Keywords
drug space, NSAID, molecular libraries
This article is included in the Emerging Diseases and Outbreaks gateway.
This article is included in the Ebola Virus collection.
drug space, NSAID, molecular libraries
The recent Ebola virus outbreak in West Africa caused (as of April 8, 2015) a total of 25,556 confirmed cases, including 10,587 deaths (World Health Organization, Ebola data and statistics). Although reports of new, confirmed cases of Ebola seemed to decrease in April of 2015 to approximately 30 cases per week, medical and aid organizations are warning that the current crisis is not over. Furthermore, there remains a risk for future epidemics. Therefore, there is an urgent need to develop new preventive and therapeutic approaches that can be rapidly utilized. New drugs for the treatment of EVD should be safe, efficacious, easy to manufacture and inexpensive in order to be successfully deployed in African countries. In contrast to the significant progress recently achieved in development of an effective Ebola virus vaccine1, therapeutic options are still limited2. The main obstacle represents identification of an appropriate therapeutic target, which has been largely hampered by the time and money-consuming development of new drugs, especially for neglected diseases. Furthermore, the registration of newly identified drugs, like favipiravir, for potential usage in EVD patients takes a relatively long period of time, even though it has been shown to be effective in non-human primate models. In order to avoid these obstacles, several approaches for repurposing of approved drugs for the treatment of EVD patients have been proposed in literature3–7.
Recently, we proposed a relatively simple theoretical criterion for the fast virtual screening of molecular libraries for candidate inhibitors of Ebola virus infection8. Using this criterion, which is based on calculation of the average quasi-valence number (AQVN) and the electron-ion interaction potential (EIIP) - parameters determining long-range interaction between biological molecules9–13 - we selected 267 approved and 382 experimental drugs as candidates for treatment of EVD. Further detailed analysis of these drugs, including molecular docking, revealed ibuprofen as an inexpensive, widely accessible and minimally toxic candidate for potential prevention and treatment of EVD. The molecular mechanism underlying the possible protective effect of ibuprofen against EVD is suggested.
Recently, we proposed a theoretical criterion for selection of candidate inhibitors of Ebola virus infection8. This criterion is based on the calculation of EIIP and AQVN which are determined by following equations:
where:
i - Type of the chemical element
Zi - Valence of the i-th chemical element
ni - Number of the i-th chemical element atoms in the compound
m - Number of types of chemical elements in the compond
N - Total number of atoms
The EIIP values calculated according to the equation (2) are in Rydbergs (Ry = 13.6 eV).
The AQVN and EIIP molecular descriptors determine the long-distance (>5Å) intermolecular interactions in biological systems14. This approach showed that molecules which potentially block Ebola virus infection are placed within AQVN range (2.3–2.7) and EIIP range (0.829–0.954 Ry), respectively. Using this theoretical criterion the drug library encompassing 267 approved drugs selected as candidate inhibitors of the Ebola virus infection (Candidate Ebola Drugs Database, CEDD) was established15. This drug library was used for selection of an approved drug, which represents the optimal candidate for prevention and treatment of EVD.
The modelling of glycoprotein GP1 from Ebola virus was previously described in 16.
Ligands were built in VEGA ZZ17, protonated according to physiological conditions and optimized on semi empirical PM6 level of theory using MOPAC 2009.
Ligand and receptor were prepared in VEGA ZZ17. The docking was carried out with Autodock Vina (version 1.1.2.)18. In both cases the whole receptor conformational space was searched, using grid boxes with dimensions 60×60×60 and 30×30×30Å3. The docking was carried out with weighting of hydrophilic interactions, and the corresponding parameter value in the docking configuration was set to -1.20 (compared to default weight: hydrogen = -0.587439). The exhaustiveness was set to 250. The conformations with lowest binding energy values were chosen. The calculations were carried out on the PARADOX Cluster computer.
We screened the CEDD library15 for optimal candidates for prevention and treatment of EVD. To achieve this end, we used the following criteria: a) high efficacy of binding of drug to the Ebola virus glycoprotein GP1; b) low toxicity and accessibility (nonprescription drugs); and c) low cost. By mining of CEDD using these criteria, we selected ibuprofen as the best candidate. Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID). Its mode of action, as for other NSAIDs, is not completely understood, but may be related to prostaglandin synthetase inhibition. It achieves this effect on prostaglandin synthesis by inhibiting cyclooxygenase (COX I and II isotypes), an enzyme that is present in at least one isoform most of the tissues of the body and which is responsible for production of not only prostaglandins but also prostacyclins and tromboxane.
Generally, ibuprofen is minimally toxic drug. A meta-analysis of eight placebo-controlled studies showed that application of non-prescription doses of ibuprofen (800–1200 mg/day) over 10 days caused no significant adverse events19. Similar results were obtained in a prospective study of ibuprofen use in healthy volunteers taking the maximum permitted, non-prescription dose of ibuprofen (1200 mg/day) for 10 days20. It was demonstrated that administration of ibuprofen in children is safe. A randomized, community-based study on the safety of ibuprofen in febrile children aged 2 years old (5 or 10 mg/kg) showed that the risk of hospitalization for gastrointestinal bleeding associated with this drug was 17 per 100,00021. It was also shown that fecal blood loss associated with application of prescription doses of ibuprofen (2400 mg/day) did not exceed the normal range22. Even in the case of post-surgical usage for pain control in children undergoing tonsillectomy, ibuprofen administration is considered to be safe and does not increase risk of hemorrhages23. Accordingly, we believe that it would be crucial to better understand the potential risk and benefits of ibuprofen usage in experimental models of EVD. Taken together, these data indicate that ibuprofen could be safely used in nonprescription doses in EVD patients, with potential antiviral effects as well as to alleviate the symptoms.
To assess specificity of ibuprofen as a potential inhibitor of the Ebola virus infection, distribution of all approved NSAIDs in AQVN/EIIP space was analyzed. Results presented in (Table 1 and Figure 1) show that only ibuprofen and its isomer dexibiprofen are located within the domain of the AQVN/EIIP space. Interestingly, most of the experimentally verified inhibitors of the Ebola virus infection are located in the same space (e.g. chloroquine, amodiaquine, brincidofovir, etc)8. This suggests that ibuprofen and dexibuprofen are the only drugs from the NSAID group that potentially have anti-Ebolavirus effects, which should be tested both in vitro and in vivo.
| NSAID | Formula | AQVN | EIIP [Ry] |
|---|---|---|---|
| Aspirin | C9H8O4 | 3.238 | 0.1179 |
| Diflunisal | C13H8F2O3 | 3.077 | 0.0720 |
| Salicylic acid | C7H6O3 | 3.250 | 0.1202 |
| Salsalate | C14H10O5 | 3.310 | 0.1296 |
| Ibuprofen | C13H18O2 | 2.485 | 0.0954 |
| Dexibuprofen | C13H18O2 | 2.485 | 0.0954 |
| Naproxen | C14H14O3 | 2.839 | 0.0169 |
| Fenoprofen | C15H14O3 | 2.875 | 0.0036 |
| Ketoprofen | C16H14O3 | 2.909 | 0.0092 |
| Dexketoprofen | C16H14O3 | 2.909 | 0.0925 |
| Flurbiprofen | C15H13FO2 | 2.774 | 0.0390 |
| Oxaprozin | C18H15NO3 | 2.973 | 0.0337 |
| Loxoprofen | C15H18O3 | 2.667 | 0.0693 |
| Indomethacin | C19H16ClNO4 | 2.976 | 0.0347 |
| Tolmetin | C15H15NO3 | 2.882 | 0.0008 |
| Sulindac | C20H17FO3S | 2.905 | 0.0076 |
| Etodolac | C17H21NO3 | 2.667 | 0.0693 |
| Ketorolac | C15H13NO3 | 3.000 | 0.0439 |
| Diclofenac | C14H11Cl2NO2 | 2.867 | 0.0067 |
| Aceclofenac | C16H13Cl2NO4 | 3.000 | 0.0439 |
| Nabumetone | C15H16O2 | 2.667 | 0.0693 |
| Piroxicam | C15H13N3O4S | 3.277 | 0.1251 |
| Meloxicam | C14H13N3O4S2 | 3.333 | 0.1319 |
| Tenoxicam | C13H11N3O4S2 | 3.454 | 0.1317 |
| Droxicam | C16H11N3O5S | 3.500 | 0.1260 |
| Lornoxicam | C13H10ClN3O4S2 | 3.454 | 0.1317 |
| Isoxicam | C14H13N3O5S | 3.333 | 0.1319 |
| Mefenamic acid | C15H15NO2 | 2.788 | 0.0345 |
| Meclofenamic acid | C14H11Cl2NO2 | 2.867 | 0.0067 |
| Flufenamic acid | C14H10F3NO2 | 2.867 | 0.0067 |
| Tolfenamic acid | C14H12ClNO2 | 2.867 | 0.0067 |
| Celecoxib | C17H14F3N3O2S | 2.950 | 0.0249 |
| Rofecoxib | C17H14O4S | 3.111 | 0.0835 |
| Valdecoxib | C16H14N2O3S | 3.111 | 0.0835 |
| Parecoxib | C19H18N2O4S | 3.046 | 0.0608 |
| Lumiracoxib | C15H13ClFNO2 | 2.788 | 0.0345 |
| Etoricoxib | C18H15ClN2O2S | 2.974 | 0.0342 |
| Firocoxib | C17H20O5S | 2.884 | 0.0003 |
| Nimesulide | C13H12N2O5S | 3.333 | 0.1319 |
| Clonixin | C13H11ClN2O2 | 2.966 | 0.0308 |
| Licofelone | C23H22ClNO2 | 2.694 | 0.0626 |
Domain of inhibitors of Ebola virus infection8 is marked in red.
A previous in silico study suggested that Elastin Microfibril Interface Located Proteins (EMILINs) are involved in interaction between GP1 and endothelial extracellular matrix (ECM)16. Docking of ibuprofen to the GP1 model gave conformations which bound to EMILINs binding domain on GP116. The binding site of ibuprofen on GP1 spans the edge of this region and consists of Thr 338, Ser 340, Gln 344 and Ala 41516. The intermolecular interactions between ibuprofen and binding site amino-acids include hydrogen bonds of carboxyl group with Thr 338, Ser 340 and Gln 334. Additional stabilization is provided through aromatic and hydrophobic interaction with Ala 415. This binding site is placed between two loops, which provide the possibility of stabilizing a particular conformation, and therefore possibly blocking receptors. The appropriately high binding energy of -9.0 kcal/mol favors this assumption. The binding conformation is presented in Figure 2. At this stage it can be hypothesized that ibuprofen prevents interaction between Ebola virus and ECM by blocking the interaction between GP1 and EMILIN. There are some literature data that support our current hypothesis. EMILIN-1 is a glycoprotein expressed in the vascular tree that binds to the TGF-β1 precursor and prevents its processing by cellular protease furin24. It was shown that Emilin-1 knockout mice display increased TGF-β1 signaling in the walls of their blood vessels, leading to peripheral vasoconstriction and arterial hypertension25. These matrix-dependent changes in the vascular hemodynamics caused by TGF-β1 and EMILIN-1 are important because they ultimately affect the cardiovascular morbidity and mortality rates. Recently, it was shown that activation of the TGF-β1 signaling pathway by Ebola virus plays an important role in pathogenesis of EVD26. These findings suggest the possibility that binding of GP1 to EMILIN-1 prevents its interaction with TGF-β1, which results in activation of TGF-β1 signaling pathway. Binding of ibuprofen to GP1 could prevent GP1/EMILIN-1 interaction allowing EMILIN-1 to keep control of TGF-β1 signaling pathway.
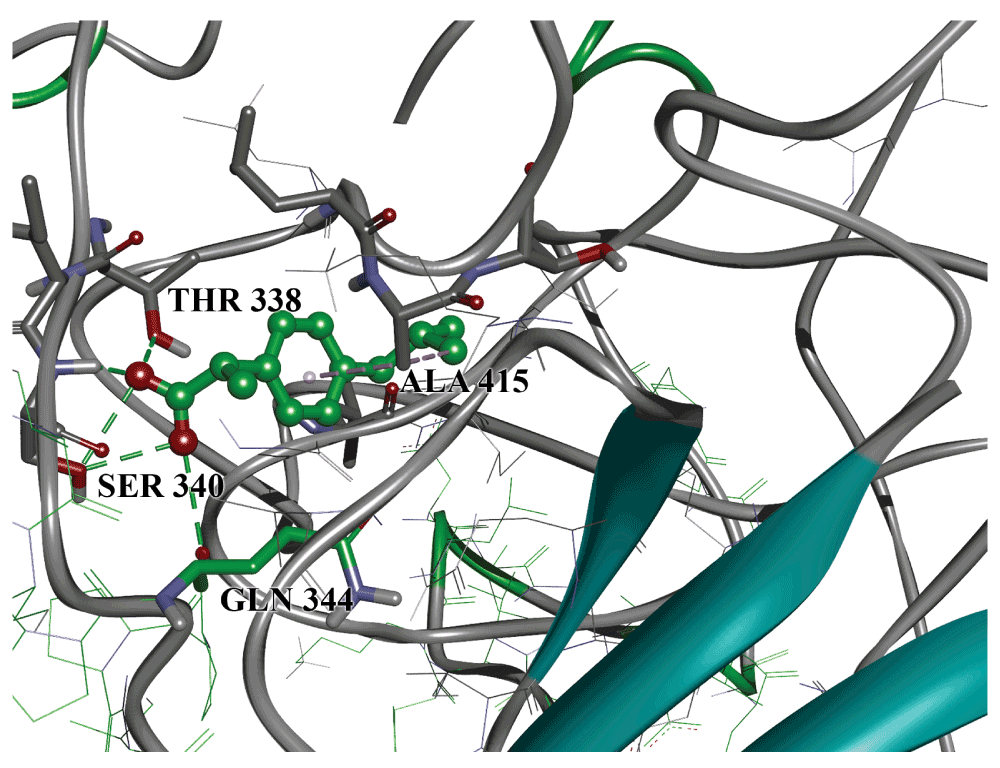
Green dotted lines: hydrogen bonds; grey: hydrophobic interactions.
In conclusion, presented results should encourage further investigation of ibuprofen and ibuprofen-inspired drugs as inexpensive, low-toxic and wide-accessible candidates for prevention and its usage in the treatment of EVD.
F1000Research: Dataset 2. Approved and experimental drugs selected as candidate for treatment of EVD, 10.5256/f1000research.6110.d4287715
VV, SP and MG conceived and designed the study. VP developed the analysis tools. VV, SG, NV, MS and DB analyzed the data. VV, SP and MG wrote the paper. All authors agreed to the final content of the manuscript.
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant no. 173001).
I confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 01 May 15 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)