Keywords
Gamma glutamyl transpeptidase, alanine aminotransferase, aspartate aminotransferase, bilirubin, liver transplantation, oxidative stress, glutathione, survival
This article is included in the University Medical Center Groningen collection.
Gamma glutamyl transpeptidase, alanine aminotransferase, aspartate aminotransferase, bilirubin, liver transplantation, oxidative stress, glutathione, survival
We have made changes to the text based on the referees’ comments, in particular emphasizing the connection to oxidative stress in the abstract and expanding the discussion.
See the authors' detailed response to the review by Michael Olausson
See the authors' detailed response to the review by Peter Jansen
Gamma glutamyl transpeptidase (GGT) is a membrane-bound enzyme that is essential for the synthesis of glutathione (GSH), a key antioxidant1. In clinical practice elevated serum GGT is generally used as an indicator of liver disease, such as biliary obstruction, alcohol consumption, and exposure to certain medical drugs1. Recently, several epidemiological studies have shown that a higher serum GGT level, even within the normal range, is associated with cardiovascular risk factors such as hypertension, hypertriglyceridemia, obesity, type 2 diabetes mellitus and stroke, as well as certain types of cancer2–10. In contrast to these studies, we observed that after surgery for ruptured abdominal aortic aneurysm11 or after liver resection12, GGT is transiently increased in patients who had a good outcome. In these short-term observational studies GGT level was inversely related to other liver laboratory parameters such as aspartate aminotransferase (ALT), alanine aminotransferase (AST) as well as total bilirubin (TBI)11,12. We observe that in the early postoperative period after a liver transplantation (LT) a transient gradual increase in GGT also occurs. How early and late postoperative changes in serum GGT are related to survival is not known.
Here we present a study in which we assessed the relationship between early (postoperative day seven) elevated GGT levels with early and late survival (i.e. survival within the 90 days and five years post-LT, respectively). We also evaluated the relationship between late (six months postoperatively) elevated GGT levels with late survival.
In addition, we studied the early and late post-LT kinetics of GGT, AST, ALT, and TBI in patients who survived more than 90 days and in patients who did not. Likewise, these kinetics were compared with long-term survival.
We conducted a single center cohort study on 522 first liver transplant patients. All LTs performed between January 1990 and August 2009 were included; excluded were pediatric patients (age <17 years), second or subsequent LT, and first LT patients who underwent a re-LT within 90 days of their first LT. Since obstructive mechanisms such as non-anastomotic stricture (NAS), acute graft rejection, and cholestatic disorders might influence GGT and TBI levels, we also specifically repeated our analysis with and without such patients. This study was performed in accordance with Dutch legislation and the local ethical committee guidelines.
Patient characteristics and variables related to the perioperative management and the surgical procedure were obtained from a prospectively collected database. These included age, sex, body mass index, Karnofsky score, indication for LT, preoperative MELD (Model for End-Stage Liver Disease) score (calculated from preoperative laboratory measurements), length of hospital stay, cold ischemia time, warm ischemia time, duration of operation, combined transplant (kidney or lung), acute rejection, graft type, the number of units of allogeneic and autologous red blood cell units (RBC with 1 U containing 300 ml) and fresh frozen plasma (FFP with 1 U containing 250 ml), donor type, type of venous and bile duct anastomosis, and NAS within 90 days and within one year. When necessary, the hospital files were reviewed to complete all relevant clinical parameters.
We studied the levels of serum GGT and other liver variables postoperatively in two ways. Early postoperatively, up to postoperative day (POD) 30, we studied the levels of GGT (reference values; 0–40 U/l), ALT (0–45 U/l), AST (0–40 U/l), and TBI (0–17 µmol/l) over time in patients who survived more than 90 days after LT compared to those who did not. Late postoperatively (i.e. 90 days and beyond), we evaluated the levels of these variables at three months, six months, and one year in patients who survived more than five years compared to those patients who did not.
To evaluate the clinical relevance of early and late elevated GGT levels, we generated tertiles of low, intermediate, and high GGT levels based on equal percentiles. GGT levels at POD 7 were used to study the relationship between early elevated GGT levels and both 90-day and five-year survival. GGT levels at six months following LT were used to study the relationship of late elevated GGT with five-year survival. The last observation date for the status of patient survival for the study cohort was August 23, 2012.
Statistical analyses were performed using the statistical software package SPSS 20 (IBM SPSS, Chicago, IL). Categorical variables are shown as numbers and percentages. Continuous variables are presented as means with standard deviation (SD) or as medians with interquartile range (IQR) based on their distribution. Continuous variables that were not normally distributed were compared using the Mann Whitney U test. We studied early LT mortality based on GGT levels at POD 7 as a categorical variable, using tertiles (low, intermediate, and high). Similarly, we assessed the late LT mortality based on GGT levels at six months post-LT using tertiles.
Patient survival was analyzed with Kaplan-Meier analysis and the differences between the groups were assessed with the log-rank test. A p<0.05 was considered statistically significant.
We performed a total of 968 consecutive LTs in our center between January 1990 and August 2009. After excluding pediatric LTs (age <17 yr; n=290), patients who were re-transplanted within the 90 days of their first LT (n=39), second or subsequent LTs (n=101), patients with a lack of follow up data (n=11), and patients that died intraoperatively (n=5) due to brain death, cardiac failure, or uncontrollable bleeding, 522 patients were included in our analyses. The median age was 48 years (37–56), 54% of the patients were males, mean (SD) BMI was 24.8 (± 5.3), median Karnofsky score was 60 (30–70) and median MELD score was 17 (13–24) for the study population. Indications for LT were post necrotic cirrhosis (49%), cholestatic liver disease (30%), metabolic disease (10%), acute liver failure (7%), and miscellaneous (5%). Patient characteristics and the surgical variables of the entire group of 522 patients are summarized in Table 1.
The overall mortality within 90 days, one year, and five years for the study cohort was 8%, 12%, and 21%, respectively. Sepsis was the major cause of mortality (37%) within 90 days followed by multi organ failure (14%), and brain death (9%). Table 2 details all causes of mortality within the first 90 days.
Early postoperative laboratory variables are shown in Figure 1. Postoperatively, GGT levels increased gradually, reaching a maximum at POD 9 and decreased thereafter. Notably, the increase in GGT levels was significantly more pronounced, i.e. deviated more from the normal range, in patients who survived more than 90 days, as compared to those who did not: 297 (178–464) vs. 172 (69–271) U/l, p<0.0001, respectively. This pattern was different from that of postoperative levels of TBI, AST, and ALT. TBI was consistently and significantly lower in patients who survived more than 90 days following LT as compared to those who did. AST and ALT levels increased rapidly until POD 1 and POD 2, respectively, followed by rapid normalization thereafter. Contrary to GGT, the peak levels of AST and ALT were significantly lower in patients who survived more than 90 days following LT as compared to those who did not: AST 659 (326–1267) vs. 1201 (451–1990) U/l, p=0.01, and ALT 527 (280–1080) vs. 1082 (529–2631) U/l, p=0.001, respectively.
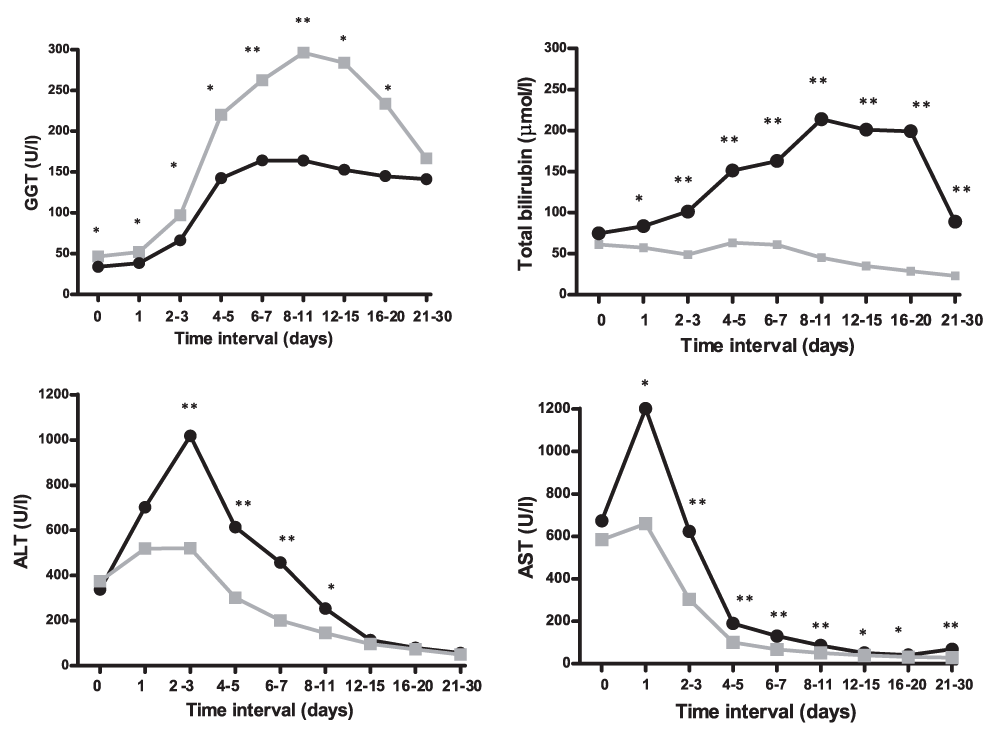
Curves represent patients who survived more than 90 days (gray) and those who did not (black). Median values are shown. * p < 0.05; ** p ≤ 0.001. GGT, gamma glutamyl transpeptidase; ALT, aspartate aminotransferase; AST, alanine aminotransferase; TBI, total bilirubin.
Thirty patients developed NAS within the 90 days post-LT. Since these patients may present with abnormal high TBI and GGT levels, we repeated the analysis with exclusion of these 30 patients with no significant affect on the GGT levels (graphical representation not shown). Also exclusion of patients who were treated for developing an acute rejection (n=103) and those who underwent LT for cholestatic liver disease (n=155) did not significantly affect the outcomes (graphical representation not shown).
Late postoperative laboratory variables are shown in Figure 2. We studied the changes in GGT levels over time in patients who survived more than five years as compared to those who did not survive. Compared to patients who died within five years post-LT, those who survived more than five years had significantly lower GGT at three months post-LT; 95 (42–244) vs. 212 (92–400) U/l, p=0.001, six months post-LT; 70 (31–189) vs. 281 (103–438) U/l, p<0.001, and 1 year post-LT; 57 (25–153) vs. 124 (45–431) U/l, p=0.003, respectively. Notably, late post-LT the GGT levels showed the same patterns as TBI, AST, and ALT, i.e. higher levels in non-survivors.
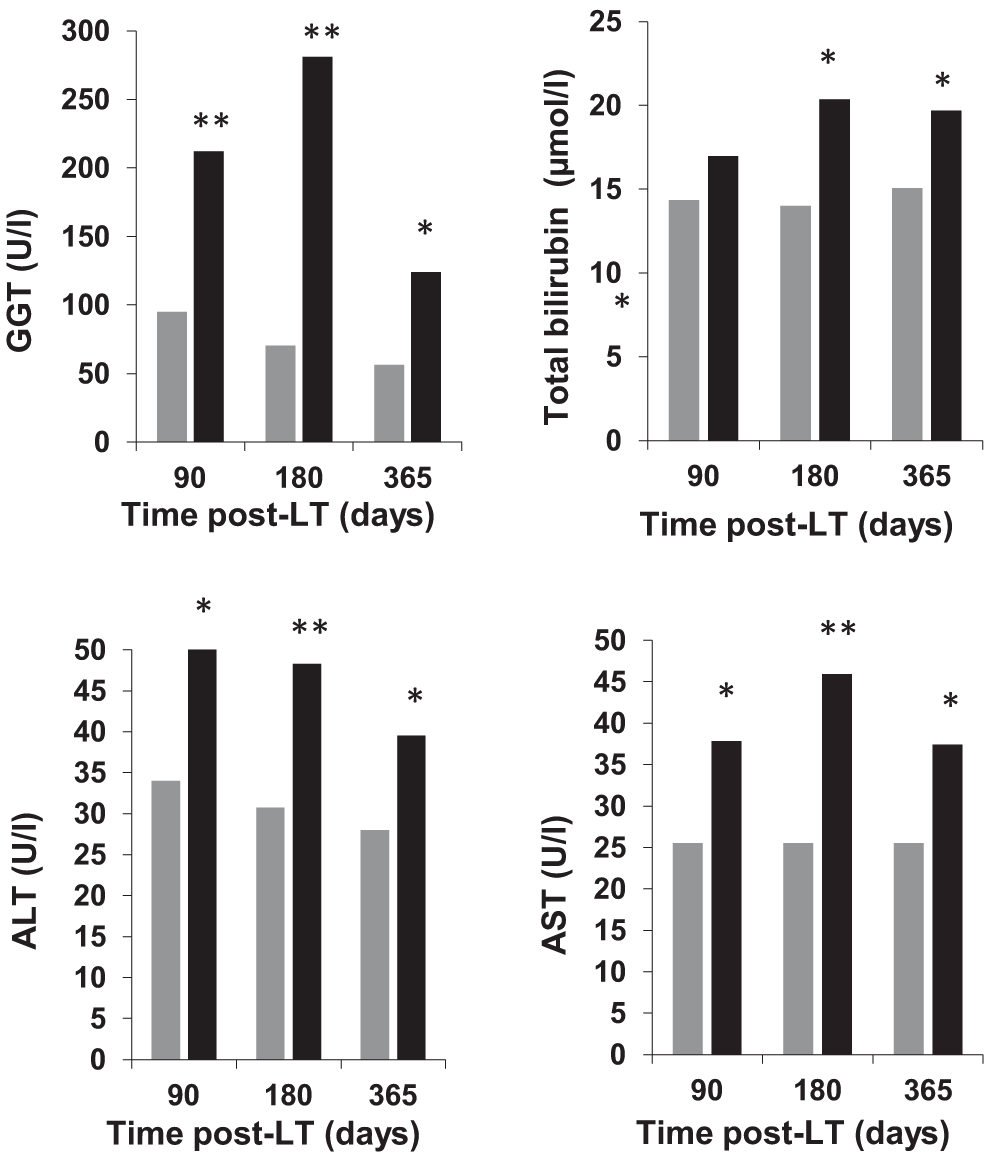
Bars represent patients who survived more than five years (gray) and those who did not (black). Median values are shown. *p<0.05; **p≤0.001. GGT, gamma glutamyl transpeptidase; ALT, aspartate aminotransferase; AST, alanine aminotransferase; TBI, total bilirubin.
We also studied the clinical relevance of early versus late elevated GGT levels with Kaplan-Meier survival analysis. Figure 3 plots the overall 90-day and 5-year overall survival for the study cohort based on GGT-tertiles. A high GGT level at POD 7 was significantly associated with better early survival following LT (Figure 3A). The overall 90-day survival was 98% for high GGT (≥ 351 U/l), compared to 94% for intermediate GGT levels (188 and 350 U/l), and 87% for the low GGT (≤ 187 U/l), at POD 7 (Figure 3A). Similarly, five-year overall survival was 86%, 83%, and 73% for high, intermediate, and low GGT at POD 7 (p=0.003; Figure 3B), respectively. Remarkably, the differences in five-year survival mainly developed during the first three months post-LT with almost no difference in survival curves thereafter (Figure 3B). In sharp contrast with early GGT, a high GGT level six months post-LT was associated with lower five-year survival (Figure 3C). The overall survival within five years was 71% for elevated GGT (> 163 U/l), compared to 91% for intermediate GGT levels (44 and 163 U/l), and 93% for the low GGT (< 43 U/l), p<0.001.
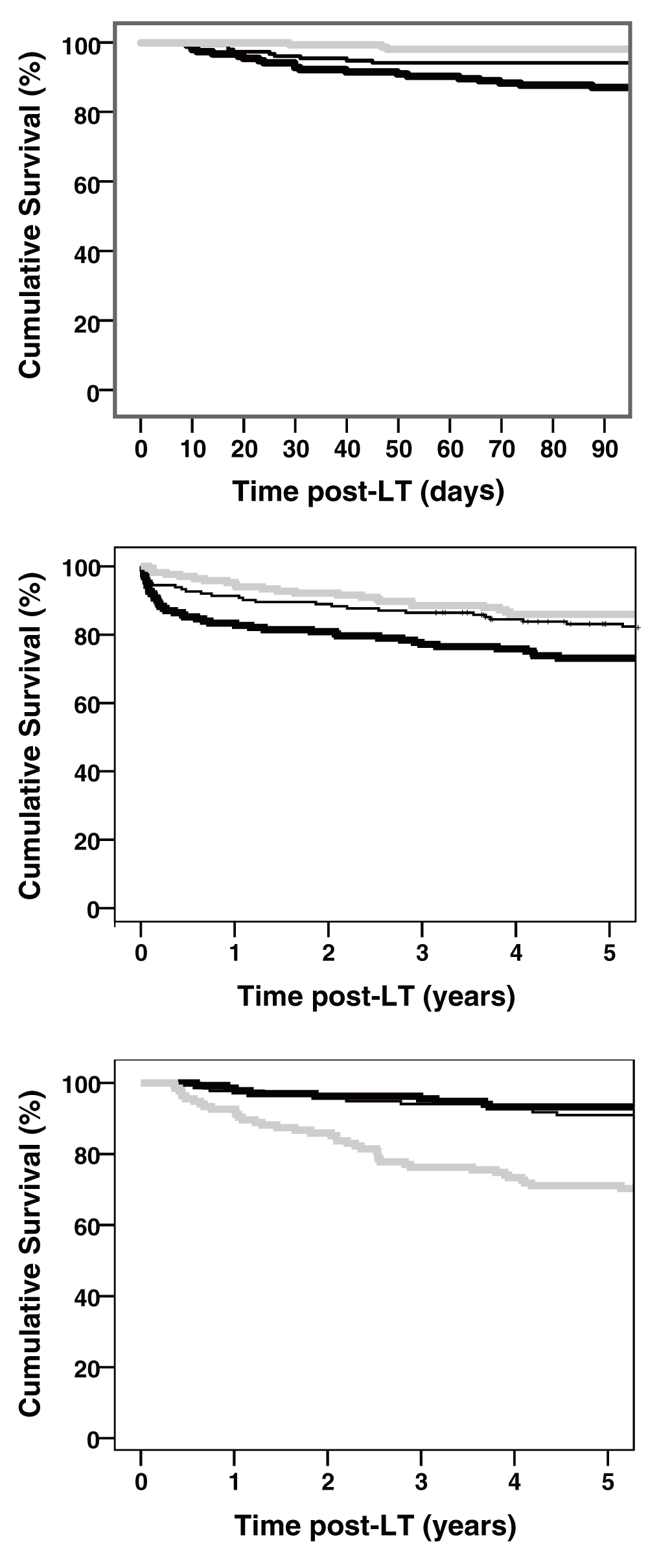
Panels A and B represent survival analysis post-LT in relation to GGT tertiles at POD 7, within 90 days and five years respectively (p=0.003). Panel C demonstrates five-year survival in relation to GGT tertiles at six months post-LT (p<0.001). Curves represent low (thick black), intermediate (thin black), and high GGT (gray).
In this study, we evaluated the changes in GGT over time following liver transplantation and the clinical relevance of these changes for early and late survival. We found that a transiently elevated GGT early after LT was associated with increased survival rates within the first 90 days. In contrast, late elevation of GGT was associated with decreased five-year survival following LT. Although the early GGT elevations was also associated with five-year survival, this difference mainly developed during the first 90 days post-LT.
This peculiar effect was not observed for TBI, AST, and ALT since higher levels for these parameters at POD 7 and six months were associated with increased mortality at both 90 days and five years after LT.
To our knowledge, this is the first study showing the short and long term kinetics of GGT and the clinical relevance of an early elevated serum GGT in LT recipients. Previously, we have reported improved outcome in patients with significantly increased levels of GGT in the early post-operative period following liver resection12 and surgical repair of a ruptured abdominal aortic aneurysm11. However, those studies were not designed to address changes in GGT progression over time.
While we acknowledge that association does not necessarily indicate causation, these data support the hypothesis that high GGT in an early post-LT setting may be a marker of some protective process.
Although the precise mechanism responsible for an elevated serum GGT early after LT is yet to be determined, experimental studies have demonstrated that cellular GGT modulates crucial redox-sensitive functions, such as antioxidant and antitoxic defenses, cellular proliferation, and apoptotic balance13. Cellular GGT is a key enzyme in the gamma-glutamyl cycle resulting in production of intracellular GSH14–16, an important antioxidant agent that protects the cells against reactive oxygen species (ROS)17–19. GSH has been shown to protect the liver against ischemia reperfusion injury in animal models16,20,21. Hepatic ischemia can cause elevation of serum GGT with peak blood levels within 20 and 30 hours after restoration of hepatic arterial blood flow18,22. Reperfusion is associated with a surge of ROS, which may overwhelm host natural antioxidant defenses21. The oxidative stress from the ROS formed after reperfusion may lead to increased cellular death by damaging membrane lipids through peroxidation, disrupting normal enzymatic activities, and diminishing mitochondrial oxidative metabolism23. Cardin and colleagues24 studied oxidative stress in patients with chronic hepatitis C virus infection. Surprisingly, the authors observed an association between GGT and 8-hydroxydeoxyguanosine (8-OHdG), a marker of oxidative DNA damage. Patients who had a high level of 8-OHdG had significantly higher GGT levels but normal ALT levels24.
Thus, a transient increase in GGT level post-LT may reflect the host compensatory mechanism against oxidative stress and toxic metabolites generated by hypoxia, reperfusion, and surgical stress21. Therefore, an increased GGT early after LT may reflect the ability of the host to initiate an appropriate systemic response.
Elevated serum GGT in the early post-LT period might also be a marker of better liver regeneration. Eisenbach and colleagues25, showed that an early increase in serum GGT after LT was associated with a better outcome and the authors reasoned that this rise could be due to liver regeneration. Although the liver might regenerate to some extent after LT, there is no conclusive evidence to support this hypothesis. As we mentioned earlier, we observed a transient increase in serum GGT levels in patients who survived a surgical repair for a ruptured abdominal aortic aneurysm11. In the latter group, it is less likely that significant liver regeneration occurs.
Furthermore, elevated serum GGT early post-LT may also be an indicator of better early secretory function of the liver. GGT in part comes from the surface of the bile ducts and is released from the anchor, which attaches this enzyme to the cell surface, by bile acids in bile. Bile acid/phospholipid ratio in bile has shown to be elevated after LT26. Thus elevated serum GGT immediately after transplantation may be an indicator or an active bile flow. Hence, it may protect hepatocytes from cytotoxic bile acids.
Contrary to early elevated GGT, but in line with published literature2–10, we observed that a late (i.e. six months post-LT) elevated GGT was significantly associated with decreased survival within the 5-years following LT. Although the finding that normal GGT levels in the late post-operative period is predictive of good outcomes is obvious and intuitive, the contrasting influences of GGT levels between early and late post-LT periods on survival may be compatible with the physiologic function of intracellular GGT. Notably, at three months, six months, and one year post-LT, the relative increase in serum GGT was two to four times higher in patients who did not survive for more than five years compared to those who did survive. This proportion was much higher than that of AST, ALT and TBI, which might imply that an elevated serum GGT is not only a marker of harm to the liver but it could be seen as a systemic response to harmful environmental factors. Indeed, in two studies, Lee and colleagues27,28 postulated that serum GGT in the general population might be a marker of increased exposure to environmental stress, internal xenobiotics, or other unknown factors that cause oxidative stress in the long run.
To avoid possible bias we performed our analysis after excluding obstructive mechanisms such as NAS, cholestatic disorders, and acute graft rejection early postoperatively. Exclusion of these cases did not change our results significantly, suggesting that the elevation in serum GGT early post-LT is independent of obstructive disorders.
A practical clinical consequence of our findings may be that care providers in hospitals should realize that an abnormally high GGT early post-LT is not a cause for alarm or specific diagnostic procedures. In fact, a GGT activity four to five times above the normal range during the second post-operative week might even be considered beneficial.
We acknowledge some considerable limitations in this study. First, due to the retrospective design of the study we can only identify association rather than causation. This could only be established by specific (intervention) studies that measure the interaction between serum GGT and ROS markers post-LT. However, there is a strong correlation between GGT and oxidative DNA damage in cirrhotic patients24. Next, we cannot entirely exclude that liver regeneration plays a role in the early post-LT rise of GGT levels since GSH and to some extent GGT are mainly produced by the liver1. Hence a high serum GGT in patients who survived more than 90 days can also be a reflection of a well-functioning graft in LT patients. It will be important to understand the relationship of serum GGT and cellular GGT in the period immediately after surgery. Besides, cumulative evidence suggests that there is a relationship between the induction and release of ROS and ischemia reperfusion injury after other types of abdominal surgery18,21,22.
In conclusion, an elevated GGT level early after LT was associated with a better short-term outcome. However, chronically elevated GGT was associated with poor long-term outcome in the outpatient setting after LT. This peculiar switch in the prognostic meaning of GGT may result from the superposition of several mechanisms. Apparently, higher expression of GGT reflects some protective process in the acute phase but reflects chronic damage over the long term.
figshare: Serum gamma glutamyl transpeptidase levels post-liver transplantation and survival data, http://dx.doi.org/10.6084/m9.figshare.90034329
The study was conceived by EMA, TJL, RJP, and MWN. Data acquisition and analysis were performed by EMA and MWN. The paper was written by EMA, TJL, RJP and MWN.
This study was sponsored in part by a Mozaiek grant of the Dutch Organisation of Scientific Research (017.007.115) to Edris Alkozai.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
This is an interesting paper providing novel insights into the value of traditional serum markers. It is quite true that the understanding of elevated GGT values is very limited, despite the fact that this value is one of the most requested 'liver function tests'. Most clinicians who request this test probably have no clear understanding what is being tested. It is very interesting that elevated GGT shortly after liver transplantation is associated with a better outcome.
The title, abstract, data section, statistics and figures of the paper are OK, the discussion section could be a little more to the point.
In my opinion there might be two reasons for an elevated GGT early after transplantation: 1) Elevated GGT is associated with oxidative stress, and some oxidative stress may prompt the hepatocyte to activate innate protective mechanisms; 2) Elevated GGT after transplantation may be an indicator of good early secretory function of the liver. GGT in part comes from the surface of the bile ducts and is released from the anchor which attaches this enzyme to the cell surface, by bile acids in bile. These same authors have shown that immediately after transplantation the bile acid/phospholipid ratio in bile is elevated and (as in MDR3 deficient/LPAC patients) this leads to elevated GGT. Thus elevated GGT immediately after transplantation indicates an active bile flow. This protects hepatocytes from cytotoxic bile acids. Later after transplantation when the BA/PL ratio has normalized, bile has lost some of its detergent action, with lower basal serum GGT levels as a result. When GGT is elevated at this stage it has become the traditional indicator of liver damage associated with a bad outcome, as the authors indicate.
If the authors agree with this reasoning they could include some of these thoughts in the discussion section, in order to provide a little bit more insight into why elevation under some conditions may be beneficial as this, in my opinion, is the main and novel message of this paper.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 15 Aug 14 |
||
|
Version 1 03 Apr 14 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)