Keywords
consomic, genetic, inbred mice, treadmill running
This article is included in the Sports cardiology collection.
consomic, genetic, inbred mice, treadmill running
We thank the reviewers for their comments. In this version we include a simple linear regression of body mass on intrinsic exercise capacity and have expanded the discussion regarding the effect of sex-specific QTLs on exercise capacity.
See the authors' detailed response to the review by Reuben Howden
See the authors' detailed response to the review by Michael Turner
Cardiorespiratory fitness measured during a graded exercise test is inversely related to the relative risk of cardiovascular disease1,2. Results from human cross-sectional, twin, and prospective studies indicate that genetic factors account for 25–65% of the variation in exercise capacity3,4. Because having higher levels of exercise capacity has been shown to be beneficial for reducing the onset of cardiovascular disease, the physiological factors determining exercise capacity have been widely studied5. However, the genetic contribution to exercise capacity is not completely understood. Presently, several candidate genes contributing to improved exercise capacity have been proposed based on genome wide studies6,7, but these genes account for only a small portion of the variability in exercise capacity or training responses8.
Several studies have investigated the genetic factors contributing to exercise capacity using inbred rodent models9–13. One common approach has been to screen multiple rodent strains for exercise capacity, followed by quantitative trait loci (QTL) analyses to identify loci linked to exercise capacity. This approach has been used to identify QTL for exercise capacity in rats10 and mice11,13,14. Research from our laboratory previously identified significant and suggestive QTL on several chromosomes that may house candidate genes that influence variation in exercise capacity13,14. These identified regions overlap with other mouse and human QTL, suggesting that these regions and/or genes are conserved among species13. Mouse Chromosome 14 (Chr 14), for example, contained a significant QTL for intrinsic (pre-training) exercise capacity, a significant QTL for exercise capacity after training, and a suggestive QTL for the change in exercise capacity in response to exercise training13. Several linkage markers for maximal oxygen consumption in the sedentary state in humans map to these exercise-related QTL on mouse Chr 1413,15. Therefore, the present study focused on characterizing the role of Chr 14 in regulating intrinsic exercise capacity.
In the current study we employed a relatively new mouse model, chromosome substitution strains (CSS) to assess the contribution of individual chromosomes to endurance exercise capacity16,17. CSS mice are made by substituting a single chromosome from a donor inbred strain on the genetic background of a host inbred strain (recipient). Therefore phenotypic differences between the recipient or background strain mice and CSS mice support the presence of a QTL on the substituted chromosome for the phenotype being measured. Results from a previous study identified the A/J strain as having low exercise capacity in comparison to the C57BL/6J (B6) strain14. Therefore we chose to use CSS mice based on A/J and B6 inbred strains.
Utilizing this CSS model, the main purposes of the present study were to investigate the effect of chromosome substitution on intrinsic exercise capacity and to identify QTL regulating intrinsic exercise capacity in mice. We hypothesized that chromosome substitution would significantly affect exercise capacity and therefore confirm the importance of Chr 14 in the genetic regulation of intrinsic exercise capacity in mice. Furthermore, we utilized linkage analysis to map QTL on Chr 14 in progeny from a cross between the CSS and host B6 strain.
All procedures adhered to the established National Institutes of Health guidelines for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee at Texas A&M University. Seven week-old inbred mice (A/J, C57BL/6J (B6), and Chr 14 substitution mice (C57BL/6J-Chr 14A/J/NaJ, abbreviated B6.A14)) (n = 12/strain, 6 male and 6 female mice) were purchased from Jackson Laboratory (Bar Harbor, ME.). Upon arrival at Texas A&M, all mice were given one week to acclimatize to their new environment before assessing exercise capacity. A separate group of male B6 mice were crossed with a separate group of female chromosome substitution B6.A14 mice to generate (B6.A14 × B6) F1 mice. The F1 mice were then intercrossed to produce 155 F2 generation mice (67 male and 88 female mice). All mice were housed in standard hanging polycarbonate cages (43 cm long × 21.5 cm wide × 15 cm high) with hardwood chip bedding and allowed food (Standardized Laboratory Rodent Diet) and water ad libitum. Mice were housed 1–5 mice per cage depending on sex and lineage and maintained on a 12 hr light:dark schedule at an ambient temperature of 22–24°C.
At 8 weeks of age, all mice were familiarized for two days at 9.0 m/min and 10.0 m/min at 10° for 10 minutes to run on a motorized rodent treadmill (Columbus Instruments, Columbus, OH), with an electric grid (160 V, 0–2 mA) at the rear of the treadmill as described previously13,14. Each mouse then completed two graded exercise tests separated by 48 hrs. Mean values for each mouse were used for statistical analyses. For each performance test, the treadmill was started at 9.0 m/min at 0° grade for 9 minutes as a warm-up. The grade was then increased 5° every 9 minutes up to a final grade of 15° and speed was increased 2.5 m/min from a starting speed of 10 m/min every three minutes until exhaustion. Exercise continued until each mouse refused to run, defined as an inability to maintain running speed in spite of repeated contact with the electric grid13,14. At exhaustion, each mouse was immediately removed from the treadmill and returned to its home cage. Exercise capacity was estimated for each animal using time (minutes) and work (kg·m). Work performed (kg·m) or vertical work was calculated as a product of body weight (kg) and vertical distance (meters), where vertical distance = (distance run)(sinθ), where θ is equal to the angle of the treadmill from 0° to 15°13,14.
At least 24 hours after the last graded exercise test, all mice were anesthetized by intraperitoneal injection of a ketamine (80 mg/kg) - xylazine (5 mg/kg) cocktail. Mice were subsequently euthanized by exsanguination due to removal of the heart and aorta. Heart, gastrocnemius, plantaris, soleus muscle and liver tissue were excised from mice, washed in ice-cold (4°C) saline, blotted dry to remove excess liquid, and snap frozen in liquid nitrogen. DNA was isolated from 25 mg of liver tissue with a DNeasy Blood and Tissue kit (Qiagen Science, Germantown, Maryland) according to the manufacturer’s instructions and quantified using NanoDrop spectrophotometry. Genotyping was performed using competitive allele-specific polymerase chain reaction (PCR) single nucleotide polymorphism (SNP) genotyping (KBiosciences, Hoddesdon, UK)13. All 155 F2 mice were genotyped using 12 SNPs spaced at approximately 5 cM intervals18.
QTL analyses were performed using R/qtl19. One-dimensional scans were performed on the entire F2 cohort with no additional covariates and with sex included as an additive and interactive covariate20. Permutation tests (1,000 repetitions) were used to identify threshold values for logarithm of odds (LOD) scores for each condition (i.e., with or without covariates) and exercise phenotype21. LOD scores were defined as significant if they surpassed the P < 0.05 threshold and suggestive if they surpassed the P < 0.63 threshold. If suggestive or significant QTL were identified using sex as interactive covariate, then one-dimensional scans were performed on male and female mice separately to identify potential sex-specific QTL. A two-dimensional scan also was performed on the entire F2 cohort to identify additive or interacting QTL on Chr 14. QTL confidence intervals were determined using the 1.5 LOD support interval19.
All data are represented as mean ± SE. Statistical significance for phenotype comparisons was denoted by P < 0.05. Two-way analysis of variance was used to determine the effect of sex and strain on exercise capacity, which is defined as time (minutes), or work (kg·m) (JMP 9.0, SAS, Cary, NC). If significant main effects were found for strain, Dunnett’s post hoc test was used to determine significant strain differences compared with B6. If significant main effects were found for sex, t-tests were used to identify sex differences within each strain. Comparisons among parental strains and F2 offspring and across genotypes for allelic effects were made using one way analysis of variance (strain or genotype) followed by Tukey’s post-hoc analysis. T-tests were used to identify sex differences in F2 offspring. Linear regression was used to determine the contribution of body mass to exercise performance.
Inbred strains. Exercise capacity, defined as mean run time during two graded exercise treadmill tests, for inbred and CSS mice is shown in Figure 1. Exercise times in A/J and B6.A14 mice were significantly less (P < 0.0001) than that in B6 mice. A significant effect of sex also was identified in all strains (A/J, B6.A14, and B6). For each strain, female mice ran significantly longer than male mice from the same strain (Figure 1A). When exercise capacity was expressed as work, A/J and B6.A14 strains were significantly different from B6 (P < 0.0001), with mice from both strains performing less work than B6 mice (Figure 1B). In contrast to exercise time, there was no significant main effect for sex (P = 0.1) on exercise capacity defined as work. Significant differences among the strains were primarily limited to differences in exercise phenotypes. Body mass was significantly less in B6.A14 mice compared with B6 (P < 0.0008) (Table 1). There were no significant differences in absolute tissue mass among A/J, B6, and B6.A14 strains (Table 1). Within each strain, body mass was significantly lower in females compared to males. Accordingly, tissue masses were lower in female mice compared to male mice from the same strain (Table 1). For each strain there was a significant negative correlation between body mass and exercise time (B6, r = -0.71, P = 0.0096; A/J, r = -0.77, P = 0.0035; B6.A14, r = -0.86, P = 0.0003).

Exercise capacity was assessed using a graded exercise test and expressed as (A) time in minutes or (B) work in kg·m. Values are expressed as mean ± SE. n = 6 mice per group. *P < 0.05 compared to B6; †P < 0.05 compared to females.
The sex-specific distributions for exercise time and work in F2 mice are shown in Figure 2. Both time and work varied significantly between male and female F2 mice (Table 2). On average, female mice ran approximately 2.5 min longer than male mice. These differences in run time between male and female mice were offset by a significantly higher body mass in male mice (P < 0.0001) resulting in comparable levels of work in male and female mice. Body mass was approximately 5 g higher in male mice compared with females (P < 0.0001), which likely accounts for the similar levels of work performed. Similar to body mass, tissue masses were significantly smaller in female F2 mice compared to their male counterparts.
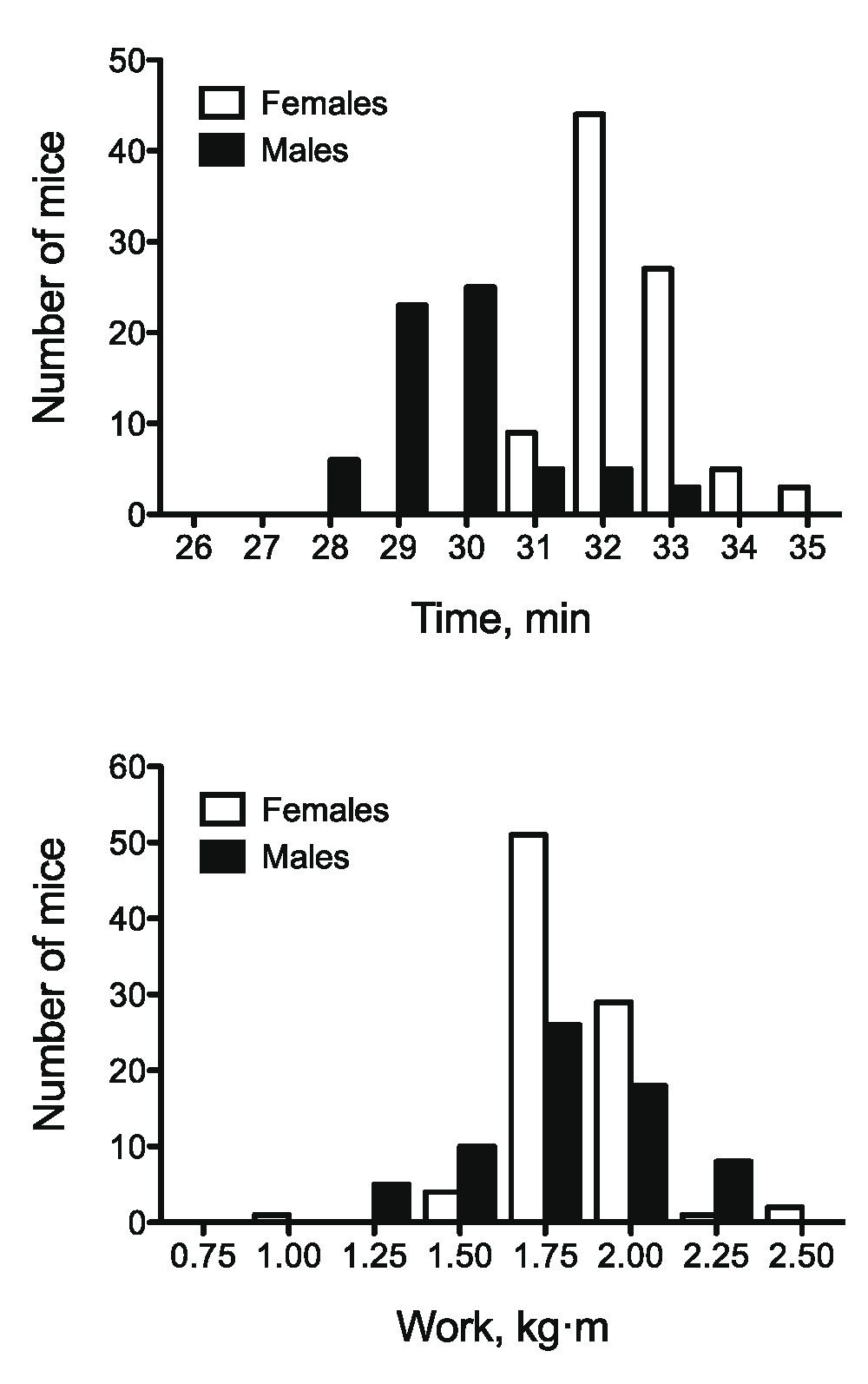
Frequency distribution for (A) time and (B) work in male and female (B6.A14 × B6) F2 mice. All F2 mice (n = 155) performed a graded exercise test to exhaustion to assess exercise capacity. Mice assorted into 1min/0.25 kg·m buckets. n = 67 for males and n = 88 for females.
Relative to the progenitor strains, eight week old F2 mice ran an average of 30.8 ± 0.1 min, which was significantly longer (P < 0.0001) than B6.A14 (28.5 ± 0.2 min) and not different from B6 (31.0 ± 0.1 min) mice. F2 mice also performed significantly more work (1.69 ± 0.02 kg·m, P < 0.0001) than B6 (1.39 ± 0.04 kg·m) and B6.A14 (0.95 ± 0.02 kg·m) strains. Body mass also was compared across strains and generations. F2 mice had average body mass of 22.1 ± 0.2 g, which was significantly greater than (P < 0.0001) the progenitor B6 (20.7 ± 0.8 g), and B6.A14 (18.9 ± 0.6 g) strains, respectively. Similar to the inbred strains, there was a significant negative correlation between exercise time and body mass (r = -0.74, P = 0.02). However, this relation was not maintained when the F2 population was divided by sex. In male F2 mice the correlation between exercise time and body mass was -0.47 (P = 0.001), but there was no significant relation between these variables in female F2 mice (r = 0.14, P = 0.18).
QTL analysis. Significant differences in exercise capacity between B6.A14 and B6 strains indicate the presence of a QTL on Chr 14. To fine-map the QTL, F2 mice were generated from B6.A14 and B6 strains. Single chromosome-wide scans for time and work are shown in Figure 3. Suggestive QTL for time (LOD = 1.75, P = 0.131) and work (LOD = 2.08, P = 0.063) with no covariates were identified on Chr 14. When sex was included as an interacting covariate, a significant QTL was identified at 56 cM for time (LOD = 3.8, P = 0.048) (Figure 3A). A suggestive QTL for work (LOD = 3.69, P = 0.07) was identified at the same location (Figure 3B). Because significant and suggestive QTL were identified using sex as an interacting covariate, chromosome-wide scans were performed on male and female mice separately. In male mice, a significant QTL for time (LOD = 2.28, 1.5 LOD = 49.0 – 58.9 cM, P = 0.049) and a suggestive QTL for work (LOD = 2.19, 1.5 LOD = 38.0 – 58.9 cM, P = 0.056) were identified at 55 cM. In female mice, no QTL were identified for time (Figure 4A). However, a suggestive QTL for work was identified at 16 cM (LOD = 1.8, P = 0.106) (Figure 4B). The two-QTL analyses for time showed limited evidence for additive QTL at 0 cM and 58 cM (LOD = 2.74, P = 0.19) on Chr 14. No significant additive or interacting QTL were identified for work. QTL scans also were performed for all physical characteristics and no significant QTL were identified.
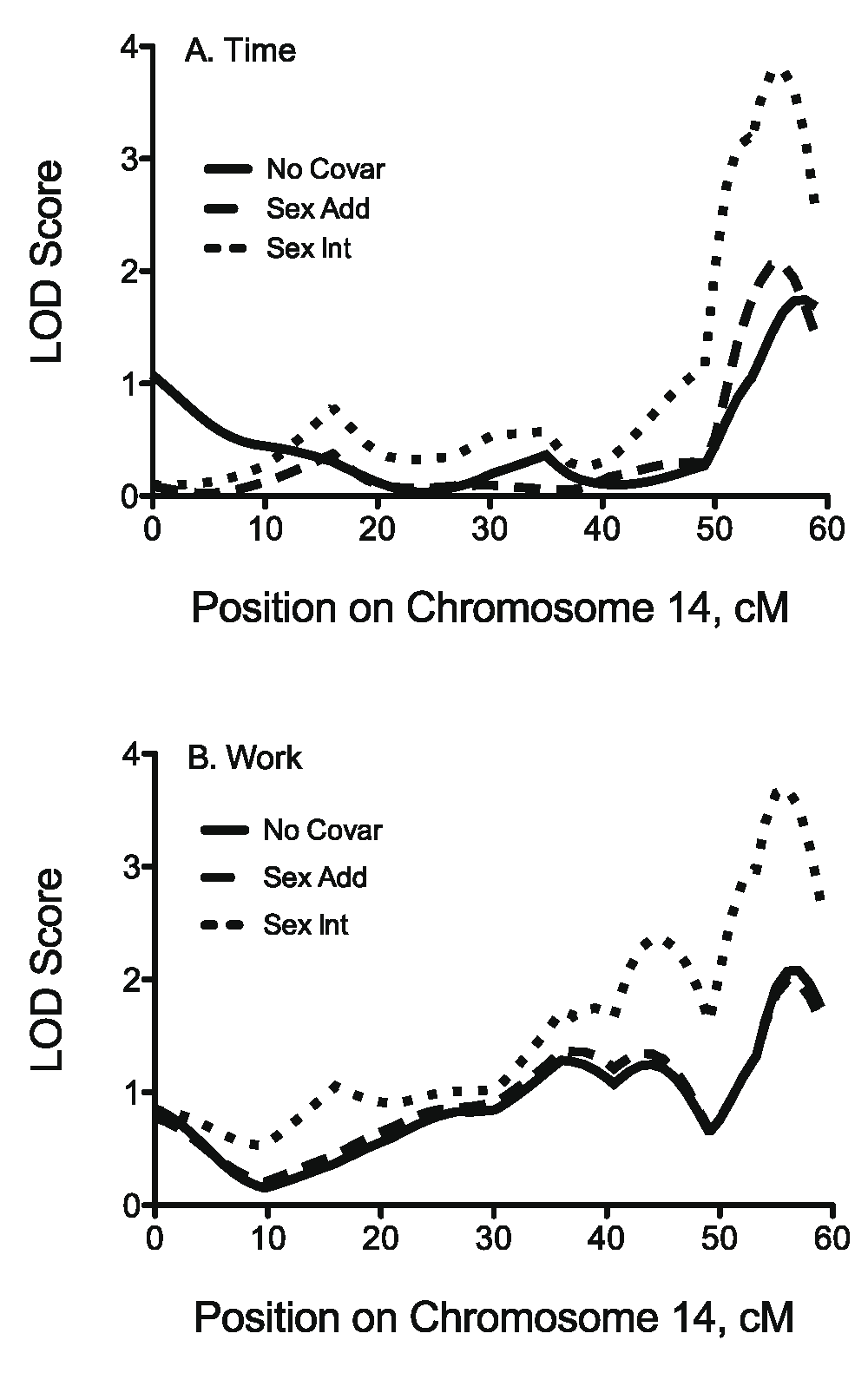
QTL analyses on mouse Chromosome 14 for intrinsic capacity expressed as time (min, A) and work (kg·m, B) in 155 (B6.A14 × B6) F2 mice. Three analyses were performed for each phenotype: 1) with no covariates, 2) with sex as an additive covariate, and 3) with sex as an interactive covariate. For time, significant (P = 0.05) logarithm of odds (LOD) thresholds are 2.22 with no covariates, 2.12 with sex as an additive covariate, and 3.78 with sex as an interactive covariate. For work, significant (P = 0.05) LOD thresholds are 2.22 with no covariates, 2.27 with sex as an additive covariate, and 3.95 with sex as an interactive covariate. LOD thresholds were determined using 1000 permutations. Chromosome-wide scans and permutation analyses were performed using R/qtl.

QTL analyses for the effect of sex on intrinsic exercise capacity in male and female (B6.A14 × B6) F2 mice expressed as time (A) and work (B). Single chromosome-wide scans for time (in min) and work (in kg·m) were performed separately on male and female F2 mice. Dashed lines in the upper graph represent the suggestive (0.82, P = 0.63) and significant (2.21, P = 0.05) logarithm of odds (LOD) thresholds for time in males. Dashed lines in the lower graph represent the suggestive (0.82, P = 0.63) and significant (2.25, P = 0.05) LOD thresholds for work in males. In females, suggestive and significant LOD thresholds for time were 0.84 (P = 0.63) and 2.15 (P = 0.05), respectively; and for work 0.81 (P = 0.63) and 2.08 (P = 0.05), respectively. LOD thresholds were determined using 1000 permutations. Chromosome-wide scans and permutation analyses were performed using R/qtl.
The allelic effects for suggestive and significant QTL are shown in Table 3. In the entire F2 cohort, heterozygous mice had the highest average exercise time and work. For both phenotypes there was no significant difference between homozygous A and B groups. A similar pattern was observed for time and work in the male F2 cohort. In this group, mice with parental genotypes had significantly lower exercise time than mice carrying the heterozygous genotype (Table 3). In the female F2 cohort, work was significantly higher in homozygous A mice compared with homozygous B mice. Heterozygous female F2 mice had an intermediate phenotype.
Position, location of peak marker in cM; Marker, SNP marker closest the LOD peak; Genotype, genotype at peak marker; A, homozygous for A/J allele; B, homozygous for B6 allele; H, heterozygous; p-value, p-value from means comparison using Tukey post-hoc analysis with specific allelic comparisons indicated (e.g., A vs. B), significant p-values are indicated by*.
The purpose of the current study was to determine the role of mouse Chr 14 in the genetic regulation of exercise capacity and to fine map this chromosome to identify QTL for exercise capacity. Significant differences in exercise time and work were observed between inbred B6 mice and mice carrying Chr 14 from the A/J strain on the B6 background (B6.A14). These differences suggest the presence of one or more QTL on Chr 14 underlying variation in exercise capacity. Utilizing a (B6.A14 × B6) F2 population, suggestive QTL for exercise time and work were localized to a position of ~58 cM on Chr 14. Further analysis revealed putative sex-specific QTL for exercise time and work. QTL identified in the male cohort was similar to that in the entire F2 cohort, but the suggestive QTL for work identified in the female F2 mapped to an alternative position. Collectively, these data suggest that one or more genes on Chr 14 contribute to variation in exercise capacity and that the genetic architecture for exercise-related traits might be different in males and females. Given the complexity of the trait, genome-wide mapping strategies should be employed to identify additional QTL underlying the variation in exercise capacity.
B6 and A/J strains show significant phenotypic differences across many traits16,22–25. We, and others14,26,27 have demonstrated that exercise capacity assessed by treadmill running is one of these traits. Although testing protocols varied, A/J mice repeatedly show low exercise capacity, having running times approximately 60% or less than that of B6 mice. In the current study, A/J mice ran 10 minutes less than B6 mice and performed only 25% of the work of B6 mice during a graded exercise test (Figure 1). These observations replicated our previous finding that A/J mice had the lowest exercise capacity among 34 strains tested14. Although B6 were in the lowest third of that survey, their run time was about 60% higher than A/J mice. This disparity in exercise capacity between inbred strains suggests that genetic variation contributes to these phenotypic differences.
Chromosome substitution strains were developed to facilitate genetic analysis of complex traits by partitioning the genome into individual chromosomes16. Phenotypic differences between a CSS and the background strain suggest the presence of at least one QTL on the substituted chromosome. To begin to identify the genetic factors contributing to variation in exercise capacity, mice from a chromosome substitution strain based on A/J and B6 strains were used. In the current study we focused on Chr 14 because we had previously identified several exercise-related QTL on this chromosome13. B6.A14 mice had significantly lower exercise capacity expressed as time or work compared with B6 mice (Figure 1). Exercise time was 2.5 minutes less in B6.A14 mice, which corresponds to 24% of the difference between parental A/J and B6 strains (10.6 min). The difference in work was 0.44 kg·m, which is approximately 43% of the difference between parental strains (1.03 kg·m). Although the substituted chromosome shifted the phenotype toward the donor strain, the effect of chromosome substitution on exercise capacity was less than expected. Based on previous CSS surveys, chromosome substitution can produce phenotypic effects of 75% or more of the difference between parental strains16,23,24. Nevertheless, the significant difference between B6 and B6.A14 for exercise time and work suggest the presence of one or more QTL on Chr 14 for exercise capacity.
To localize the QTL on Chr 14, linkage analysis was performed in F2 mice from B6 and B6.A14 strains. Suggestive QTL were identified for both time and work at 58 cM (Figure 3). The 1.5 LOD interval for each of these QTL spanned nearly the entire chromosome, so these QTL overlapped with previously reported QTL for pre-training and post-training work13. However, the peak markers for pre-training work (4 cM) and post-training work (26 cM) QTL localize to positions distant from the QTL identified in the current study and likely represent different QTL. Further analysis using sex as an interactive covariate provided evidence for sex-specific QTL; therefore, male and female F2 cohorts were analyzed separately. Significant and suggestive QTL for time and work, respectively, were identified in the male cohort only and were similar to those identified in the entire cohort. Conversely, there was less evidence for exercise-related QTL on Chr 14 in female mice. This is somewhat surprising given the differences between B6 and B6.A14 female mice were comparable to those in male mice (Figure 1). However, the peak marker for the suggestive QTL for work in the female F2 cohort is in close proximity to a syntenic human region linked to maximal oxygen consumption in the sedentary state in the HERITAGE Family Study15. We previously reported a significant effect of sex on exercise capacity after 4 weeks of exercise training and the responses to training in mice13. Sex-specific QTL also have been reported for voluntary wheel running28 and exercise-related traits such as muscle mass29,30. Furthermore, Wang et al. identified several QTL related to fat mass in the mouse which were influenced by sex31. Global gene expression analysis of liver tissue in the same population of mice revealed that a large percentage of expression QTL also were influenced by sex. These data suggest that sex can affect the genetic regulation of gene expression as well as clinical phenotypes. Therefore sex-specific affects should be considered when investigating the genetic regulation of phenotypes, especially those such as exercise capacity that are known to differ between males and females.
One potential explanation for the limited evidence for exercise QTL in the F2 cohort is that the number of animals was insufficient for detecting multiple QTL with small effects. However, the number of mice included in the entire F2 cohort or each sex-specific cohort is comparable to most intercross populations utilizing a CSS and B6 strains and should have been sufficient to detect at least 1 QTL16,24,25. Similar to the current study, Burrage et al. were also unable to localize QTL in CSS × B6 intercross populations for several traits showing significant differences between parental CSS and B6 mice25. They concluded that multiple QTL with opposing effects might be present on individual chromosomes and that congenic strains might be more advantageous for QTL detection and mapping than larger intercross populations. Alternatively, a close inspection of the allelic effects for each exercise QTL suggests that alleles derived from the A/J stain contribute to increasing exercise capacity (Table 3). This was most evident in the female F2 cohort. The suggestive QTL for work identified in this population mapped to a position (16 cM) that was different from that observed in the entire F2 and male-only cohorts. In females, mice homozygous for the parental A allele performed significantly greater work that mice homozygous for the parental B allele. Heterozygous mice were intermediate and not significantly different from either parental genotyping suggesting an additive inheritance pattern with the A allele conferring increasing exercise capacity. In the full F2 and male-only cohorts, there was no significant difference between mice homozygous for the parental genotypes and heterozygous mice had the highest exercise capacity. Thus, at some locations A and B alleles can interact to elicit a phenotype greater that either parental genotype.
Collectively, these data support the use of CSS as a model for the genetic analysis of exercise capacity. They also provide evidence that genetic factors on Chr 14 contribute to the variation in exercise capacity. Based on the complexity of the exercise phenotype, a survey of the complete C57BL/6J-ChrA/J/NaJ CSS panel will likely identify multiple chromosomes of interest and potential QTL for exercise capacity. Furthermore, the sex-dependent differences in exercise capacity and the putative sex-specific QTL imply that the genetic architecture underlying exercise capacity might be different between males and females. Thus, any such survey should be conducted in male and female mice to elucidate the potential genotype by sex interaction underlying differences in exercise capacity between males and females. Once strong candidate genes are identified, the link between exercise capacity and cardiorespiratory fitness, and the mechanistic basis for diseases associated with low cardiorespiratory fitness can be explored.
Figshare: Effect of chromosome 14 substitution on intrinsic exercise capacity in mice: R/qtl linkage analysis and phenotype data, http://dx.doi.org/10.6084/m9.figshare.89358132.
SC designed and conducted the study, analyzed the data, and wrote the manuscript. SC critically revised the manuscript and agreed to its publication. MM designed the study, analyzed the data, and wrote the manuscript. MM critically revised the manuscript and agreed to its publication.
This work was supported by NIH grant HL-085918 (MPM) and the Sydney and J.L. Huffines Institute for Sports Medicine and Human Performance (SC).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors would like to thank Jean Kovar for management of the breeding colony for this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
References
1. Howley E: How much exercise is enough?. Huffines Discussion. 2013. Reference SourceCompeting Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 28 May 14 |
read | |
|
Version 1 13 Jan 14 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)