Keywords
Hax1, neutrophil, PLB-985, tubulin
This article is included in the Antibody Validations gateway.
Hax1, neutrophil, PLB-985, tubulin
This revision incorporates suggestions from the referees and includes all updated figures along with additional data sets 1 and 2. The following updates and revisions have been incorporated into the publication:
See the authors' detailed response to the review by Mautusi Mitra
See the authors' detailed response to the review by Lawrence L. LeClaire III
See the authors' detailed response to the review by Andrew D. Chalmers
HS1-associated protein X-1 (Hax1) is a 35 kDa protein consisting of 279 amino acids that is ubiquitously expressed1. Hax1 has been demonstrated to be a negative regulator of apoptosis in many immune cell types2–4. Furthermore, Hax1 has been shown to have additional roles in regulating cell motility and adhesion5,6, and is overexpressed in many types of cancer7. Patients with autosomal recessive mutations in the HAX1 gene have a form of severe congenital neutropenia called Kostmann syndrome. Severe congenital neutropenia is characterized by early recurrent bacterial infections and decreased neutrophil counts in the blood stream8.
Because of the recent increase in Hax1 investigations, it is important to identify reliable antibodies directed against Hax1. Using the human neutrophil model cell line PLB-985 cells, which can be terminally differentiated into neutrophil-like cells after treatment with DMSO, we demonstrate the applicability and selectivity of two commercially available antibodies against Hax1. A mouse Hax1 monoclonal antibody (BD Biosciences) that is routinely used in publications investigating Hax15,6,9–11 directed against Hax1 amino acids 10–148, and a rabbit polyclonal antibody (Proteintech Group, Inc.) directed against the full length Hax1 protein6.
Details of all reagents used in the Western blotting procedures can be found in Table 1.
| Process | Reagent | Manufacturer | Catalogue Number | Concentration |
|---|---|---|---|---|
| 6× Laemmli Protein Loading Buffer | Tris-HCL SDS Glycerol Bromophenol blue DL-Dithiothreitol | Fisher Scientific Fisher Scientific Fisher Scientific Sigma-Aldrich Sigma-Aldrich | Tris base BP152 BP166-100 G33-1 B0126 D0632 | 375mM 9% 50% 0.03% 0.6M |
| Protein blotting | 0.45μm nitrocellulose pure transfer membrane | Santa Cruz Biotechnology | Sc-201705 | |
| SDS-PAGE Transfer Buffer | 1× Tris-Gylcine Electroblotting buffer Methanol | National Diagnostics Fisher Scientific | EC-800 A412-4 | 1× (25mM Tris-HCL, 192mM glycine) 20% v/v |
| Wash Buffer, blocking buffer, and Antibody Diluent | Tween-Tris/Saline (T-TS) | Fisher Scientific | Tris base BP152 NaCl S271 Tween-20 BP337 | 50mM Tris 150mM NaCl 1% Tween -20 |
| Blocking | Bovine Serum Albumin, heat shock fraction | Sigma-Aldrich | A9647 | 5% in T-TS |
| Pre-Immune Serum Incubation (Figure 5A) | Rabbit pre-immune serum Mouse pre-immune serum | Sigma-Aldrich Sigma-Aldrich | R9133 M5905 | 1:1000 1:1000 |
Anti-tubulin (beta-) is a mouse monoclonal IgG1 [E7 was deposited to the DSHB by Klymkowsky, Michael (DSHB Hybridoma Product E7)] and was used as a loading control for all Western blots at a dilution of 1:1000 resulting in a final concentration of 45 ng/mL. Rabbit anti-Hax1 (Proteintech Group, Inc, Table 2) is a polyclonal antibody generated to full length Homo sapiens Hax1. The lot number used was 1, and a dilution of 1:1000 was used for all Western blots resulting in a final concentration of rabbit anti-Hax1 of 230 ng/mL. Mouse anti-Hax1 (BD Biosciences) is a mouse monoclonal IgG1 raised against Homo sapiens Hax1 amino acids 10–148. The lot number used was 3266979, and a dilution of 1:1000 was used for all Western blots resulting in a final concentration of 250 ng/mL. Goat anti-rabbit IgG IRDye 680LT and Goat anti-mouse IgG IRDye 800CW (Li-Cor Biosciences, Table 2) were used at a dilution of 1:40,000 (25 ng/mL).
PLB-985 cells were maintained in RPMI 1640 (Mediatech, Inc.) supplemented with 10% fetal bovine serum, 60 μg/mL penicillin, and 100 μg/mL streptomycin (Mediatech, Inc.) at a concentration of 0.1–1 × 106 cells/mL. To differentiate PLB-985 cells into “neutrophil-like” cells 1.25% DMSO (Fisher Scientific) was added to 2 × 105 cells/mL for 6 days. Lentiviral Hax1 shRNA targets were purchased from Open Biosystems. Targets used; Hax1 shRNA (5'-ACAGACACTTCGGGACTCAAT-3') and control shRNA (5'-TGTCTCCGAACGTGTCACGTT-3'). HEK293-FT cells were grown to 70% confluency in a 10cm tissue culture dish for each lentiviral target and transfected using 6μg Hax1, 0.6μg vesicular stomatitis virus (VSV)-G, and 5.4μg cytomegalovirus (CMV) 8.9.1. 72 hour viral supernatant was collected and concentrated using Lenti-X concentrator (Clontech, Inc.) following the manufacturer’s instructions. 1 × 106 PLB-985 cells were infected with viral supernatant for 3 days in the presence of polybrene (4 μg/mL, Santa Cruz Biotechnology). Stable cell lines were generated with puromycin (1 μg/mL, Sigma Aldrich) selection.
Differentiated PLB-985 cells were counted and 0.1 × 106, 0.5 × 106, and 1 × 106 cells were pelleted by centrifugation.
Cells were lysed in Triton X-100 lysis buffer with protease inhibitors (25 mM HEPES, pH 7.5, 150 mM NaCl2, 1% TX-100, 10 mM MgCl2, 1 mM EDTA, 10% glycerol, 1 μg/mL pepstatin A, 2 μg/mL aprotinin, 1 μg/mL leupeptin) on ice for 10 minutes and clarified by centrifugation.
Cellular lysate was then removed and added to 6× Laemmli sample buffer, boiled at 90°C for 5 minutes, and run on 10% SDS-PAGE gels.
Proteins were then transferred to 0.45μm nitrocellulose membranes (Santa Cruz Biotechnology) at 400mA for 1 hour at 4°C.
Following transfer, the membrane was blocked in 5% BSA in 1× T-TS for 1 hour at room temperature with gentle rocking.
Membranes were then probed with mouse anti-tubulin [(beta-) (45 ng/mL)], and either mouse anti-Hax1 (BD Biosciences, 250 ng/mL) or rabbit anti-Hax1 (Proteintech Group, Inc., 230 ng/mL) at room temperature for 1 hour.
After primary antibody incubation the membranes were washed 3 × 5 minutes with 1× Tris-HCL/NaCl saline buffer (1× T-TS), see Table 1.
The membranes were incubated with goat anti-rabbit IgG IRDye 680LT and goat anti-mouse IgG IRDye 800CW (Li-Cor Biosciences, 25 ng/mL) at room temperature for 1 hour.
After secondary antibody incubation the membranes were washed 3 × 5 minutes with 1× T-TS.
Blots were imaged with an infrared imaging system (Odysssey Fc; Li-Cor Biosciences) using a 2-minute exposure time.
To determine the reproducibility and sensitivity of the mouse and rabbit anti-Hax1 antibodies on the PLB-985 cells, we performed Western blot analysis using three separate cell densities, 0.1 × 106, 0.5 × 106, and 1 × 106 cells. In our research using the PLB-985 cell system, we routinely use 1 × 106 – 10 × 106 cells in a Western blot. Using beta-tubulin as a loading control our Western blots illustrate an increasing protein concentration in the three samples as would be expected with increasing cell densities. We found that the mouse anti-Hax1 antibody (BD Biosciences) is visible as low as 0.5 × 106 cells, binding to a protein band at the expected Hax1 size with a relative mobility of 35 kDa (Figure 1). In six different experiments (Figure 1 and Figure 4) we found inconsistency in protein detection with the Ms anti-Hax1 antibody. In all blots Hax1 was visible, however with varying degrees of intensity. Conversely, when the rabbit anti-Hax1 antibody (Proteintech Group, Inc.) was used, the antibody gave consistent and robust detection (Figure 2 and Figure 4). In some cases, Hax1 can be detected in as low as 0.1 × 106 cells using the rabbit anti-Hax1 antibody (Figure 2C). We do not believe the difference between the two antibodies is due to variations in the cell extract or imaging software because when the same cell extract is immunoblotted on two different blots and scanned simultaneously the difference in sensitivity can be observed (Figure 3A). Using the Odyssey imaging system (Li-Cor Biosciences) to measure the intensity of each band, we calculated the intensity ratio of Hax1 relative to the tubulin loading control from three independent blots for each antibody (Figure 3B). In both blots the levels of tubulin are similar, however it is evident that the rabbit anti-Hax1 antibody exhibits a stronger signal compared to the mouse monoclonal antibody. Nevertheless, it should be noted that both antibodies reliably detect Hax1 in differentiated PLB-985 cells.
To demonstrate the specificity of both Hax1 antibodies we generated stably-expressing control shRNA and Hax1 shRNA PLB-985 cells (Figure 4). As described previously using the mouse anti-Hax1 antibody the control shRNA cells show inconsistent staining intensity, however in these samples the mouse anti-Hax1 antibody is more robust than in the wild-type PLB-985 cells. Both the mouse anti-Hax1 and rabbit anti-Hax1 antibodies show reduced detection in the Hax1-deficient PLB-985 cells. Quantification of the level of Hax1 knockdown is consistent using the two antibodies at 1 x 106 cells. This demonstrates that the antibodies are highly specific for Hax1. In many of the experiments we observed additional background bands in the rabbit 680nm channel. To determine the source of these background bands rabbit and mouse pre-immune serum were tested (Figure 5A). Our results show a unique background pattern using the pre-immune serum that we do not observe on the Hax1 blots. We next performed a Western blot on cells using only the rabbit and mouse secondary antibodies (Figure 5B). The mouse channel does not display any significant background, however the rabbit secondary antibodies shows a background staining that we observe in the previous blots as well. These blots were then subsequently probed with the rabbit and mouse Hax1 antibodies (Figure 5C). Comparison of the secondary only blot before and after Hax1 antibody incubation demonstrates that the Hax1 antibodies are highly specific and the background we are observing can be attributed to the goat anti-rabbit IgG secondary antibody.
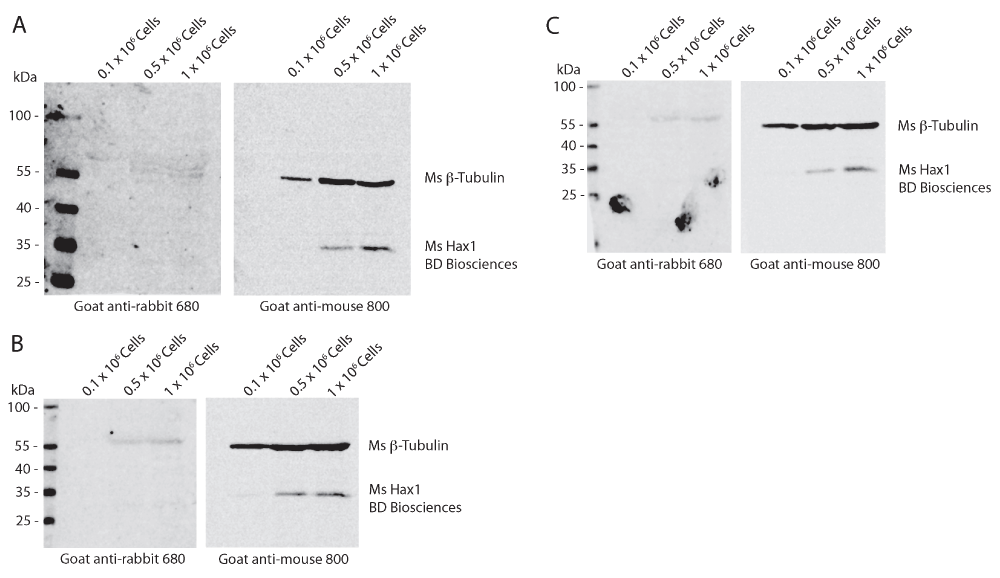
Western blot analysis of differentiated PLB-985 cell lysates from 0.1 × 106, 0.5 × 106, and 1 × 106 cells from three independent replicates. Mouse anti-tubulin (beta-) is used as a loading control and can be seen present at a relative mobility of 55 kDa in the goat anti-mouse 800 channel. Mouse anti-Hax1 detects a band with a relative mobility of 35 kDa as predicted. Hax1 can be detected in densities of 0.5 × 106 and 1 × 106 cells.
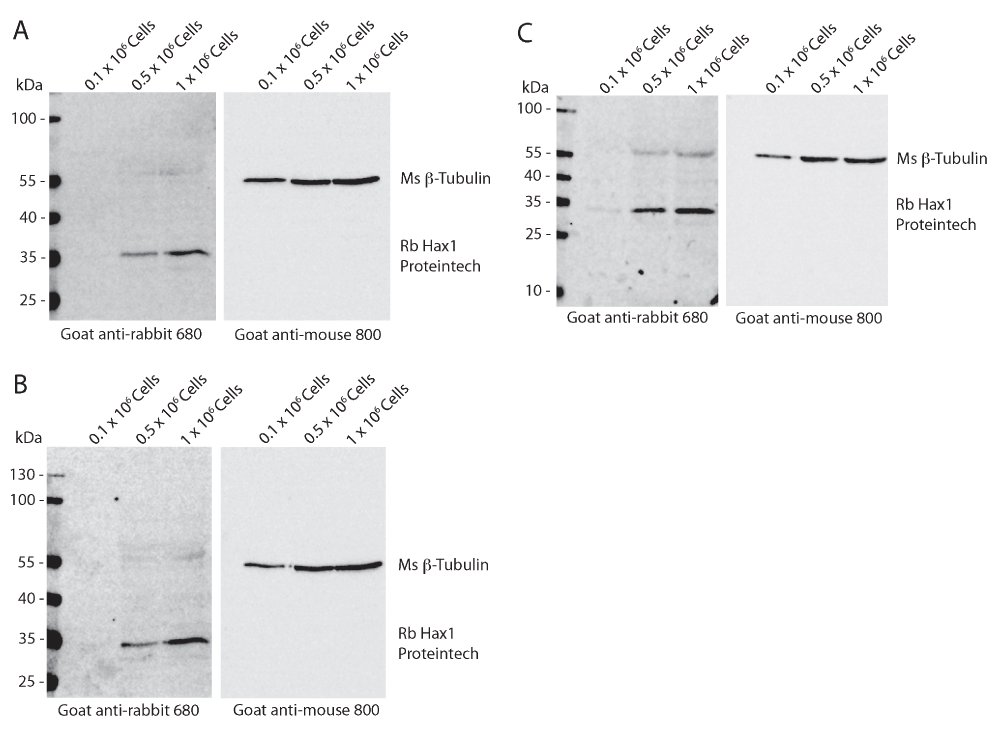
Western blot analysis of differentiated PLB-985 cell lysates from 0.1 × 106, 0.5 × 106, and 1 × 106 cells from three independent replicates. Mouse anti-tubulin (beta-) is used as a loading control and can be seen present at a relative mobility of 55 kDa. Rabbit anti-Hax1 detects a band at a relative mobility of 35 kDa as predicted. Hax1 can be detected in densities as low as 0.1 × 106 cells (C).
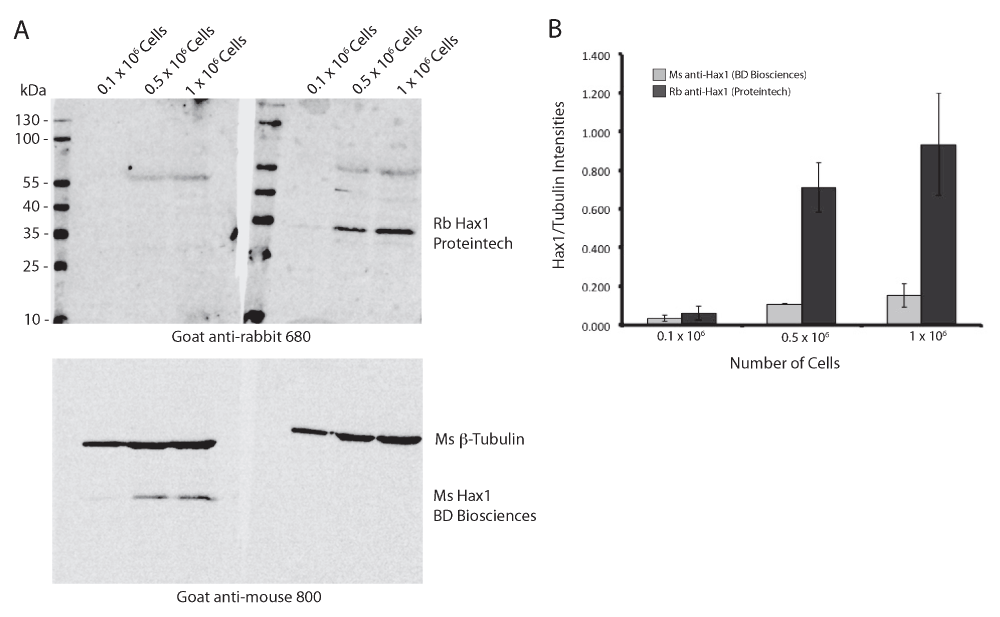
(A) Western blot analysis of differentiated PLB-985 cell lysates from 0.1 × 106, 0.5 × 106, and 1 × 106 cells comparing mouse and rabbit anti-Hax1 antibodies. Lysates from the same cell extractions were run on a single SDS-PAGE gel and blotted onto a single nitrocellulose membrane. After transfer, the membrane was divided and probed with either mouse anti-Hax1 or rabbit anti-Hax1. The membranes were imaged simultaneously. (B) Quantification of the ratios of Hax1 and tubulin band intensities from three independent blots were measured and plotted. Error bars indicate standard deviation.
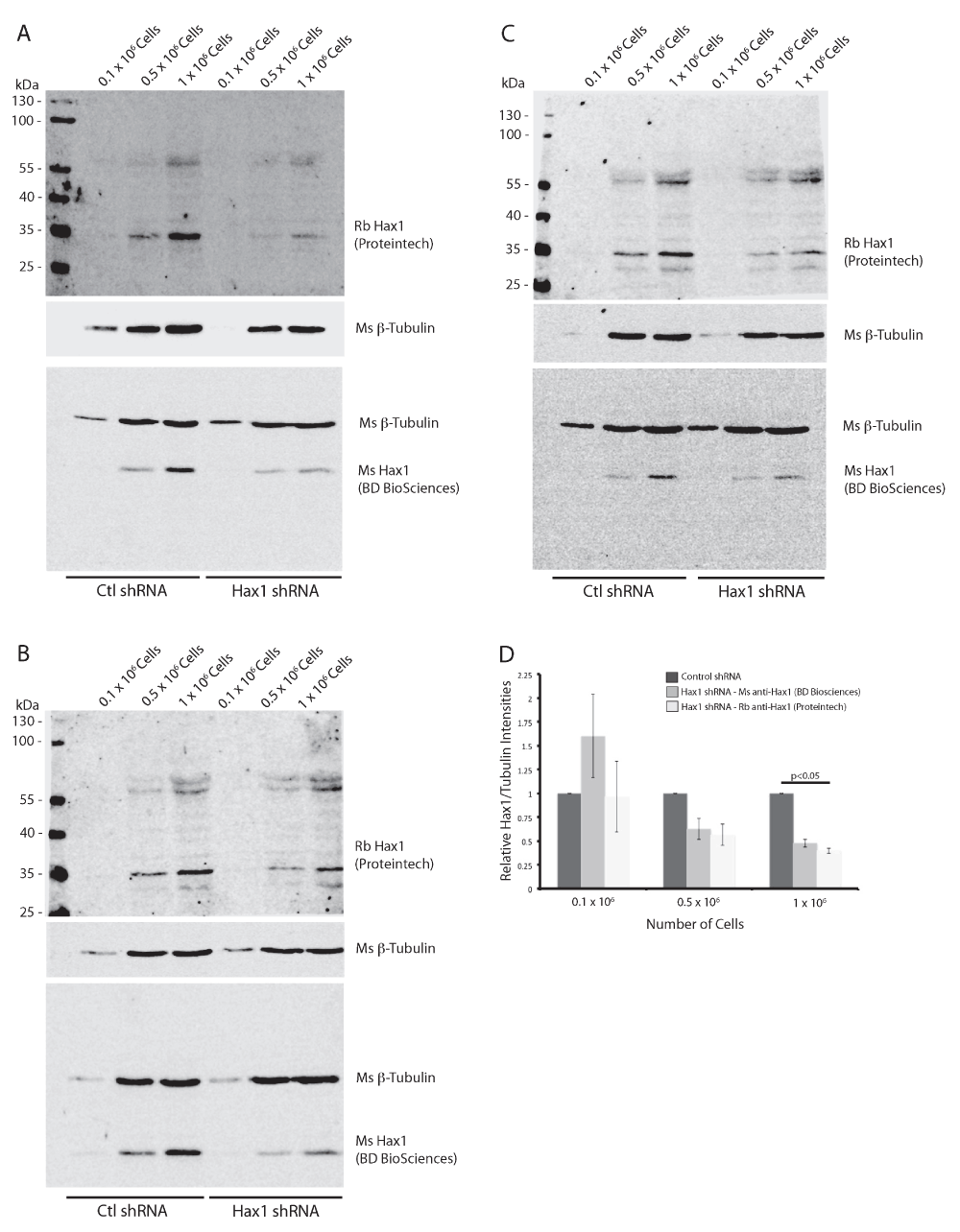
(A–C) Western blot analysis of differentiated PLB-985 cell lysates from 0.1 × 106, 0.5 × 106, and 1 × 106 cells expressing either control shRNA or Hax1 shRNA from three independent replicates. Mouse anti-tubulin (beta-) is used as a loading control and can be seen present at a relative mobility of 55 kDa. Both mouse and rabbit anti-Hax1 detects a band at a relative mobility of 35 kDa as predicted. (D) Quantification of the band intensities of tubulin and Hax1 relative to control shRNA from three independent Western blots. Error bars indicate standard error of the mean. p values were calculated using paired t-test to assess significance relative to control shRNA.
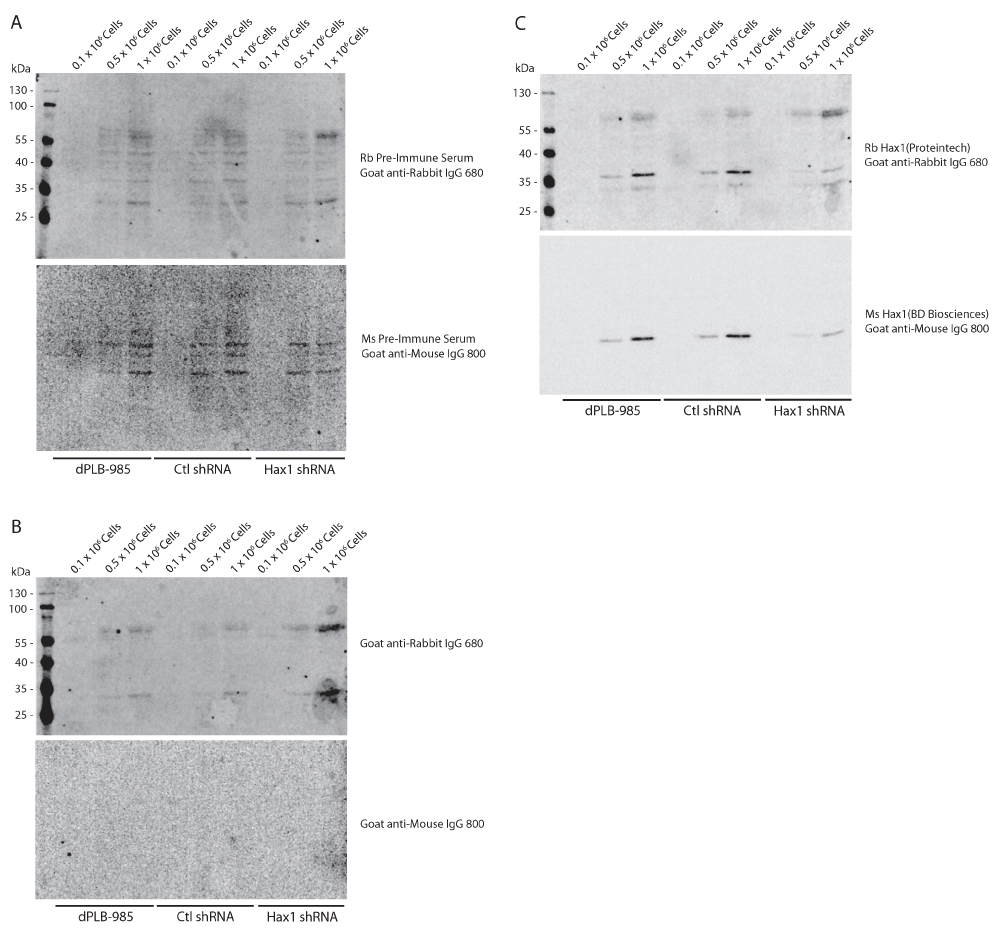
(A) Western blot analysis using rabbit and mouse pre-immune serum from 0.1 × 106, 0.5 × 106, and 1 × 106 differentiated PLB-985, control shRNA, and Hax1 shRNA cells. (B) Western blot analysis using goat anti-rabbit IgG 680LT only on cell lysates from 0.1 × 106, 0.5 × 106, and 1 × 106 differentiated PLB-985, control shRNA and Hax1 shRNA expressing PLB-985 cells. Two predominant background bands can be observed at a relative mobility of 60 and 70 kDa, and one band around 30 kDa. These background bands can also be seen in Figure 1, Figure 2, and Figure 4. (C) Subsequent incubation with rabbit and mouse anti-Hax1 from Western blots shown in B demonstrate the appearance of the Hax1 band at the predicted 35 kDa size.
Here we show validation and comparison results of two commercially available antibodies generated against HS1-associated protein X-1 (Hax1), an anti-apoptotic protein that has a multi-factorial role in regulating cell proliferation and differentiation, cell motility, and cancer. Homozygous loss-of-function of Hax1 results in severe congenital neutropenia, a life threatening loss of circulating neutrophils in the blood stream. Studying the function of Hax1 in primary neutrophils and the neutrophil model cell line PLB-985 will help elucidate the disease pathogenesis of neutropenia syndromes. We demonstrate that mouse anti-Hax1 (BD Biosciences) and rabbit anti-Hax1 (Proteintech Group, Inc.) are both specific for Hax1. Furthermore we show that as little as 0.5 × 106 differentiated PLB-985 cells can be used to reliably detect Hax1 expression with both of the antibodies. We have evidence that the rabbit anti-Hax1 (Proteintech Group Inc.) results in a more robust and consistent detection of Hax1, likely due to the polyclonal nature of the antibody. Finally, lentiviral knockdown of endogenous Hax1 expression results in loss of Hax1 detection by both mouse anti-Hax1 and rabbit anti-Hax1 demonstrating the specificity of each antibody. In our quantification of Hax1 knockdown we observed variation when the cell densities were low, with 1 × 106 cells giving us the most reliable quantification. In our experiments we observed background bands that we attributed to the goat anti-rabbit 680nm secondary antibody. Therefore we are confident that these antibodies are very specific.
In conclusion we recommend the use of either mouse or rabbit anti-Hax1 antibodies shown here for studies using the PLB-985 cells as a neutrophil model cell line. It is our conclusion that a minimum cell density of 0.5 × 106 neutrophils should be used as a starting point for immunoblotting of Hax1, with greater than or equal to 1 × 106 cells being optimal.
F1000Research: Dataset 1. Raw data for Figure 3 quantification., 10.5256/f1000research.6516.d9934312
F1000Research: Dataset 2. Raw data for Figure 4 quantification., 10.5256/f1000research.6516.d9934413
PC and KI co-wrote and conceived of the article. PC developed the figures. KI performed Western blotting and cell culture. All authors agreed to the final content of the manuscript.
KI and PC are funded by the University of West Florida.
I confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors would like to acknowledge the University of West Florida, for resources and lab support to do this study. The authors would like also like to thank John Steele and Roberta Palau for tissues culture assistance and aid in preparing the materials for this publication.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 10 Aug 15 |
read | read | |
|
Version 1 10 Jun 15 |
read | read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)