Keywords
salivary sIg-A, children, tuberculosis, antigen Ag38-rec
This article is included in the World TB Day collection.
salivary sIg-A, children, tuberculosis, antigen Ag38-rec
Tuberculosis (TB) among children has been a serious threat in Indonesia, primarily due to a lack of accurate methods to diagnose the disease. Clinical examination, the acid-fast bacillus (AFB) test, and culture are the gold standard methods for the diagnosis of TB in adults. However, these methods have limited use for diagnosing TB in children in a clinical setting because collecting sputum samples from children is difficult. Moreover, children with TB do not exhibit the typical symptoms as TB observed in adults. The diagnosis of pediatric TB in Indonesia is determined according to the TB Scoring System1. This system is based on the clinical signs and symptoms found in suspected TB in children1. Children with a total score of ≥ 6 are considered to have TB and are treated for the disease. However, the accuracy of this scoring method is constrained due to the non-specificity of TB symptoms in children, and may result in over or under-diagnosis2. In consequence, a rapid, simple, and inexpensive method to confirm Mycobacterium tuberculosis (Mtb) infection is required to reduce the spread of the disease. One such promising method, a serodiagnostic approach focusing on the detection of specific TB antibodies in patients, has been extensively studied3–5. A non-invasive diagnostic method like this would be highly valued, particularly with regards to pediatric TB patients.
Secretory immunoglobulin A (sIg-A) antibodies and presentations by antigen presenting cells (APC) are the first line of defense against bacterial invasion. These antibodies are a major class of immunoglobulin in external secretions and provide specific immunological protection in all mucosal surfaces that prevent the entry of bacteria6. Saliva contains a significant amount (85%) of sIg-A. This antibody is produced by B lymphocytes found near the salivary glands7. The 38-kDa protein from Mtb contains B-cell epitopes and has been shown to have high specificity for the Mycobacterium complex8. Previous study by Raras et al. demonstrated an immune response of salivary sIgA against recombinant Ag38 (Ag38-rec) Indonesian strain in adult pulmonary TB patients with a sensitivity of up to 80%, but with a low specificity (38%)9. Another study investigating Mtb specific antibodies, including sIgA, in saliva from children of the Warao Amerindian tribes in Venezuela, reported significantly greater reactivity to the purified protein derivative (PPD) antigen with a sensitivity of 26.5% and specificity of 97%10. In this study, the saliva from children diagnosed with TB (those scoring ≥ 6 in the TB Scoring System) and from children with suspected TB (those scoring < 6) was tested against Ag38-rec produced in our laboratory. The term ‘Indonesian strain’ is used throughout this article to refer to M. tuberculosis that was isolated from an Indonesian patient who was suffering from severe pulmonary TB, from which the pab gene was amplified. We evaluated whether Ag38-rec from the Mtb Indonesian strain could be used to differentiate between children with pulmonary TB with scores of ≥ 6 and children with suspected TB with scores of < 6.
Production of Ag38-rec was conducted according to a previous study with slight modifications11. The pab gene coding for Ag38 was amplified via a PCR method using chromosomal DNA from M. tuberculosis which was isolated from a severe pulmonary TB patient in Malang, Indonesia. The fragment was inserted into a plasmid producing pMBhis. We do not know the strain of this Mtb, but alignment of the nucleotide sequence of the pab gene showed 95% homology to the pab gene from M. tuberculosis H37Rv. Escherichia coli BL21-(DE3) containing plasmid pMBhis was grown in Luria broth medium until OD600 of 0.5 and induced with IPTG. After 3 h the cells were then harvested by centrifugation. The pellet was mixed with phosphate buffer containing protease inhibitor phenyl methyl sulphonyl fluoride (Sigma, USA) and was broken using a sonicator. Protein purification was performed using a Protino®Ni-TED column according to the manufacturer’s protocol (Protino, Dueren, Germany). Following centrifugation for 15 min at 10,000 × g the supernatant was loaded onto an Ni-TED 1000 column and washed twice with phosphate buffer containing 10 mM imidazole. The antigen Ag38-rec and other proteins that bound to the nickel column were eluted using phosphate buffer containing 250 mM imidazole. The eluted proteins were collected and loaded once more on the new Ni-TED column. The eluent was then dialyzed against the elution buffer in order to remove imidazole. The purity of Ag38-rec was analyzed using 12.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
A total of 78 children (n=78) were included in this study: 26 patients with recently diagnosed pulmonary TB (total scores of ≥ 6), 26 patients with suspected TB (scores of < 6) from primary health care clinics in Malang City, Indonesia, and 26 healthy children to serve as negative controls. All of the participants were between the ages of 6 and 15 years, and their parents signed informed consent forms. The inclusion criteria for the healthy controls included absence of illness in the three weeks prior to the study and a lack of contact with adult TB patients. The study was initiated after being approved by the Ethical Commission from the Faculty of Medicine, Universitas Brawijaya, Malang Indonesia (No.341/EC/KEPK-OPDS/05/2014). Saliva samples were collected from June to October 2014. The diagnosis of TB was conducted by a pediatrician based on the eight parameters of the scoring system1 i.e., contact with TB patients; fever; cough; enlarged lymph nodes of the neck, groin, and armpits; nutritional status; bone and joint swelling; chest X-ray (CXR); and Mantoux tuberculin skin test (TST).
The dot blot method was applied according to a previous experiment9. Before mounting onto the dot blot apparatus, a nitrocellulose membrane was pre-wetted using sterile H2O. A 20 L volume containing 1 g of antigen Ag38-rec in Tris-Cl buffer [pH 7.4] was dropped onto the membrane following overnight incubation at 4°C with blocking buffer. PPD (protein purified derivative) from Mtb (Serum Staten Institute, Denmark) served as a control. The next day, the blocking buffer was removed and replaced with TBS and gently shaken for 10 min at 4°C. After the blocking agent was removed, 50 μL of primary antibody was applied to the membrane and incubated for 12 h at room temperature with gentle shaking. The solution was then discharged and the membrane was washed three times with 0.05% TBS-Tween-20 and subsequently shaken in Tris-Cl buffer containing secondary antibody (1:500) (mouse anti-Mycobacterium tuberculosis Ag38 monoclonal antibody, AbD, Serotec, England, cat.no.0100-0519) at room temperature for 1 h, followed by three washing steps. Finally, a chromogenic substrate (BCIP-NBT) was applied to the membrane in the darkroom at room temperature for 30 min. The reaction was stopped by the addition of H2O. The Corel Draw graphic suite X4 (Corel, USA) program was used to interpret the color range of the spot(s). The gradation of color was quantified to numerical value using Corel Photopaint 11. Positive and negative value was based on the cut-off of the median value of the positive control (PPD) after reaction with saliva of the TB group. A dot was regarded as positive when the value was below the cut-off point and as negative when the value was higher than the cut-off point. A positive result was defined as a dark blue or dark purple spot (>50%) on the blot.
The procedure of saliva collection was conducted according to Chiappin et al.7. For patients with cough, saliva collection was preceded by induction with nebulized 3% NaCl to thin phlegm. Subjects were asked to transfer the collected saliva (a minimum amount of 5 ml) from the mouth to a falcon tube. Saliva samples were immediately deposited into a thermos of ice at 4°C for up to 2 h to protect the unstable analyte and prevent the growth of bacteria prior to long-term storage in a refrigerator at -40°C until use.
To avoid the possibility of contamination from substances in the saliva that may interfere with the immunoassay, the following precautions for participants were applied: avoid consumption of a meal 1 h before sample collection; avoid dairy products 20 min before sample collection; avoid foods with high acidity, high sugar, or caffeine immediately before sample collection; and rinse mouth with water to remove food residue before sample collection.
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of an immune response to Ag38-rec in children with a TB score of ≥ 6 and those with a score < 6 were determined and compared to those values for PPD. The statistical significance of the differences between the sensitivity and specificity of the immune response to Ag38-rec and to PPD was analyzed using the Student’s t-test. A p-value of less than 0.05 was considered statistically significant. All data were analyzed using SPSS version 15.
In all three groups, the number of male patients was slightly higher than the number of female patients. The mean age of the subjects of all three groups was 8 years old. In the case of the TB group (score ≥ 6), most have had contact with an adult TB patient with a positive smear test. A positive TST result had been obtained from the majority of children from the TB group (22/26). In contrast, only a single positive TST result was found in the group of children with a suspected TB (score of < 6). Persistent cough for more than 3 weeks dominated the patients in both groups (score of ≥ 6 and < 6). All subjects in the group with pulmonary TB had X-rays suggestive of pulmonary TB, while only 6 subjects (6/28) in the suspected TB group had X-rays suggestive of pulmonary TB.
To explore whether there is an immune response of sIgA from the saliva of children against Ag38-rec, we tested two samples from each group against purified antigens. PPD was used as a positive control, and a negative control was performed without antigen. A dark blue color indicates a reaction between Ag38-rec Mtb antigens with sIgA, while a pale color or white indicates no reaction. After the optimization process with the checker board, the best concentration of antigen was 250 ng Ag38-rec, 125 ng PPD and 25 μL of saliva. All samples from the three different groups were tested and the results of the dot blot are shown in Figure 1.
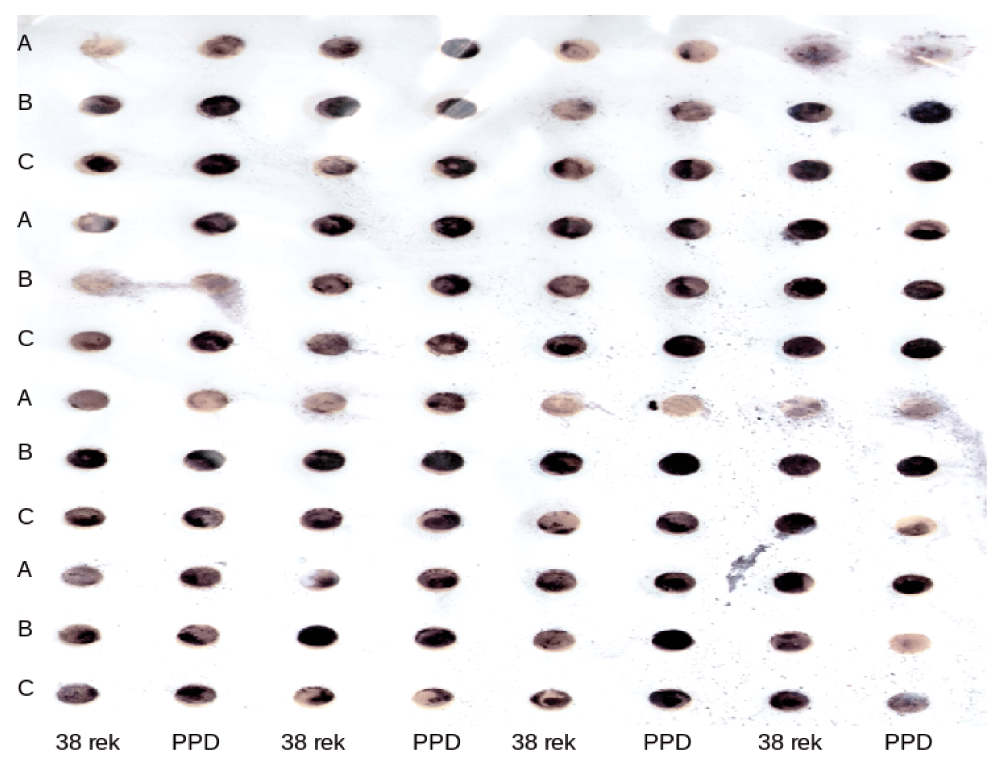
The response of salivary sIg-A of TB group scores ≥ 6 (A) non TB group scores < 6 (B) and healthy children (C) against Ag38-rec (labeled 38 rek) and PPD using the dot blot method.
Comparison between response of sIg-A from TB group to Ag38-rec and the standard antigen PPD showed that sIg-A recognized PPD better than Ag38-rec (Table 1).
| Group | Ag38-rec | PPD | ||||
|---|---|---|---|---|---|---|
| TB | Non TB | Healthy | TB | Non TB | Healthy | |
| Positive Response | 17 | 19 | 18 | 15 | 11 | 17 |
| Negative Response | 9 | 7 | 8 | 9 | 15 | 7 |
| Total Tested | 26 | 26 | 26 | 24 | 26 | 24 |
The strength of Ag38-rec as an immunodiagnostic agent to detect sIgA was determined based on the sensitivity and specificity. It was found that the sensitivity of sIg-A antibody response in saliva against Ag38-rec Mtb is significantly higher than PPD (70.8% vs 62.5%) (p < 0.05), although with lower specificity (26.9% vs 34.62%) (p < 0.001).
Comparison of sIgA responses to Ag38-rec between the confirmed TB group (score ≥ 6) and the suspected TB group (score < 6) (70.8% vs 73%), suggested that Ag38-rec could be recognized among TB suspected children, although this difference was not statistically significant (p = 0.861) (Table 2).
We then compared the response of sIgA towards Ag38-rec in both groups based on the TST result. It was clear that patients with a positive TST responded significantly better to Ag38-rec than did patients with a negative TST result (77.2% vs. 41%, p = 0.003) (Table 3).
The diagnosis of TB in children in Indonesia is conducted using a scoring system1. This system is not a gold standard to diagnose TB in children, but rather a consensus by the WHO for areas that do not have the facilities for complete TB screening7. Therefore, it may be beneficial to add another complementary parameter to support the scoring determination.
The objective of the current study was to observe whether the Mtb Ag38-rec antigen could be used to discriminate children that are diagnosed with TB (scoring ≥ 6) from the suspected TB (scoring < 6) based on their immune response in salivary sIgA. However, we found that the positive response of salivary sIgA against Ag38-rec was lower for children with pulmonary TB than for children with suspected TB, albeit this difference was not statistically significant (73% vs 70.8%, p = 0.861). It could be that because the children with scores < 6 were recruited from Malang, Indonesia where TB is endemic; therefore these children may be infected with TB but still in the incubation stage of the disease, resulting in negative TST results12. When we consider the potential of the Ag38-rec antigen as a serodiagnostic agent in pediatric TB compared to the PPD antigen, Ag38-rec has a significantly higher sensitivity than PPD (70.83% vs. 62.5%, p<0.001).
Although the 38-kDa Mtb antigen is the most widely studied antigen for serological purposes and is a major component in commercial TB tests13, the serum antibodies showed high heterogeneity against Mtb antigens so that specific antibody response requires high purity of antigen14. In this study, the purity of Ag38-rec was not very high (80%). Nevertheless, the sensitivity of Ag38-rec compared to PPD may indicate that the immunogenicity value of Ag38-rec is slightly better than that of PPD. A study conducted in children of the Warao Amerindian tribes in Venezuela against Mtb-specific antibodies, including sIgA in saliva, demonstrated that PPD and Ag38-rec had a higher reactivity than other antigens10. This immunodominant antigen in patients with smear-positive TB is apparently specific for Mtb bacteria. A humoral immune response against this protein is often associated with active TB disease. Since our research participants were children that could not produce sputum, we could not confirm whether the low sensitivity of our Ag38-rec antigen was a result of negative sputum smears from the children. A previous study using the same antigen demonstrated that the response of sIgA from AFB-positive adult TB patients had a sensitivity of up to 80%, although, with a lower specificity (38%)9. This suggests that the immunogenicity of Ag38-rec is better than that of PPD. The explanation for this may be the fact that the salivary sIgA antibody is part of the mucosal immune response that belongs to the naive immune response. These immune responses are produced earlier in infection than the cellular immune response (the immune response of TST). When the naive immune response alone is sufficient to eradicate a pathogen, consequently the cellular immune response will not be developed. The route of TB infection is initiated via the mucosal immune response, followed by an adaptive immune response. If the Mtb bacteria have been eliminated by the mucosal immune response, there would be no cellular immune response produced14.
With respect to the specificity of the sIgA response to Ag38-rec, it showed that it was significantly lower than that of PPD (26.9% vs. 34.6%, p < 0.001). Considering that all subjects in this study were recruited from TB endemic areas therefore it is possible that the healthy subjects may have had contact with TB patients without being infected and produced antibodies against Mtb. Interestingly the variation in specificity was also observed even when the study subjects came from non-endemic areas15.
The positive control PPD also produced a low specificity despite PPD being a mixture of antigens secreted by the bacteria Mtb. This suggests that the problem of low specificity is not simply a property of Ag38-rec, but also was affected by sample population and variations in antibody levels. These results are consistent with a previous study using serum samples in which the largest proportion of positive antibody responses was detected from TB endemic areas10.
The reactivity of antibodies against Mtb antigens in children can vary slightly due to an immature immune system, and severity of the disease16. Therefore, their antibody response has a widely ranging sensitivity and specificity, from 14% to 85% and from 86% to 100%, respectively12,16. Moreover, several factors such as the purity of the protein and its immunogenicity play an important role in antibody response. Patient characteristics, the severity of disease, the presence or absence of Mtb bacteria in sputum, and the type and origin of the antibody should be considered13. Finally, last but not least is the process of taking the saliva sample. In previous experiments, whole saliva exhibited a greater antibody response than the supernatant or pellet9. Although, Hagewald et al. found that centrifugation of saliva resulted in a greater sIgA antibody response in saliva supernatant17. In this study, due to the limited volume of saliva in children, we used whole saliva. It is perhaps worthwhile to test saliva supernatants for comparison.
An interesting phenomenon occurred as we connected a positive immune response to the Ag38-rec antigen in children with suspected TB (score < 6) and a positive TST result. Considering that a positive TST indicates an infection with Mtb bacteria18, the fact that sIgA in saliva showed a greater reactivity against Ag38-rec in children with a positive TST (77.2%) compared to children with a negative TST (41%) indicates that the Ag38-rec antigen is able to differentiate infected children within the group of suspected TB patients (score < 6). Similar results showed a significant difference between the levels of anti-Ag38-rec sIgA in individuals with a positive TST and a negative TST within the control group19. However Arauzo et al., found that there was no significant difference in the antibody response between patients with a positive TST and a negative TST19.
The specificity of Ag38-rec among healthy children compared to that of PPD suggests that Ag38 may potentially be used to identify TB infected children among children with suspected TB (scores < 6). Most of these children had a negative TST result (90%); however, 30% had a positive response to Ag38-rec.
This study has several limitations. The ideal method for saliva collection is passive drooling; however, we were unable to use this method because the children refused it. The criteria used for the determination of healthy child controls are also a limitation of this study. The control group was determined based on the criteria laid out in the scoring parameters for TB. They were children who did not meet the criteria for suspected TB and were very healthy.
F1000Research: Dataset 1. Raw data for Raras et al. 2015, ’Evaluation of immunologic response of salivary sIg-A in pediatric tuberculosis patients to antigen Ag38-rec of Mycobacterium tuberculosis Indonesian strain’, 10.5256/f1000research.7234.d10695520
Written informed consent for publication of their clinical details was obtained from the parents of the patients.
TMR, DE and CK conceived the study. TMR and DE designed the experiments. DE and TMR carried out the research. CK provided expertise in clinical analysis. DE and TMR prepared the first draft of the manuscript. All authors were involved in the revision of the draft manuscript and have agreed to the final content.
We thank Suci Megasari, MS from Central Laboratory of Biomedical Science, Faculty of Medicine, Brawijaya University for technical assistance.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
References
1. Uma Devi KR, Ramalingam B, Brennan PJ, Narayanan PR, et al.: Specific and early detection of IgG, IgA and IgM antibodies to Mycobacterium tuberculosis 38kDa antigen in pulmonary tuberculosis.Tuberculosis (Edinb). 2001; 81 (3): 249-53 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 5 (revision) 24 Oct 16 |
||
|
Version 4 (revision) 30 Jun 16 |
read | |
|
Version 3 (revision) 18 Feb 16 |
read | read |
|
Version 2 (revision) 01 Feb 16 |
read | |
|
Version 1 16 Nov 15 |
read | |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)