Keywords
EGFR and KRAS mutation, multiplex-PCR, concatenation of PCR products, Clinical diagnostics
EGFR and KRAS mutation, multiplex-PCR, concatenation of PCR products, Clinical diagnostics
We are particularly grateful to Reviewer 1 for describing the study as “a well conducted proof of principle report”, and Reviewer 2 for their comments that, “The methods are well described and the test is of clinical relevance, particularly in settings with limited resources and without access to tumor next generation sequencing”. Further, incorporating the suggestions made by the reviewers have contributed to an improved version of the manuscript. Specifically, we have, in the revised version:
In response to Reviewer 1
a) We have incorporated the suggestion of the reviewer by correcting the original submission in response their comments 1, 3, 4 and 7.
b) We have included the relevant references as pointed by the reviewer comment 2.
c) We have detailed our response to rest of the queries.
In response to Reviewer 2
d) We have incorporated the suggestion of the reviewer by correcting the original submission in response to both of their comments 1 and 2.
See the authors' detailed response to the review by Bob T. Li
See the authors' detailed response to the review by Chandan Kumar
The growing significance of identifying EGFR and KRAS mutations in lung cancer using molecular diagnostic approaches underlines the emphasis on the use of personalized medical care by physicians to help design optimal therapeutic regimens (Lynch et al., 2004; Paez et al., 2004; Pao et al., 2004; Pao et al., 2005a; Pao et al., 2005b). While EGFR and KRAS mutations largely occur mutually exclusively in non-small cell lung cancer (NSCLC), and predict contrasting response rate to tyrosine-kinase inhibitors (TKI) (Chougule et al., 2013; Fukuoka et al., 2011; Ihle et al., 2012; Lynch et al., 2004; Mao et al., 2010; Mok et al., 2009), some recent studies, including ours, suggest co-occurrence of EGFR and KRAS mutations in the same patients, albeit at low frequency (Choughule et al., 2014; Li et al., 2014). While no direct evidence exists as yet, these studies may have implications for carrying out routine KRAS molecular testing along with EGFR mutations for precluding a patient with NSCLC from therapy with EGFR inhibitors, as approved for colorectal cancer (Lievre et al., 2006). Such information is especially important for lung cancer patients at an advanced-stage, who are not candidates for surgical intervention—wherein biopsy specimens obtained through fine-needle aspiration (FNA) may represent the only opportunity to obtain tissue material for diagnosis and molecular diagnostic analysis.
EGFR mutations in NSCLC are characterized by approximately 39 unique mutations present across exons 18-21. Of these, most common are activating mutations, which account for approximately 90% of all EGFR mutations and are closely related to the efficacy of EGFR-TKIs. These activating mutations include point mutations G719S, T790M, L858R, and L861Q in exons 18, 20 and 21 respectively and in-frame deletions/insertions in exon 19 (Kosaka et al., 2004). The most common mutations that result in an amino acid substitution at position 12 and 13 in KRAS are G12V and G13D (Choughule et al., 2014). Several screening and target based methods are currently in use for to infer the EGFR and KRAS hot spot mutations, viz; PCR-RFLP (Restriction fragment length polymorphism), Amplification Refractory Mutation System (ARMS), PCR-Invader, TaqMan PCR, allele specific qPCR, high resolution melting analysis and ultra-deep pyrosequencing, SNaPshot analysis and co-amplification at lower denaturation temperature (COLD)-PCR (Angulo et al., 2012; Borràs et al., 2011; Ellison et al., 2013; Santis et al., 2011; van Eijk et al., 2011; Zinsky et al., 2010). Of these, direct sequencing is the most commonly used method worldwide (Yatabe et al., 2015). However, a typical PCR reaction that precedes the sequencing step to amplify 4 EGFR and 1 KRAS exon(s) essentially consists of five rounds of independent PCR requiring separate aliquots of genomic DNA template for each reaction, followed by ten rounds of sequencing reactions. With a limited amount of genomic DNA from clinical FFPE specimens or fine biopsies of lung tumors, multiple rounds of PCR and sequencing reactions can often be challenging to perform.
In-frame concatenation or assembly of individually amplified exons from genomic DNA to generate a coding fragment has been described in earlier research, wherein the total number of PCR reactions corresponds to the number of exons to be concatenated (An et al., 2007; Fedchenko et al., 2013; Mitani et al., 2004; Tuohy & Groden, 1998). Here, we describe a novel methodology to co-amplify all four EGFR exons 18-21 along with KRAS exon 2 in a single multiplex PCR followed by directional or ordered concatenation of the products in the form of a single linear fragment. This concatenated product can be used to detect mutations by direct sequencing, at a much reduced cost and duration, and with a much smaller amount of template.
Genomic DNA was isolated from human NSCLC cell line NCI-H1975 and primary fresh frozen tumor tissue using QIAamp DNA blood mini kit (Qiagen). Genomic DNA from FFPE blocks was isolated using QIAamp DNA FFPE tissue kit (Qiagen) as per manufacturer’s instructions. DNA concentration was determined by absorbance at 280 nm (NanoDrop 2000, Thermo Scientific).
PCR primers were designed for KRAS exon 2 and EGFR exons 18-21. Supplementary Table S1 represents all the primers used for PCR amplifications. With the exception of the OAD176 and OAD152 primers, all internal primers contain an additional overhang of 15 nucleotides, such that the tail sequence of forward and reverse primers of two subsequent exons are complementary to each other to allow ordered and directional concatenation of KRAS and EGFR exons. The full length concatenated product of 915 bases was amplified using OAD176 and OAD152 primers.
Multiplex PCR (50 µl per reaction) was carried out in a single tube by using multiplex PCR kit (Qiagen) containing either 10 ng of genomic DNA from the NSCLC cell line or fresh frozen primary tumor, or 50 ng of genomic DNA from FFPE blocks with 0.2 µM each of the five primer pairs using Applied Biosystems Veriti 96-Well Thermal Cycler. PCR was carried out with initial hot-start denaturation at 95°C for 15 min, followed by 35 cycle of denaturation at 94°C for 30 seconds, annealing at 57°C for 90 seconds, polymerization at 72°C for 60 seconds, and final incubation for 30 min at 60°C. The multiplex PCR products were analyzed by agarose gel electrophoresis.
For concatenation of KRAS exon 2 and EGFR exons 18-21, 2 µl of multiplex PCR product was used as template in a 50 µl PCR reaction containing 0.2 µM of each OAD176 and OAD152 primers. PCR was carried out in a Verity thermal cycler (Applied Biosystems) with an initial hot-start denaturation at 95°C for 15 min, followed by 35 cycle of denaturation at 94°C for 30 seconds, annealing at 57°C for 90 seconds, polymerization at 72°C for 60 seconds, and final incubation for 30 min at 60°C. Concatenated PCR product was analyzed by agarose gel electrophoresis. Sequencing of concatenated PCR products were performed by Sanger sequencing. Sequences were analyzed using Mutation Surveyor software V4.0.9 (Minton et al., 2011).
CRE (Co-amplification of KRAS and EGFR) exons is a cost-effective multiplex-PCR based method followed by concatenation of the PCR product as a single fragment for direct sequencing (Figure 1). It is a robust methodology to determine the mutation status of KRAS and EGFR with reduced variability, cost and turnaround time, requiring a minimal amount of template DNA extracted from FFPE or fresh frozen tumor samples.

The flowchart represents the workflow for CRE methodology. KRAS and EGFR primers are shown along with complementary tail overhangs that prime with consecutive exons in an ordered manner. 2 µl PCR products, amplified with a cocktail of primers, as shown and described in Supplementary Table S1, for KRAS and EGFR exons in a single multiplex reaction is transferred to a fresh tube and concatenated in a separate reaction using OAD 176 and OAD 152 primers. The concatenated product obtained is a single product of 915 bp with all individual exons amplified from multiplex PCR ligated together in an ordered manner as a single fragment. 2x sequencing using the forward primer OAD 176 and reverse primer OAD 152 of the concatenated product is adequate to scan the mutation status across all the KRAS and EGFR exons.
Following CRE-based multiplex PCR of KRAS exon 2 and EGFR exons 18-21 with overlapping PCR bands (Figure 2A, lane 6), concatenation of the PCR product was performed with OAD176 and OAD152 primers using genomic DNA extracted from NCI-H1975 cells, a non-small-cell lung adenocarcinoma cell line. Concatenation PCR resulted in the enrichment of a concatenated product of about 915 base pairs (Figure 2B). This concatenated, gel purified PCR product of 915 base pair was used for Sanger sequencing. Sequencing analysis of the concatenated PCR product confirmed concatenation as a single fragment (Figure 3) along with the presence of EGFR T790M and L585R mutations in NCI-H1975 cells (Supplementary Figure S1). A similar concatenation of a 915 bp single fragment was performed with genomic DNA extracted from fresh frozen tumor cells (Figure 2C).
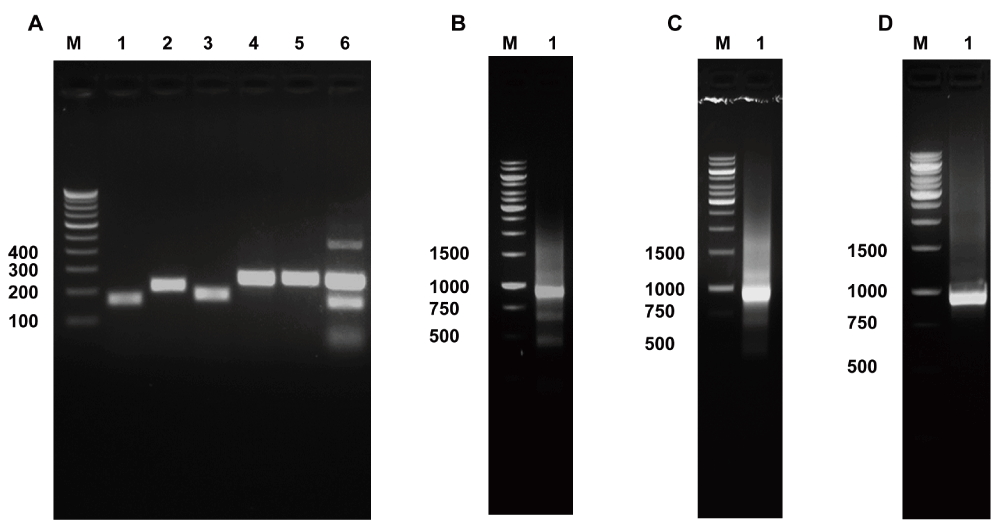
Panel A. PCR amplification of KRAS and EGFR exons using NCI-H1975 genomic DNA: Lane 1, KRAS exon 2 (151 bp) amplified with OAD176 and OAD177; Lane 2, EGFR exon 18 (209 bp) amplified with OAD 178 and OAD 144; Lane 3, EGFR exon 19 (178 bp) amplified with OAD 145 and OAD 146; Lane 4, EGFR exon 20 (246 bp) amplified with OAD 147 and OAD 150; Lane 5, EGFR exon 21 (251 bp) amplified with OAD 151 and OAD 152; Lane 6, Multiplex PCR of KRAS exon 2 and EGFR exons 18-21 with cocktail of primers used in Lanes 1–5.
Concatenated KRAS and EGFR (CRE) product of ~915 bp amplified with OAD 176 and OAD 152 using multiplex PCR product as template derived from NCI-H1975 genomic DNA (shown in Panel B, Lane 2); derived from fresh frozen primary tumor genomic DNA (shown in Panel C, Lane 2); using tumor genomic DNA extracted from FFPE block (shown in Panel D, Lane 2).
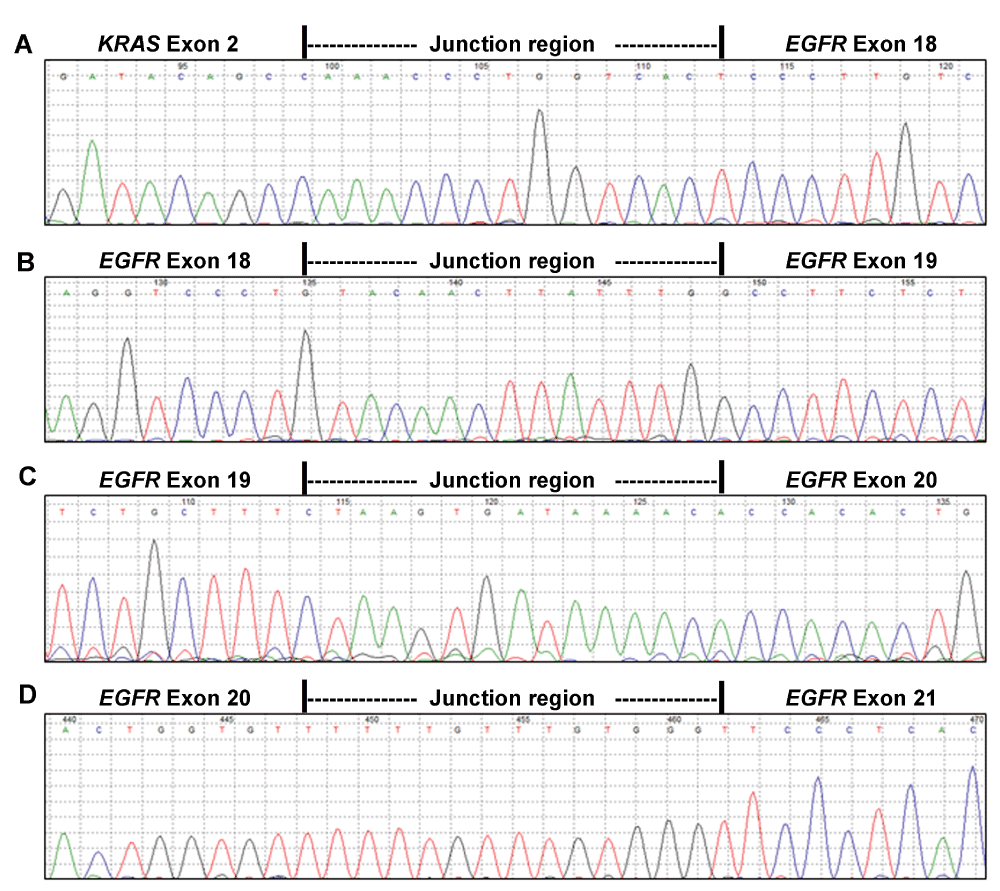
Reverse complements of the forward sequencing reads of the 915 bp KRAS-EGFR concatenated product are displayed as generated by Mutation Surveyor V4.0.9. Panel A displays 15 nucleotide junction region flanked by KRAS exon 2 and EGFR exon 18 sequence; Panel B displays 15 nucleotide junction region flanked by EGFR exons 18 and 19; Panel C displays 15 nucleotide junction region flanked by EGFR exons 19 and 20; and displays 15 nucleotide junction region flanked by EGFR exons 20 and 21 is shown in Panel D.
The amount of genomic DNA obtained from FFPE tissue is always limiting and thus there is a substantial need to develop a technique with a limited amount of starting DNA as a template for mutation detection. CRE demonstrates the ability to co-amplify all five exons (KRAS exon 2 and EGFR exon 18-21) in a single multiplex PCR reaction with a limited amount of starting template DNA followed by the enrichment of concatenated product (Figure 2D) by concatenation PCR using first multiplex PCR product as a template. The concatenated product confirmed EGFR L858R mutation in the FFPE tissues (Supplementary Figure S2), as reported earlier (Choughule et al., 2014). Thus our CRE method can be routinely used for the mutational analysis of KRAS and EGFR genes.
CRE is a novel, simple and effective strategy to concatenate multiple amplicons obtained from a multiplex PCR, using primers with overlapping complementary overhangs. Compared to ARMS, and other genotyping technologies, CRE is relatively inexpensive with faster turnaround time involving lesser amount of template genomic DNA.
Using CRE, in vitro tandem reconstitution of KRAS exon 2 with EGFR exons 18-21 can be effectively performed to generate a concatenated single PCR product of 915 bp, as a template for sequencing. Most commercially-available allele-specific and genotyping technologies are restricted by their ability to probe only for eight out of the approximately 39 known commonly occurring EGFR and KRAS activating mutations. However, growing clinical data on the less common mutations are now emerging to fully inform their predictable outcomes on EGFR TKIs (Lohinai et al., 2015; Yang et al., 2012). Currently available methodologies, if extended to genotype all known 39 mutations would not only be cost-prohibitive but challenging to perform due to a limiting amount of template genomic DNA available from clinical cancer specimens that are mostly available in the form of formalin-fixed, paraffin-embedded (FFPE) tissue. While a directed sequencing approach –classical or next-generation sequencing (NGS) -based—can determine a whole spectrum of rare and co-occurring mutations in an individual, the question of template genomic DNA availability still remains. CRE circumvents the issue of a limiting amount of template genomic DNA with increased affordability by multiplexing PCR for all exons to a single reaction and concatenating the PCR product as a single fragment, thereby further reducing the cost of multiple sequencing reactions.
In this era of genome sequencing, applicability of the CRE strategy could be of immense significance to effectively reduce the cost and turnaround time taken to determine the mutational status across the whole KRAS exon 2 and EGFR kinase domain exons. As the limitation of the CRE strategy is defined by the sensitivity and resolution of the sequencing methodology adopted, concatenated EGFR and KRAS PCR products from multiple individuals—each tagged with unique bar code sequence—can be pooled and deep-sequenced using a NGS platform. The CRE strategy described here can reduce the labor and cost of performing individual PCR for all exons for each patient and effectively circumvent the noise due to variation in normalization for equimolar pooling of exons within the same sample at a resolution of single base. Additionally, the current version of CRE is limited by exclusion of fewer number of exons of EGFR and KRAS. Inclusion of known extracellular EGFR and KRAS exon 3 codon 61 mutation may help to immediately expand the scope of its application to other cancers, such as glioblastoma.
F1000Research: Dataset 1. Raw gel electrophoresis images for Figure 2: Multiplex PCR amplification and concatenation of KRAS and EGFR exons generates CRE product, 10.5256/f1000research.6663.d50236
F1000Research: Dataset 2. Sequencing traces for Figure 3: Full length sequencing of the CRE product, 10.5256/f1000research.6663.d50237
F1000Research: Dataset 3. Sequencing traces for Figure S1: Detection of EGFR T790M and L858R mutations from NCI-H1975 CRE product, 10.5256/f1000research.6663.d50238
F1000Research: Dataset 4. Sequencing trace for Figure S2: Detection of EGFR L858R mutation in a CRE product derived from FFPE primary tumor sample, 10.5256/f1000research.6663.d50239
M.P.R. and K.J.P. contributed equally to this work. M.P.R., K.J.P, K.P. and A.D. conceived and designed the experiments. M.P.R., K.J.P, M.G., M.V., and K.K. performed the experiments. M.P.R., K.J.P. and A.D. analyzed the data. A.C. and K.P. contributed reagents/materials/analysis tools. M.P.R., K.J.P. and A.D. wrote the paper.
A.D. is supported by an Intermediate Fellowship from the Wellcome Trust/DBT India Alliance (IA/I/11/2500278), by a grant from DBT (BT/PR2372/AGR/36/696/2011), and intramural grants (IRB project 55, 88, 92, 107, 108, 116).
I confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Sequences in italics indicate extra 15 nucleotide tail sequences (junction region). Sequences in bold denotes complementary region between reverse primer of one exon with forward primers of successive exon. 5′ and 3′ represents forward and reverse primer respectively.
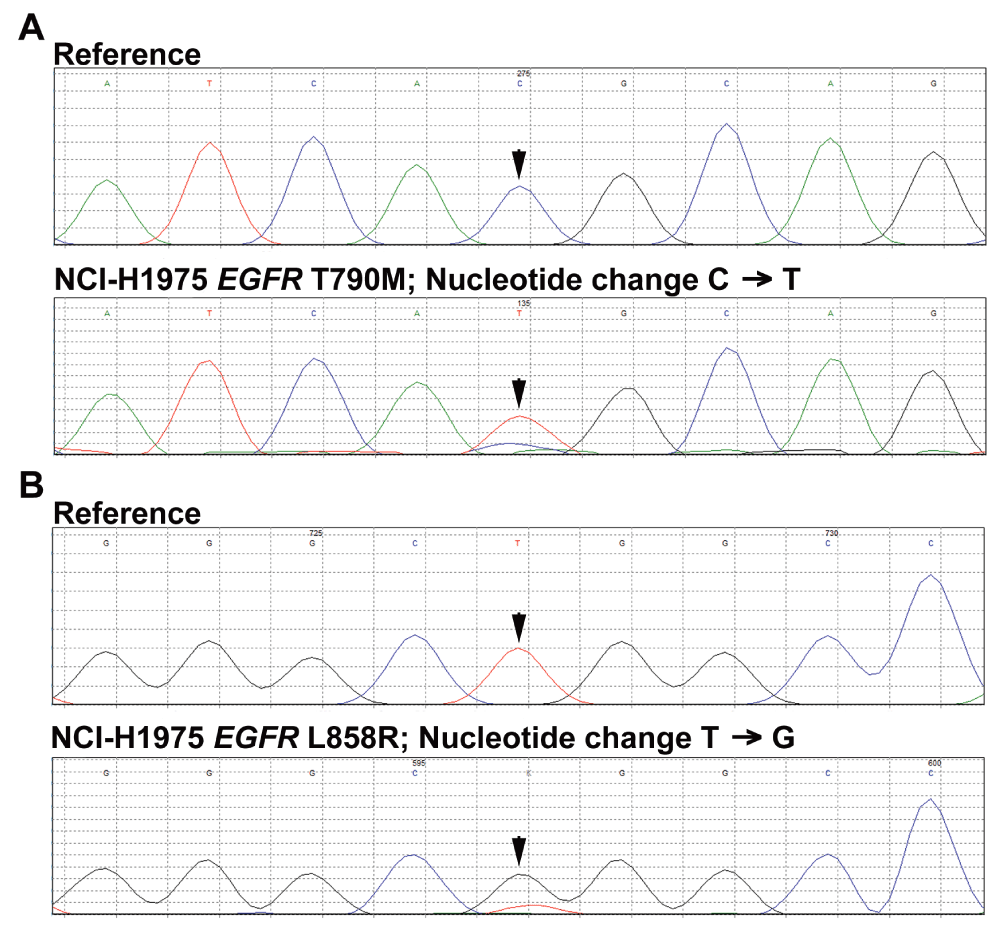
Reverse complements of the forward sequencing reads of the 915 bp CRE product using genomic DNA extracted from NCI-H1975 cells are displayed as generated by Mutation Surveyor. Panel A: The arrow indicates expected location of the wild-type and T790M mutant allele peak. Panel B: The arrow indicates expected location of the wild-type and L858R mutant allele peak.
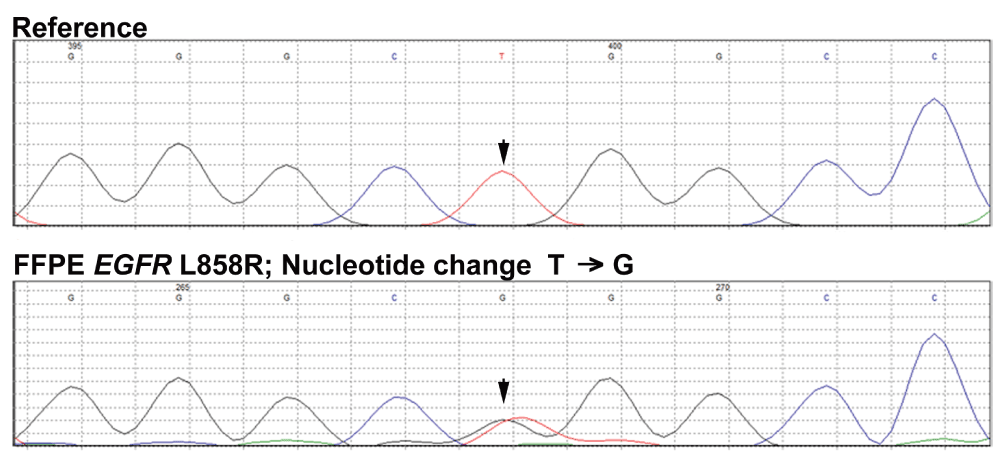
Reverse complements of the forward sequencing reads of the 915 bp CRE product using genomic DNA extracted from FFPE primary tumor are displayed are displayed as generated by Mutation Surveyor. The arrow indicates expected location of the wild-type and L858R mutant allele peak.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 08 Mar 16 |
read | |
|
Version 1 23 Jun 15 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)