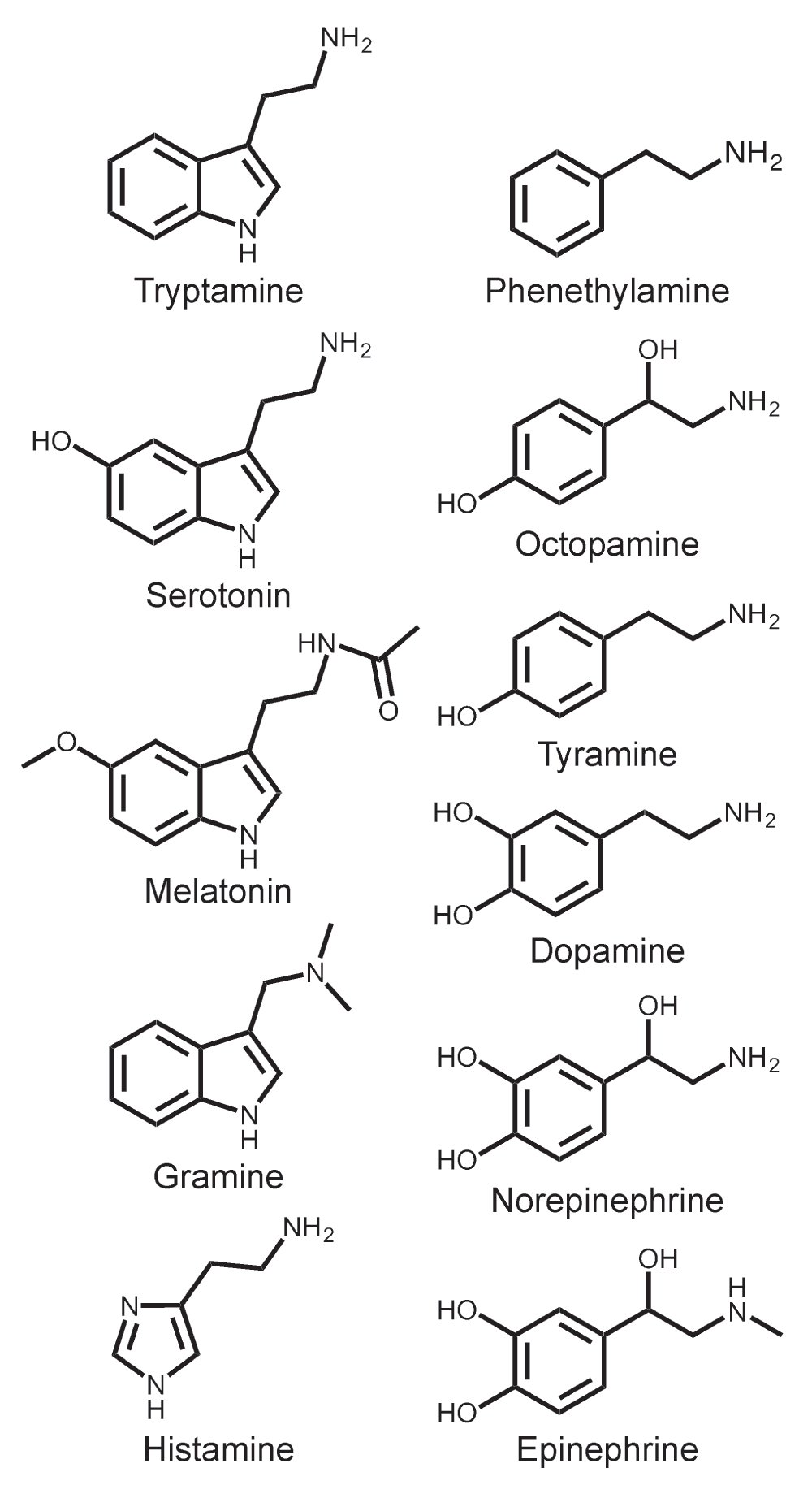
Figure 1. Structures of amines tested in this study.
Expression of insect ORs in Xenopus oocytes
Mature Xenopus laevis frogs were anesthetized by submersion in 0.1% 3-aminobenzoic acid ethyl ester. Depth of anesthesia was judged by loss of nasal flare and swallow reflexes. Oocytes were surgically removed. The incision was treated with gentamicin sulfate (two subcutaneous injections of 0.1 mL 10 mg/mL gentamycin at the surgical site) and sutured. Immediately following surgery (and before recovery from anesthesia), as an analgesia agent, one subcutaneous injection of Meloxicam solution (0.1 mg/mL) (0.1 mg/kg body weight) was administered to the dorsal lymph sac of the frogs. The frogs were allowed to recover from surgery in a humid chamber before being placed back in the holding tank. Surgeries were performed on individual frogs no more often than once every 3 months. Following the fourth surgery, frogs were anesthetized as described above and then pithed.
Follicle cells were removed by treatment with collagenase B (Boehringer Mannheim) for 2 hours at room temperature. Capped cRNA encoding each OR subunit was generated using mMessage mMachine kits (Ambion). For heteromeric ORs, 25 ng of cRNA encoding each OR subunit was injected into Stage V-VI Xenopus oocytes. For expression of Orco homomers, 50 ng of cRNA was injected. Oocytes were incubated at 18°C in Barth's saline (in mM: 88 NaCl, 1 KCl, 2.4 NaHCO3, 0.3 CaNO3, 0.41 CaCl2, 0.82 MgSO4, 15 HEPES, pH 7.6, and 150µg/ml ceftazidime) for 2–5 days prior to electrophysiological recording.
Electrophysiology and data capture
Odorant and Orco ligand induced currents were recorded under two-electrode voltage clamp, using an automated parallel electrophysiology system (OpusExpress 6000A, Molecular Devices). Oocytes were perfused with ND96 (in mM: 96 NaCl, 2 KCl, 1 CaCl2, 1 MgCl2, 5 HEPES, pH 7.5). Orco ligands were prepared as 50 or 100 mM stock solutions in DMSO and then diluted into ND96 on the day of the experiment. Odorants were prepared as 100 mM stock solutions in DMSO and then diluted into ND96. Unless otherwise noted, applications were for 60 sec at a flow rate of 1.0 ml/min, with extensive washing in ND96 at 4.6 ml/min between applications. Micropipettes were filled with 3 M KCl and had resistances of 0.2–2.0 MΩ. The holding potential was -70 mV. Current responses, filtered (4-pole, Bessel, low pass) at 20 Hz (-3 db) and sampled at 100 Hz, were captured and stored using OpusXpress 1.1 software (Molecular Devices).
Experimental protocols and data analysis
To screen for agonist activity, oocytes were exposed to 30 sec applications of candidate compounds with 5 min washes between applications (Figure 2A). For the concentration-response protocol (Table 1), applications were for 20 sec at a flow rate of 1.65 ml/min. To measure antagonist activity at Orco (Figure 2B, 2C, Figure 3, Figure 4A and Figure 5A), oocytes were exposed to two 60 sec applications of the synthetic Orco agonist OLC12 (2-((4-Ethyl-5-(4-pyridinyl)-4H-1,2,4-triazol-3-yl)sulfanyl)-N-(4-isopropylphenyl)acetamide) with 4 min washes between applications. Oocytes were then exposed to a 90 sec application of antagonist candidate, immediately followed by a 60 sec co-application of antagonist candidate and OLC12. The current response in the presence of antagonist candidate was compared to the mean of the preceding two responses to OLC12 alone and is presented as a percentage.
Table 1. Odorant and Orco agonist concentration-response curve values for Orco homomers and heteromeric ORs from several insect species.
Concentration-response data was fit as described in Methods. nH is the apparent Hill coefficient. Values are presented as mean ± SEM (n = 3-14).
| Receptor | Ligand
(Normalizing Conc.) | EC50
µM | nH | Fit Max |
|---|
| Agam\Orco | OLC12 (30µM) | 124 ± 9 | 2.4 ± 0.3 | 47 ± 2 |
| Agam\Orco + Agam\Or31 | Geranyl Acetate (30µM) | 65 ± 23 | 1.0 ± 0.3 | 2.9 ± 0.3 |
| Agam\Orco + Agam\Or65 | Eugenol (1µM) | 0.08 ± 0.01 | 0.9 ± 0.1 | 1.0 ± 0.03 |
| Agam\Orco + Agam\Or65 | OLC12 (30µM) | 67 ± 6 | 2.0 ± 0.3 | 5.7 ± 0.3 |
| Cqui\Orco | OLC12 (30µM) | 95 ± 6 | 2.5 ± 0.3 | 48 ± 2 |
| Dmel\Orco | OLC12 (10µM) | 36 ± 4 | 3.9 ± 1.9 | 36 ± 4 |
| Dmel\Orco + Dmel\Or35a | OLC12 (10µM) | 20 ± 5 | 1.9 ± 0.8 | 4.7 ± 0.4 |
| Onub\Orco + Onub\Or6 | OLC12 (100µM) | 100 ± 4 | 2.1 ± 0.2 | 2.0 ± 0.1 |
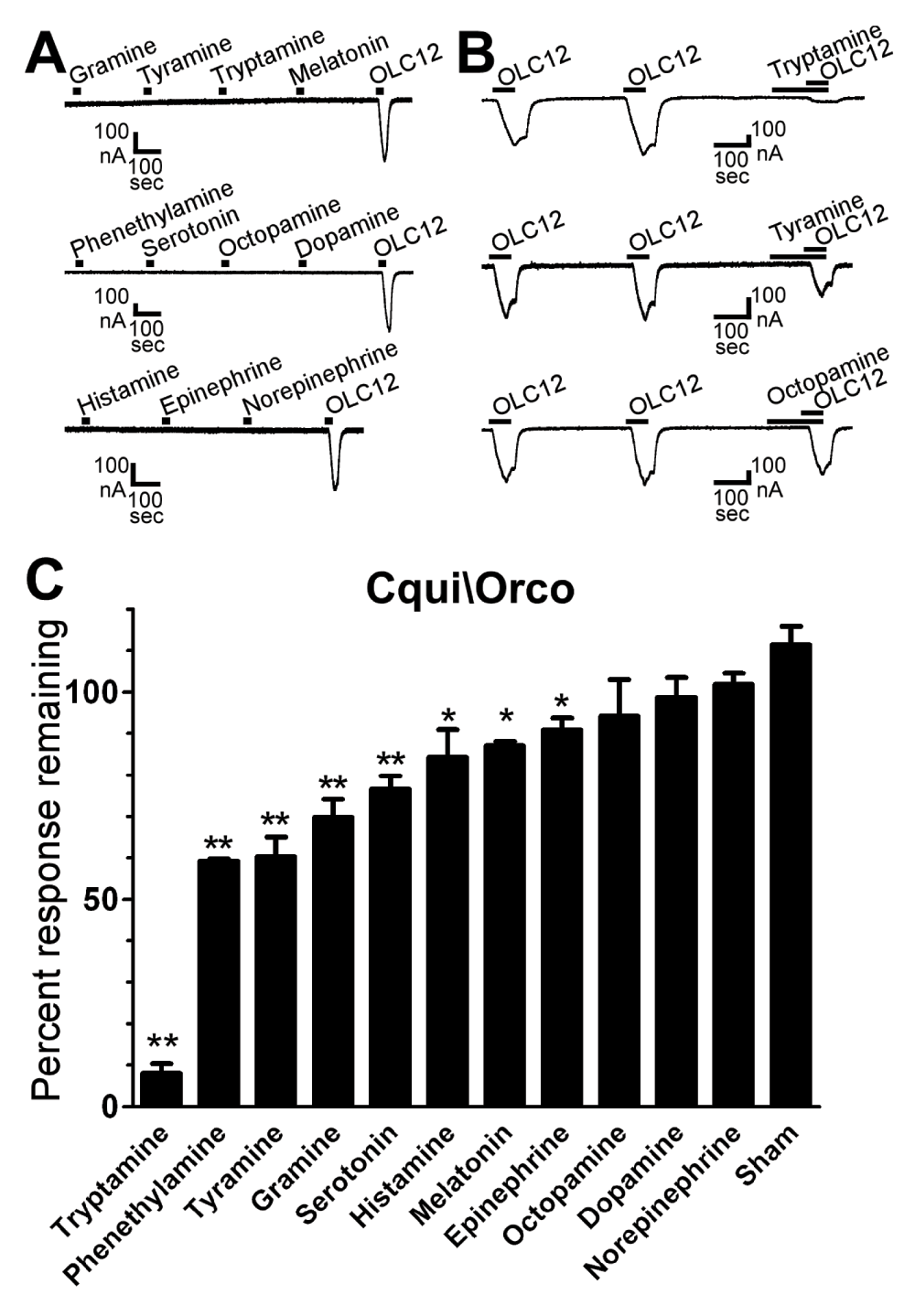
Figure 2. Tryptamine and several other amines are antagonists of Cqui\Orco.
A) The tested amines do not display Orco agonist activity. Oocytes expressing Cqui\Orco were challenged with 30 sec applications of 100µM gramine, tyramine, tryptamine and melatonin (top trace), phenethylamine, serotonin, octopamine and dopamine (middle trace), or histamine, epinephrine and norepinephrine (bottom trace), with 5 min washes between applications. 30µM OLC12 (Orco agonist) was applied at the end of each trace. B) Tryptamine and tyramine are antagonists of Cqui\Orco. Oocytes expressing Cqui\Orco were exposed to 60 sec applications of 30µM OLC12 with 4 min washes between applications. 100µM tryptamine (top trace), tyramine (middle trace), or octopamine (bottom trace) were applied and incubated for 90 sec preceding the third application of OLC12 and then co-applied during the OLC12 application. C) Screen of 11 amines for Orco antagonism. Responses of Cqui\Orco to 30µM OLC12 (~EC5) in the presence of 100µM of each compound are presented as a percentage of the average of two preceding responses to OLC12 alone (mean ± SEM, n = 3-10). Statistical significance was assessed by one-way ANOVA, followed by Dunnett's post-test comparing to sham treated oocytes (*p<0.01; **p<0.001).
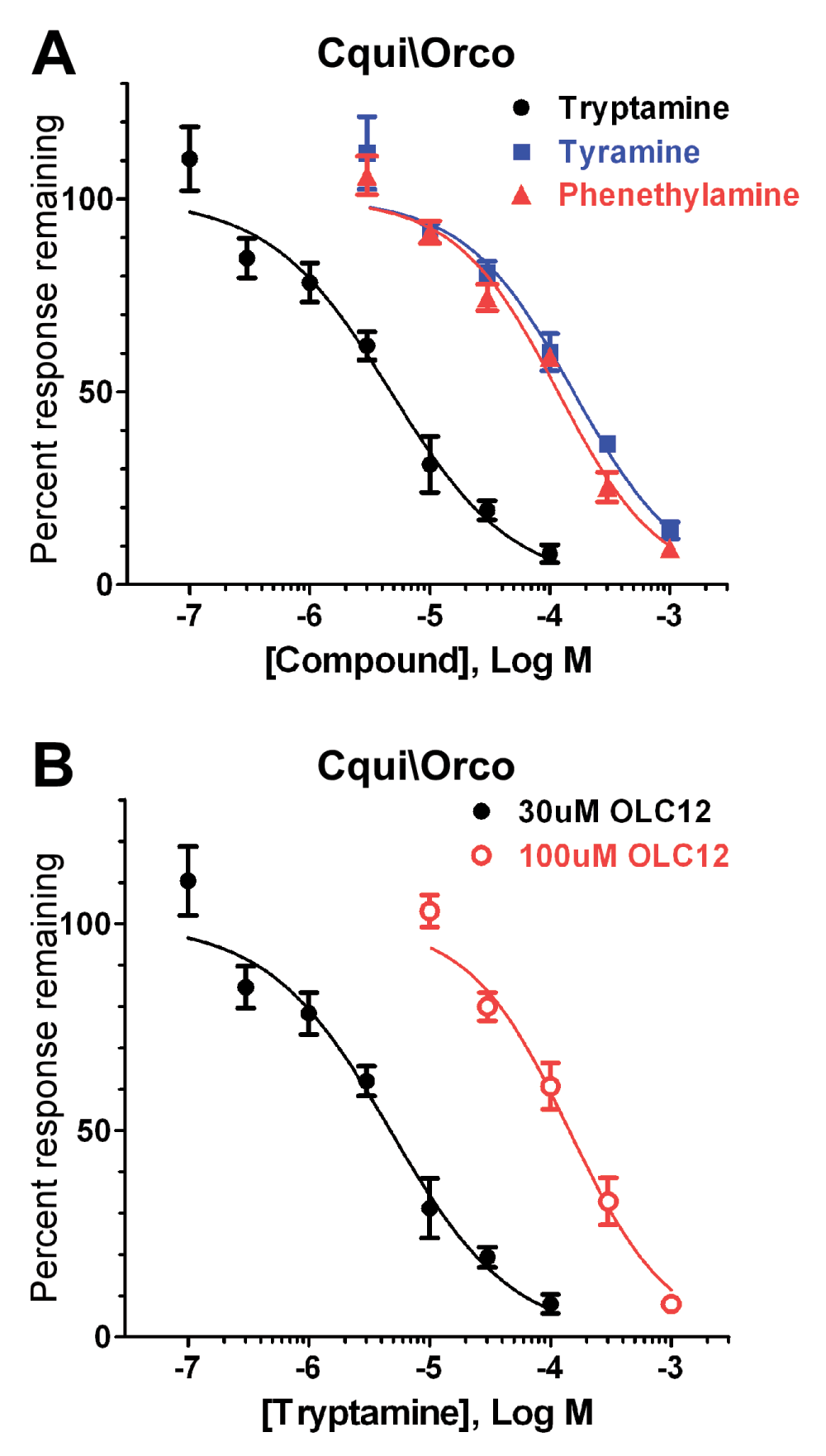
Figure 3. Trace amine antagonists of Cqui\Orco.
A) Concentration-inhibition curves for tryptamine, tyramine and phenethylamine inhibition of Cqui\Orco activated by 30µM OLC12. B) Altering the concentration of Orco agonist (OLC12) shifts the tryptamine inhibition curve. The IC50 for tryptamine inhibition of Cqui\Orco activation by 30µM OLC12 (4.7 ± 0.7µM, n = 5) is significantly different (p<0.0001, F-test) from the IC50 for tryptamine inhibition of Cqui\Orco activation by 100µM OLC12 (143 ± 18µM, n = 6).
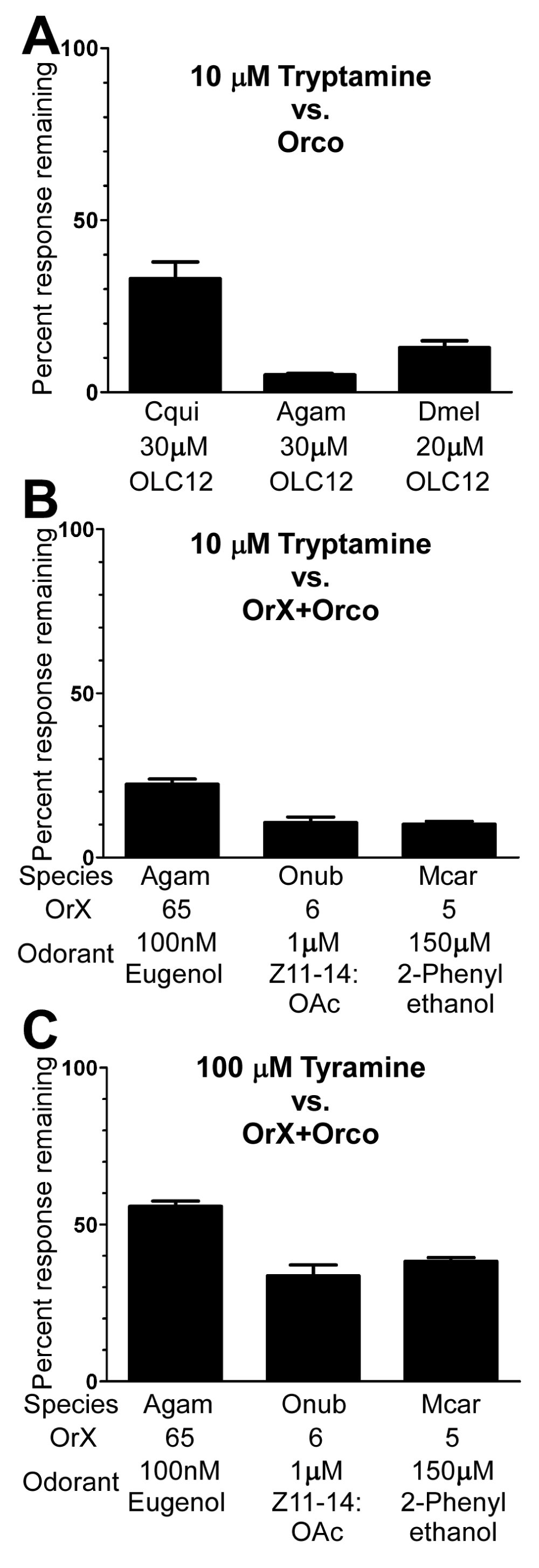
Figure 4. Tryptamine and tyramine inhibit odorant activation of ORs from different insect species.
A) Oocytes expressing Orco from each of three different species were activated by the indicated concentration of OLC12. For Cqui\Orco from Cx. quinquefasciatus, 30µM is the ~EC5; for Agam\Orco from An. gambiae, 30µM is the ~EC3; for Dmel\Orco from D. melanogaster, 20µM is the ~EC10. Current responses in the presence of 10µM tryptamine were compared to the average of two preceding responses to OLC12 and are presented as mean ± SEM (n = 4-9). B–C) Tryptamine and tyramine inhibit odorant activation of heteromeric ORs from different insect species. Oocytes expressing an OR from An. gambiae (Agam\Orco+Agam\Or65) were activated by 100nM eugenol, oocytes expressing an OR from O. nubilalis (Onub\Orco+Onub\Or6) were activated by 1µM Z11-14:OAc, oocytes expressing an OR from M. caryae (Mcar\Orco+Mcar\Or5) were activated by 150µM 2-phenylethanol. Current responses in the presence of 10µM tryptamine (B) or 100µM tyramine (C) were compared to the preceding response to odorant alone and are presented as mean ± SEM (n = 3).
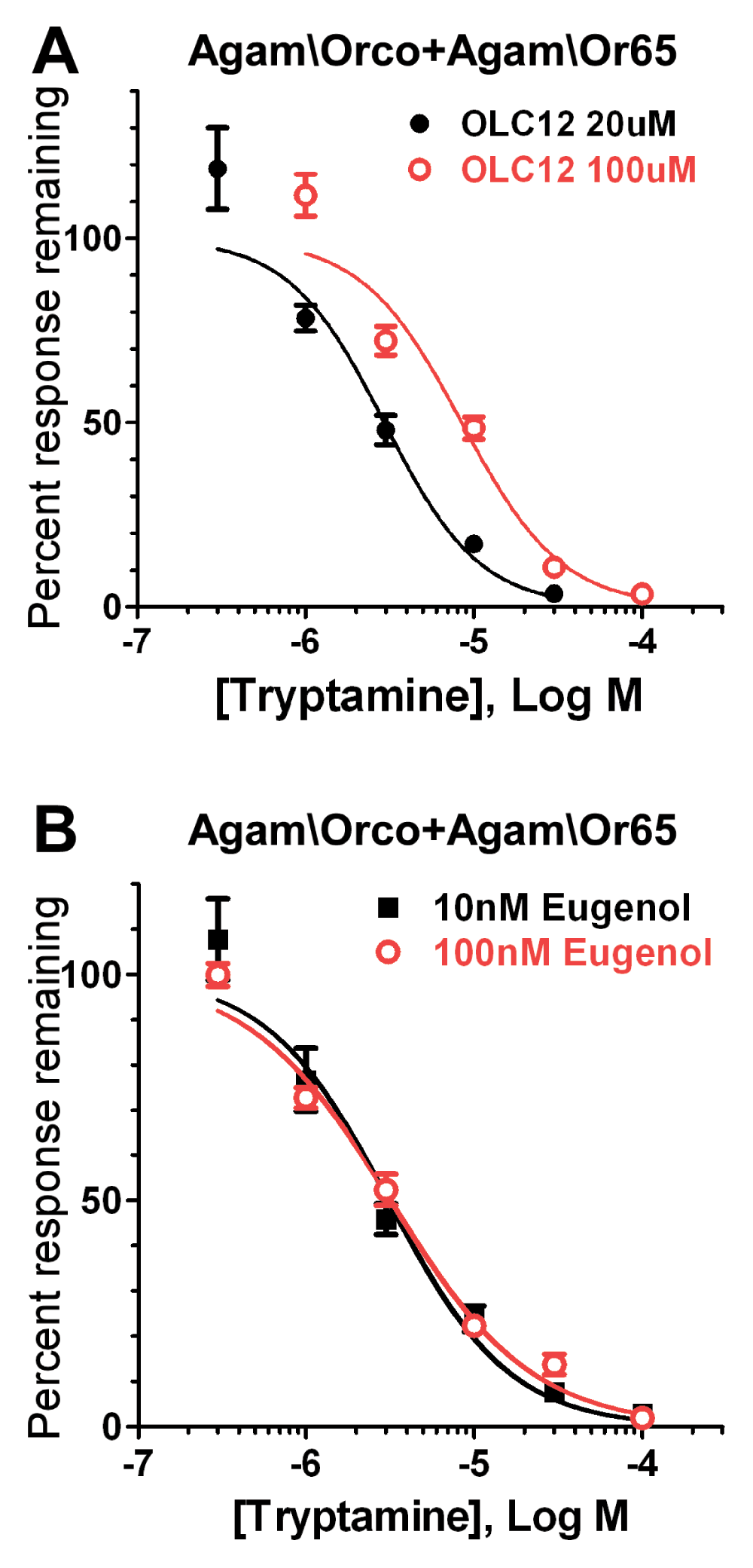
Figure 5. Tryptamine antagonism of odorant activation of an Agam\OR is non-competitive.
A) Tryptamine competitively inhibits OLC12 activation of Agam\Orco+Agam\Or65. Altering the concentration of Orco agonist (OLC12) shifts the tryptamine inhibition curve. The IC50 for tryptamine inhibition of Agam\Orco+Agam\Or65 activation by 20µM OLC12 (2.9 ± 0.5µM, n = 3) is significantly different (p<0.0001, F-test) from the IC50 for tryptamine inhibition of Agam\Orco+Agam\Or65 activation by 100µM OLC12 (8.5 ± 1.1µM, n = 3). B) Tryptamine non-competitively inhibits odorant activation of Agam\Orco+Agam\Or65. Altering odorant (eugenol) concentration fails to shift the tryptamine inhibition curve. The IC50 values for tryptamine inhibition of responses to 10nM eugenol (3.1 ± 0.4µM, n = 4), and 100nM eugenol (3.2 ± 0.3µM, n = 3) did not differ (p=0.7172, F-test).
To measure inhibition of odorant activation of heteromeric ORs (Figures 4B, 4C, Figure 5B and Figure 6), oocytes were exposed to a 30 sec application of odorant followed by a 10 min wash. Oocytes were then exposed to a 90 sec application of tryptamine or tyramine, immediately followed by a 30 sec co-application of tryptamine or tyramine and odorant. The current response in the presence of antagonist candidate was compared to the preceding response to odorant alone and expressed as a percentage. In our previous work, we found that repeated odorant applications to some ORs could cause a progressive decrease in response amplitude31,33. For this reason, we then re-normalized antagonism data to the value obtained when the assay was run in the absence of antagonist candidate (sham). In the “sham” assay, oocytes were exposed to a 30 sec application of odorant followed by a 10 min wash and then exposed to a 90 sec application of ND96 (no antagonist candidate), immediately followed by a 30 sec application of odorant. The second odorant response was compared to the first response and expressed as a percentage. In Figure 4B, 4C, Figure 5B and Figure 6, the sham value for 100nM eugenol was 57 ± 3% (mean ± SEM, n = 3). In Figure 5B, the sham value for 10nM eugenol was 93 ± 4% (n = 4). In Figure 4B and C, the sham value for 1µM Z11-14:OAc was 82 ± 6% (n = 6) and the sham value for 150µM 2-phenylethanol was 92 ± 2% (n = 3). In Figure 6, the sham value for 3µM l-fenchone was 83 ± 1% (n = 3), the sham value for 40µM acetophenone was 92 ± 1% (n = 3), the sham value for 70µM geranyl acetate was 97 ± 1% (n = 3), the sham value for 10µM 6-methyl-5-hepten-2-one was 94 ± 2% (n = 3) and the sham value for 3µM 2-nonanone was 81 ± 1% (n = 3).
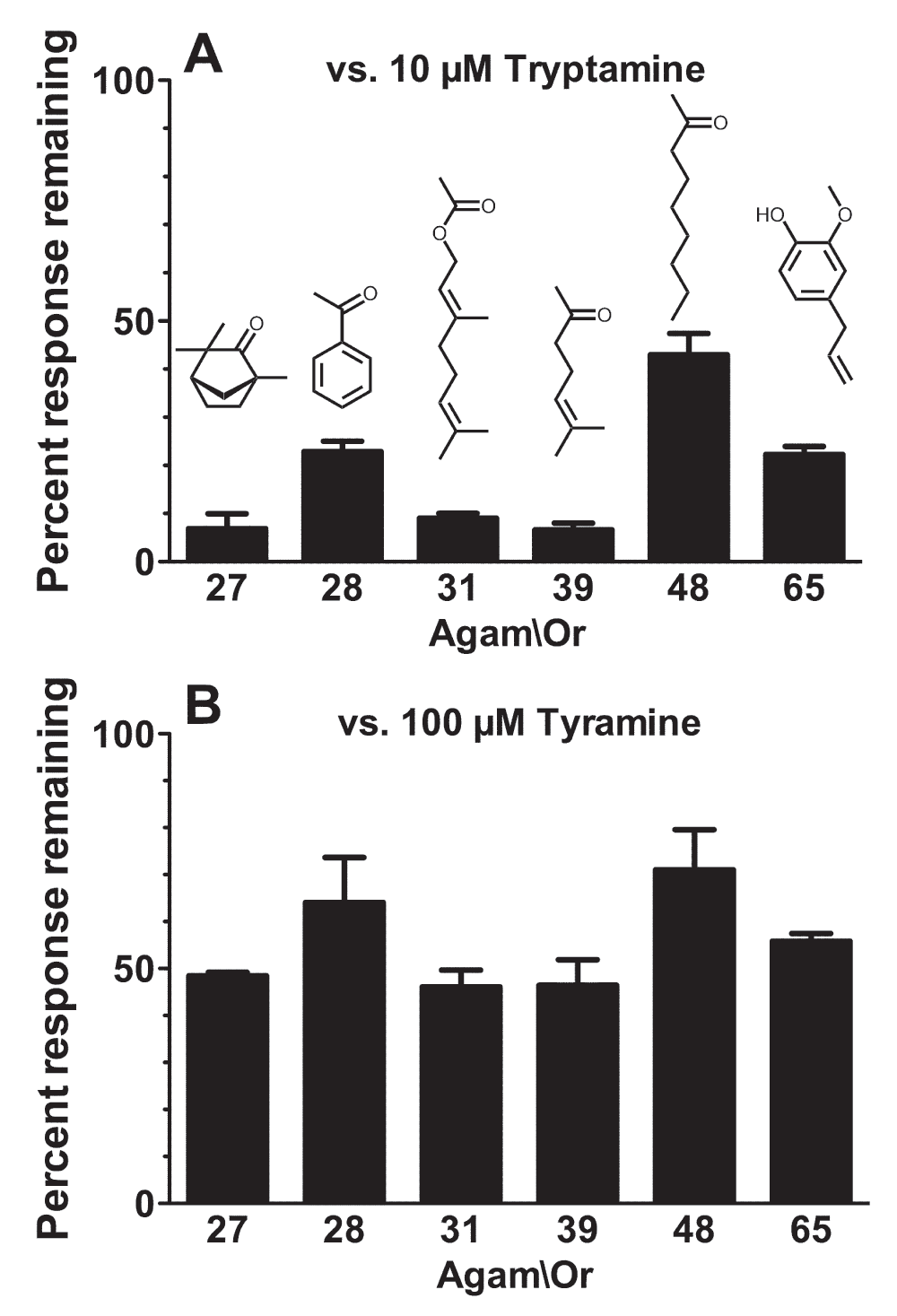
Figure 6. Tryptamine and tyramine inhibit odorant activation of multiple Agam\ORs.
Current responses of oocytes expressing Agam\Orco+Agam\Or27 (activated by 3µM L-fenchone), Agam\Orco+Agam\Or28 (activated by 40µM acetophenone), Agam\Orco+Agam\Or31 (activated by 70µM geranyl acetate), Agam\Orco+Agam\Or39 (activated by 10µM 6-methyl-5-hepten-2-one), Agam\Orco+Agam\Or48 (activated by 3µM 2-nonanone), or Agam\Orco+Agam\Or65 (activated by 100nM eugenol) in the presence of 10µM tryptamine (A) or 100µM tyramine (B) were compared to the preceding response to odorant alone and are presented as mean ± SEM (n = 3). Odorant structures are shown.
Initial analysis of electrophysiological data was done using Clampfit 9.1 software (Molecular Devices). Curve fitting and statistical analyses were done using Prism 5 (Graphpad). Concentration-inhibition data were fit to the equation: I = Imax/(1+ (X/IC50)n) where I represents the current response at a given concentration of inhibitor, X; Imax is the maximal response in the absence of inhibitor; IC50 is the concentration of inhibitor present that still allows a half maximal response from odorant; n is the apparent Hill coefficient. Concentration-response data were fit to the equation: I = Imax/(1+(EC50/X)n) where I represents the current response at a given concentration of odorant, X; Imax is the maximal response; EC50 is the concentration of agonist yielding a half maximal response; n is the apparent Hill coefficient. Statistical significance (p<0.05) was assessed using a two-tailed unpaired t test, an F test, or a one-way analysis of variance followed by the Dunnett's post-test, as appropriate.
Results
To screen a panel of biogenic and trace amines (Figure 1), we expressed Orco from Culex quinquefasciatus (Southern House Mosquito) in Xenopus oocytes and recorded ligand-induced current responses using two-electrode voltage clamp electrophysiology (see Methods). Orco subunits from several species, including Cqui\Orco, have been shown to form homomeric channels when heterologously expressed in the absence of odorant-binding subunits27,33. This convenient property of Orco allowed us to perform the initial screen without potentially confounding interactions with odorant-binding subunits. Successful functional expression of Cqui\Orco was confirmed by application of OLC12, a previously identified Orco specific agonist31. While OLC12 elicited robust current responses, none of the amines displayed agonist activity at Cqui\Orco (Figure 2A). Next we screened the amines for antagonist activity by applying 30µM OLC12 (∼EC5) to activate Cqui\Orco and co-applying 100µM of each amine (Figure 2B, C). Several amines were able to inhibit OLC12 activation of Cqui\Orco. Tryptamine was the most effective antagonist, blocking more than 90% of the OLC12 response (92 ± 2% inhibition). Highly significant inhibition (p<0.001) was also observed for phenethylamine (41 ± 1%), tyramine (40 ± 5%), gramine (30 ± 4%) and serotonin 23 ± 3%), but the extent of inhibition was less than 50%, suggesting relatively low affinity interactions. Histamine (16 ± 8%), melatonin (13 ± 1%) and epinephrine (9 ± 3%) also displayed significant (p<0.01), but modest, inhibition of the OLC12 current. Octopamine, dopamine and norepinephrine were inactive in this assay.
In Figure 3A, we constructed concentration-inhibition curves for block of Cqui\Orco activity in order to quantitatively evaluate the inhibitory potency of tryptamine, as well as phenethylamine and tyramine, representing the less effective amines. Tryptamine was clearly the most potent of these antagonists, inhibiting Cqui\Orco with an IC50 of 4.7 ± 0.7µM, a value similar to that of the most potent synthetic Orco antagonists that we identified in our previous work33. Phenethylamine (IC50 = 117 ± 12µM) and tyramine (IC50 = 157 ± 22µM) were substantially less potent than tryptamine (25-fold and 33-fold, respectively). Previously identified Orco antagonists inhibited OLC12 activation of Orco through a competitive mechanism31,33. To determine whether tryptamine was also a competitive antagonist of Orco, we measured blockade of Cqui\Orco achieved by tryptamine when the OLC12 concentration was increased from 30µM to 100µM (Figure 3B). Tryptamine was significantly less effective at inhibiting responses to 100µM OLC12 (IC50 = 143 ± 18µM, p<0.0001, F-test), indicating that tryptamine is a competitive antagonist of Cqui\Orco.
We next asked whether tryptamine could also inhibit Orco from other insect species. In addition to Cqui\Orco, we tested Agam\Orco from An. gambiae (human malaria vector mosquito) and Dmel\Orco from D. melanogaster. Co-application of 10µM tryptamine inhibited OLC12 activation of Orco from each of these three insect species (Figure 4A). We then wondered whether tryptamine could also inhibit odorant activation of heteromeric insect ORs containing both Orco and odorant binding subunits. We chose ORs from three insect orders: Agam\Orco+Agam\Or65 from An. gambiae (Order Diptera) that responds to the eugenol25; Onub\Orco+Onub\Or6 from O. nubilalis (European Corn Borer, Order Lepidoptera) that responds to the pheromone Z11-14:OAc45; and Mcar\Orco+Mcar\Or5 from M. caryae (Long-Horned Beetle, Order Coleoptera) that responds to 2-phenylethanol44. We chose to proceed with an OR from An. gambiae instead of Cx. quinquefasciatus for two reasons. The best characterized of the Cqui\Or subunits respond to indoles23,24, which are structurally related to tryptamine and might confound our experiments. Also, the Agam\Or subunit family has been more extensively characterized10,25, offering more options for OR expression (see below). Each odorant was applied at or near the EC50 concentration (Table 1,44,45). Co-application of 10µM tryptamine resulted in substantial inhibition of each receptor (Figure 4B). We also examined tyramine. While tyramine is a low potency Orco antagonist (Figure 3A), it is a major neurotransmitter in insects37. Tyramine was also able to reduce odorant activation of these ORs, but was less effective than tryptamine (Figure 4C). These results suggest that tryptamine and tyramine are broadly active antagonists of insect ORs.
Several previously identified Orco antagonists have been shown to inhibit odorant activation of insect ORs through a non-competitive mechanism31–33. To determine whether the tryptamine inhibition of odorant activation that we observed in Figure 4 was also non-competitive, we examined the effect of tryptamine on activation of the heteromeric Agam\Orco+Agam\Or65 in more detail (Figure 5). When the concentration of Orco directed agonist (OLC12) was increased, the tryptamine inhibition curve was significantly shifted to the right (Figure 5A). However, when the concentration of odorant agonist (eugenol) was increased, the tryptamine inhibition curve did not shift (Figure 5B). These results indicate that, similar to previously identified synthetic Orco antagonist compounds, tryptamine is a competitive antagonist of direct activation of Orco and a non-competitive antagonist of odorant activation of the OR.
The ability of tryptamine to interact with Orco and exert a non-competitive inhibitory effect on odorant activation of a heteromeric OR (Figure 5) suggests that tryptamine should be able to inhibit activation of a variety of ORs activated by diverse odorants. To examine this possibility, we tested the ability of tryptamine to inhibit odorant activation of ORs formed by Agam\Orco and each of six different odorant-binding subunits chosen from across the An. gambiae OR gene family46. We activated each OR with a previously identified cognate odorant25 at a concentration at or near the EC50 (Table 1,25). In addition to Agam\Orco+Agam\Or65 (activated by eugenol), we tested Agam\Orco+Agam\Or27 (activated by L-fenchone), Agam\Orco+Agam\Or28 (activated acetophenone), Agam\Orco+Agam\Or31 (activated by geranyl acetate), Agam\Orco+Agam\Or39 (activated by 6-methyl-5-hepten-2-one) and Agam\Orco+Agam\Or48 (activated by 2-nonanone). With the exception of Agam\Or39 and Agam\Or48, which display overlapping odorant specificities at 4 odorants, there is little or no similarity among the odorant specificities of these six odorant-binding subunits25. In each case, 10µM tryptamine was able to inhibit odorant activation of the receptor, despite the disparate odorant-binding subunits and diverse odorant structures (Figure 6). Tyramine was also able to inhibit odorant activation of each of these receptors, but was less effective than tryptamine (note that tyramine is applied at 100µM). We conclude that tryptamine and tyramine are general antagonists of insect ORs.
Discussion
Animals use a variety of biogenic and trace amines as neurotransmitters and neuromodulators. These include compounds derived from tyrosine (dopamine, norepinephrine, epinephrine, tyramine, octopamine and phenethylamine), tryptophan (serotonin, melatonin and tryptamine) and histidine (histamine)47. Dopamine and serotonin play a variety of roles in the insect nervous system48–50. In addition, insects use octopamine, histamine and tyramine as neurotransmitters48–53. Melatonin also appears to exert neuromodulatory effects in insects54,55. Interestingly, many of these amines modulate the olfactory system56–58.
Recent reports27,32, together with our previous findings31,33, have revealed the existence of a ligand-binding site on the Orco subunit and that inhibition of odorant activation through a non-competitive mechanism may be a general property of Orco-directed antagonists. Our current results suggest that endogenous and exogenous natural compounds serve as Orco ligands and modulate insect olfaction. While tyramine is a major neurotransmitter in insects53, its low potency in our assay (Figure 3) suggests that it might not serve as an endogenous OR modulator. However, the function of an endogenous Orco antagonist is unlikely to be the complete block of OR function. Rather, an endogenous Orco antagonist might be used to diminish olfactory sensitivity by inhibiting a fraction of the available receptors. For tyramine, such inhibition could occur at concentrations ranging from 10µM to 30µM. Alternatively, there may be additional, more potent, but as yet uncharacterized, endogenous Orco antagonists that can decrease olfactory sensitivity at lower concentrations.
In contrast to the low potency of tyramine, we found tryptamine to be a high potency Orco antagonist. Tryptamine inhibited odorant activation of an OR with an IC50 in the low micromolar range (Figure 5). While it is currently unclear whether tryptamine is endogenous to insects, tryptamine and similar compounds, such as gramine, are produced by a variety of plants and are thought to serve as a defense against insect herbivores42,59. Various tryptamine analogs have been proposed as larvicides60 and when tryptamine is caused to accumulate in poplar and tobacco, through ectopic expression of tryptophan decarboxylase, the feeding behavior of insects that target these plants is altered41. Tryptamine-based structures also act on various receptors and transporters, particularly those involved in serotonergic neurotransmission, exerting psychedelic effects in humans. Indeed, many plant derived and synthetic hallucinogens are based on the tryptamine and phenethylamine scaffolds61–63. Interestingly, the potency that we observed for tryptamine inhibition of odorant activation of an insect OR (Figure 5) is similar to the potency for tryptamine inhibition of the D. melanogaster serotonin transporter64.
Might there also be natural endogenous or exogenous Orco agonists? An endogenous Orco agonist could serve to increase olfactory sensitivity, perhaps in a circadian fashion, to alter behavior during critical foraging or mating periods. An exogenous, plant-derived Orco agonist would, by activating all ORs through Orco, serve as an olfactory “confusant” and might alter the feeding behavior of insect herbivores. The limited screen of 11 compounds that we conducted here did not identify any Orco agonists, but more extensive screening is clearly warranted.
Several synthetic Orco antagonists have been shown to inhibit odorant activation of ORs through an allosteric mechanism31–33. The ability of these compounds to inhibit multiple ORs from a variety of species is likely due to the high conservation of Orco across the insects12. Similarly, we found that tryptamine and tyramine, acting as Orco antagonists, could inhibit odorant activation of ORs from insect species chosen from three different orders: Diptera (An. gambiae), Lepidoptera (O. nubilalis) and Coleoptera (M. caryae). Furthermore, when we examined multiple ORs from a single species (An. gambiae), we found that tryptamine and tyramine blocked odorant activation of each receptor. The action of these compounds through Orco allowed blockade to occur despite the highly diverse odorant-binding subunits used to form the receptors and the different odorant structures used to activate the receptors. Interestingly, while all six receptors were inhibited, the extent of inhibition varied depending on the odorant-binding subunit present and the pattern of variation was similar for tryptamine and tyramine. This suggests differences in allosteric coupling between Orco and the various odorant-binding subunits. Also, while we showed that tryptamine is a potent inhibitor of odorant activation of Agam\Or65+Agam\Orco, the results we present in Figure 6 suggest that tryptamine is even more potent at other ORs, such as those formed by Agam\Or27, Agam\Or31 and Agam\Or39. Our current results with naturally occurring amines, together with previous reports with synthetic compounds27,31–33,35 strongly suggest that: 1) allosteric antagonism of odorant activation of ORs is a general property of Orco antagonists; 2) Orco antagonists are broadly active at ORs of many insect species; and 3) Orco is an important target for the development of novel insect repellants. The broad activity of Orco directed compounds across many insect species that has been observed to date suggests that these compounds may have limited agricultural utility, since both pests and pollinators could be affected. Determining whether species-specific Orco ligands can be developed will require further effort. What is clear, however, is that the pursuit of new, synthetic Orco directed ligands (both agonists and antagonists) is a promising direction for the development of new, more effective insect repellants that can aid in controlling the spread of insect-borne diseases.
Data availability
figshare: Inhibition of odorant and Orco agonist initiated current responses of oocytes expressing insect odorant receptors by various amines, doi: 10.6084/m9.figshare.97779165
Author contributions
SC and CWL conceived the study. SC and CWL designed the experiments. SC performed the experiments. SC and CWL analyzed the data. SC and CWL wrote the manuscript.
Competing interests
No competing interests were disclosed.
Grant information
This work was supported by a grant from the National Institutes of Health (RO1 DC011091 to CWL).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgements
We thank B. Sherman and A. Castro for Xenopus care and oocyte preparation.
Faculty Opinions recommendedReferences
- 1.
Klein AM, Vaissière BE, Cane JH, et al.:
Importance of pollinators in changing landscapes for world crops.
Proc Biol Sci.
2007; 274(1608): 303–313. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 2.
Kremen C, Williams NM, Aizen MA, et al.:
Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land-use change.
Ecol Lett.
2007; 10(4): 299–314. PubMed Abstract
| Publisher Full Text
- 3.
Abate T, Ampofo JK:
Insect pests of beans in Africa: their ecology and management.
Annu Rev Entomol.
1996; 41: 45–73. PubMed Abstract
| Publisher Full Text
- 4.
Bardner R, Fletcher KE:
Insect Infestations and Their Effects on Growth and Yield of Field Crops: a Review.
Bull Entomol Res.
1974; 64(1): 141–160. Publisher Full Text
- 5.
Gahukar RT:
Insect Pests of Millets and Their Management: a Review.
Trop Pest Manage.
35(4): 382–391. Publisher Full Text
- 6.
Gratz NG:
Critical review of the vector status of Aedes albopictus.
Med Vet Entomol.
2004; 18(3): 215–227. PubMed Abstract
| Publisher Full Text
- 7.
Sinka ME, Rubio-Palis Y, Manguin S, et al.:
The dominant Anopheles vectors of human malaria in the Americas: occurrence data, distribution maps and bionomic precis.
Parasit Vectors.
2010; 3: 72. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 8.
Pages F, Faulde M, Orlandi-Pradines E, et al.:
The past and present threat of vector-borne diseases in deployed troops.
Clin Microbiol Infect.
2010; 16(3): 209–224. PubMed Abstract
| Publisher Full Text
- 9.
Hallem EA, Carlson JR:
Coding of odors by a receptor repertoire.
Cell.
2006; 125(1): 143–160. PubMed Abstract
| Publisher Full Text
- 10.
Carey AF, Wang G, Su CY, et al.:
Odorant reception in the malaria mosquito Anopheles gambiae.
Nature.
2010; 464(7285): 66–71. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 11.
Vosshall LB, Amrein H, Morozov PS, et al.:
A spatial map of olfactory receptor expression in the Drosophila antenna.
Cell.
1999; 96(5): 725–736. PubMed Abstract
| Publisher Full Text
- 12.
Larsson MC, Domingos AI, Jones WD, et al.:
Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction.
Neuron.
2004; 43(5): 703–714. PubMed Abstract
| Publisher Full Text
- 13.
Nakagawa T, Vosshall LB:
Controversy and consensus: noncanonical signaling mechanisms in the insect olfactory system.
Curr Opin Neurobiol.
2009; 19(3): 284–292. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 14.
Neuhaus EM, Gisselmann G, Zhang W, et al.:
Odorant receptor heterodimerization in the olfactory system of Drosophila melanogaster.
Nat Neurosci.
2005; 8(1): 15–17. PubMed Abstract
| Publisher Full Text
- 15.
Benton R, Sachse S, Michnick SW, et al.:
Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo.
PLoS Biol.
2006; 4(2): e20. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 16.
Laissue PP, Vosshall LB:
The olfactory sensory map in Drosophila.
Adv Exp Med Biol.
2008; 628: 102–114. PubMed Abstract
| Publisher Full Text
- 17.
Goldman AL, Van der Goes van Naters W, Lessing D, et al.:
Coexpression of two functional odor receptors in one neuron.
Neuron.
2005; 45(5): 661–666. PubMed Abstract
| Publisher Full Text
- 18.
Wicher D, Schäfer R, Bauernfeind R, et al.:
Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels.
Nature.
2008; 452(7190): 1007–1011. PubMed Abstract
| Publisher Full Text
- 19.
Sato K, Pellegrino M, Nakagawa T, et al.:
Insect olfactory receptors are heteromeric ligand-gated ion channels.
Nature.
2008; 452(7190): 1002–1006. PubMed Abstract
| Publisher Full Text
- 20.
Nichols AS, Luetje CW:
Transmembrane segment 3 of Drosophila melanogaster odorant receptor subunit 85b contributes to ligand-receptor interactions.
J Biol Chem.
2010; 285(16): 11854–11862. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 21.
Nichols AS, Chen S, Luetje CW:
Subunit contributions to insect olfactory receptor function: channel block and odorant recognition.
Chem Senses.
2011; 36(9): 781–790. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 22.
Pask GM, Jones PL, Rutzler M, et al.:
Heteromeric Anopheline odorant receptors exhibit distinct channel properties.
PLoS One.
2011; 6(12): e28774. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 23.
Hughes DT, Pelletier J, Luetje CW, et al.:
Odorant receptor from the southern house mosquito narrowly tuned to the oviposition attractant skatole.
J Chem Ecol.
2010; 36(8): 797–800. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 24.
Pelletier J, Hughes DT, Luetje CW:
An odorant receptor from the southern house mosquito Culex pipiens quinquefasciatus sensitive to oviposition attractants.
PLoS One.
2010; 5(4): e10090. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 25.
Wang G, Carey AF, Carlson JR, et al.:
Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae.
Proc Natl Acad Sci U S A.
2010; 107(9): 4418–4423. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 26.
Xia Y, Wang G, Buscariollo D, et al.:
The molecular and cellular basis of olfactory-driven behavior in Anopheles gambiae larvae.
Proc Natl Acad Sci U S A.
2008; 105(17): 6433–6438. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 27.
Jones PL, Pask GM, Rinker DC, et al.:
Functional agonism of insect odorant receptor ion channels.
Proc Natl Acad Sci U S A.
2011; 108(21): 8821–8825. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 28.
Rinker DC, Jones PL, Jason pitts R, et al.:
Novel high-throughput screens of Anopheles gambiae odorant receptors reveal candidate behavior-modifying chemicals for mosquitoes.
Physiol Entomol.
2012; 37: 33–41. Publisher Full Text
- 29.
Carey AF, Carlson JR:
Insect olfaction from model systems to disease control.
Proc Natl Acad Sci U S A.
2011; 108(32): 12987–12995. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 30.
Ramdya P, Benton R:
Evolving olfactory systems on the fly.
Trends Genet.
2010; 26(7): 307–316. PubMed Abstract
| Publisher Full Text
- 31.
Chen S, Luetje CW:
Identification of new agonists and antagonists of the insect odorant receptor co-receptor subunit.
PLoS One.
2012; 7(5): e36784. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 32.
Jones PL, Pask GM, Romaine IM, et al.:
Allosteric antagonism of insect odorant receptor ion channels.
PLoS One.
2012; 7(1): e30304. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 33.
Chen S, Luetje CW:
Phenylthiophenecarboxamide antagonists of the olfactory receptor co-receptor subunit from a mosquito.
PLoS One.
2013; 8(12): e84575. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 34.
Jones WD, Nguyen TA, Kloss B, et al.:
Functional conservation of an insect odorant receptor gene across 250 million years of evolution.
Curr Biol.
2005; 15(4): R119–121. PubMed Abstract
| Publisher Full Text
- 35.
Bohbot JD, Dickens JC:
Odorant receptor modulation: ternary paradigm for mode of action of insect repellents.
Neuropharmacology.
2012; 62(5-6): 2086–2095. PubMed Abstract
| Publisher Full Text
- 36.
Blenau W, Thamm M:
Distribution of serotonin (5-HT) and its receptors in the insect brain with focus on the mushroom bodies: lessons from Drosophila melanogaster and Apis mellifera.
Arthropod Struct Dev.
2011; 40(5): 381–394. PubMed Abstract
| Publisher Full Text
- 37.
Cazzamali G, Klaerke DA, Grimmelikhuijzen CJ:
A new family of insect tyramine receptors.
Biochem Biophys Res Commun.
2005; 338(2): 1189–1196. PubMed Abstract
| Publisher Full Text
- 38.
Nassel DR:
Histamine in the brain of insects: a review.
Microsc Res Tech.
1999; 44(2-3): 121–136. PubMed Abstract
| Publisher Full Text
- 39.
Roeder T:
Octopamine in invertebrates.
Prog Neurobiol.
1999; 59(5): 533–561. PubMed Abstract
| Publisher Full Text
- 40.
Cai QN, Han Y, Cao YZ, et al.:
Detoxification of gramine by the cereal aphid Sitobion avenae.
J Chem Ecol.
2009; 35(3): 320–325. PubMed Abstract
| Publisher Full Text
- 41.
Gill RI, Ellis BE, Isman MB:
Tryptamine-induced resistance in tryptophan decarboxylase transgenic poplar and tobacco plants against their specific herbivores.
J Chem Ecol.
2003; 29(4): 779–793. PubMed Abstract
| Publisher Full Text
- 42.
Thomas JC, Saleh EF, Alammar N, et al.:
The indole alkaloid tryptamine impairs reproduction in Drosophila melanogaster.
J Econ Entomol.
1998; 91(4): 841–846. PubMed Abstract
- 43.
Liman ER, Tytgat J, Hess P:
Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs.
Neuron.
1992; 9(5): 861–871. PubMed Abstract
| Publisher Full Text
- 44.
Mitchell RF, Hughes DT, Luetje CW, et al.:
Sequencing and characterizing odorant receptors of the cerambycid beetle Megacyllene caryae.
Insect Biochem Mol Biol.
2012; 42(7): 499–505. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 45.
Wanner KW, Nichols AS, Allen JE, et al.:
Sex pheromone receptor specificity in the European corn borer moth, Ostrinia nubilalis.
PLoS One.
2010; 5(1): e8685. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 46.
Hill CA, Fox AN, Pitts RJ, et al.:
G protein-coupled receptors in Anopheles gambiae.
Science.
2002; 298(5591): 176–178. PubMed Abstract
| Publisher Full Text
- 47.
Zucchi R, Chiellini G, Scanlan TS, et al.:
Trace amine-associated receptors and their ligands.
Br J Pharmacol.
2006; 149(8): 967–978. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 48.
Birmingham JT, Tauck DL:
Neuromodulation in invertebrate sensory systems: from biophysics to behavior.
J Exp Biol.
2003; 206(Pt 20): 3541–3546. PubMed Abstract
| Publisher Full Text
- 49.
Grosmaitre X, Marion-Poll F, Renou M:
Biogenic amines modulate olfactory receptor neurons firing activity in Mamestra brassicae.
Chem Senses.
2001; 26(6): 653–661. PubMed Abstract
| Publisher Full Text
- 50.
Perry CJ, Barron AB:
Neural mechanisms of reward in insects.
Annu Rev Entomol.
2013; 58: 543–562. PubMed Abstract
| Publisher Full Text
- 51.
Cole SH, Carney GE, McClung CA, et al.:
Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility.
J Biol Chem.
2005; 280(15): 14948–14955. PubMed Abstract
| Publisher Full Text
- 52.
Monastirioti M:
Biogenic amine systems in the fruit fly Drosophila melanogaster.
Microsc Res Tech.
1999; 45(2): 106–121. PubMed Abstract
| Publisher Full Text
- 53.
Roeder T:
Tyramine and octopamine: ruling behavior and metabolism.
Annu Rev Entomol.
2005; 50: 447–477. PubMed Abstract
| Publisher Full Text
- 54.
Bembenek J, Sehadova H, Ichihara N, et al.:
Day/night fluctuations in melatonin content, arylalkylamine N-acetyltransferase activity and NAT mRNA expression in the CNS peripheral tissues and hemolymph of the cockroach, Periplaneta americana.
Comp Biochem Physiol B Biochem Mol Biol.
2005; 140(1): 27–36. PubMed Abstract
| Publisher Full Text
- 55.
Richter K, Peschke E, Peschke D:
Effect of melatonin on the release of prothoracicotropic hormone in the brain of Periplaneta americana (Blottodea: Blattidae).
Eur J Entomol.
1999; 96: 341–345. Reference Source
- 56.
Dacks AM, Riffell JA, Martin JP, et al.:
Olfactory modulation by dopamine in the context of aversive learning.
J Neurophysiol.
2012; 108(2): 539–550. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 57.
Huser A, Rohwedder A, Apostolopoulou AA, et al.:
The serotonergic central nervous system of the Drosophila larva: anatomy and behavioral function.
PLoS One.
2012; 7(10): e47518. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 58.
Zhukovskaya MI:
Modulation by octopamine of olfactory responses to nonpheromone odorants in the cockroach, Periplaneta americana L.
Chem Senses.
2012; 37(5): 421–429. PubMed Abstract
| Publisher Full Text
- 59.
Sun XQ, Zhang MX, Yu JY, et al.:
Glutathione S-transferase of brown planthoppers (Nilaparvata lugens) is essential for their adaptation to gramine-containing host plants.
PLoS One.
2013; 8(5): e64026. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 60.
Oliveira RR, Brito TB, Nepel A, et al.:
Synthesis, Activity, and QSAR Studies of Tryptamine Derivatives on Third-instar Larvae of Aedes aegypti Linn.
Med Chem.
2013. Epub ahead of print. PubMed Abstract
| Publisher Full Text
- 61.
McKenna DJ:
Plant hallucinogens: springboards for psychotherapeutic drug discovery.
Behav Brain Res.
1996; 73(1-2): 109–116. PubMed Abstract
- 62.
Shulgin A, Shulgin A:
Pihkal: A chemical love story. (Transform Press, Berkeley, California). 1991; p 978. Reference Source
- 63.
Shulgin A, Shulgin A:
Tihkal: The continuation. (Transform Press, Berkeley, California). 1997; p 804. Reference Source
- 64.
Adkins EM, Barker EL, Blakely RD:
Interactions of tryptamine derivatives with serotonin transporter species variants implicate transmembrane domain I in substrate recognition.
Mol Pharmacol.
2001; 59(3): 514–523. PubMed Abstract
| Publisher Full Text
- 65.
Chen S, Luetje CW:
Inhibition of odorant and Orco agonist initiated current responses of oocytes expressing insect odorant receptors by various amines.
Figshare.
2014. Data Source






Comments on this article Comments (0)