Keywords
Systemic lupus erythematosus, central nervous system, NZB/W mice, behavior, astrogliosis, cyclophosphamide
This article is included in the Lupus nephritis and neuropsychiatric lupus collection.
Systemic lupus erythematosus, central nervous system, NZB/W mice, behavior, astrogliosis, cyclophosphamide
We thank the reviewers for their constructive criticisms. This manuscript has been revised to address the reviewers’ concerns. Other than the concern regarding language and grammar, the only major concern was regarding the transport and dose of CY and PD in lupus brain. When studying the brain, it is an important concern as to whether the pharmacological agent has access to the brain. It is known that cyclophosphamide and prednisolone do cross the BBB. Higher doses in patients cause side effects, therefore that is another important facet. However, it was shown earlier that the brain pathology persisted even after the systemic disease abated suggesting that the brain has to be studied both in relation to the systemic changes and the changes occurring in the brain itself. In our study the mice got better than their control counterparts when treated with CY and PD. To determine whether the effect on brain function is direct or secondary due to prevention of systemic aberrations will be the focus of our next study. The manuscript has been revised to eliminate grammatical errors. The figures have been labelled more thoroughly.
See the authors' detailed response to the review by Christopher Reilly
See the authors' detailed response to the review by Trine N Jørgensen
Systemic lupus erythematosus (SLE) is an autoimmune disease in which one third of patients exhibit neuropsychiatric (NP) disturbances. Patients with NP-SLE have a variety of behavioral and cognitive impairments1–3. These are likely to track with pathological alterations occur in the brain, however subtle. A challenge in defining the pathogenesis of SLE is its complexity, involving affecting many systems and pathways.
Animal models have proven invaluable in our understanding of SLE4. Lupus mice mirror many of the findings seen clinically and they can be manipulated to help determine underlying pathophysiological mechanisms. Arguably the most accurate SLE model occurs in females of the F1 cross between New Zealand Black and New Zealand White mice (NZB/W)4–6. Among the finest examples of translational research are that which occurred over several decades in work done at the National Institutes of Health by Alfred Steinberg, James Balow and colleagues; they first showed cyclophosphamide (CY) and methylprednisolone (PD) were efficacious in NZB/W mice7–10, followed by clinical studies in human SLE7 with this therapy remaining the “gold standard” by which all else is compared.
NZB/W mice have an increase in anxiety behavior and decreased exploratory behavior, which is increased with advancing age, indicating these behaviors were related to the development of autoimmune disease11,12. In studies using the MRL/lpr lupus mouse model, these mice explored the open field less, spent more time at home-base, had impaired exploratory activity and defecated less in comparison to congenic MRL/+ controls11,13–17. Lupus mice have a progressive disease over time, including NP-SLE14, which is in contrast to the periods of disease quiescence punctuated by intermittent flares seen in human SLE18. Although the mice differ in this aspect from humans, they give us insights and a better understanding of the underlying mechanisms that lead to disease and enable the development of therapeutic strategies.
Several alterations have been observed affecting the central nervous system (CNS) in lupus mice19,20. The cross-talk between the peripheral immune system and the CNS predicts that, particularly when there is an alteration of the blood-brain barrier (BBB)21–23, peripheral immune signals could result in glial cell activation. Activated astrocytes could secrete factors that promote neuronal survival, and could also initiate an inflammatory response, leading to neuronal death16,24,25, which could result in behavioral changes.
The CNS responds to injury by the process of gliosis which involves astrocytes, oligodendroglial precursors and meningeal cells. Astroglia are the most abundant glial cells in the CNS. They play a crucial role in maintaining normal brain physiology, integrity of the BBB and are a key component of the CNS response to injury and disease21,24,26,27. Astrocytes contain abundant intermediate filaments dispersed in the cell body and organized as thick bundles in astrocytic processes. Depending on the region of the brain, astrocytic intermediate filaments are either homopolymers of glial fibrillary acid protein (GFAP) or heteropolymers of GFAP and vimentin28–30. Reactive astrogliosis, in which astrocytes undergo hypertrophy or proliferation along with other histological and enzymatic changes, is a prominent feature in CNS inflammation31,32. Alterations in astrocytes could affect brain circuits that include interactions between neurons and astrocytes, thereby impacting behavior. Activation of astrocytes could lead to increased expression of the proteoglycan, syndecan, to provide a supporting environment for axons to regenerate at the site of brain injury33. Coupling of astrocytic and neuronal activities gives rise to membrane potential instability and oscillations34. The presence of autoantibodies to GFAP in serum correlated with NP manifestations in SLE patients35.
Although there has been progress in our understanding of the immunology and phenotype of lupus brain disease, our current therapy is still imperfect. The continued use of non-specific and potent immunosuppressive agents like CY and PD is less than ideal. There remains a balance between drug toxicity and efficacy. Both CY and PD cross the BBB. In addition, the BBB is compromised in lupus due to ischemia, endothelial cell activation and immune-mediated attack22,23. Neuropsychiatric symptoms persist even after attenuation of the systemic symptoms36. These studies gives insight into the complexity of the process and needs further investigation to understand the kinetics of transport and dose of the CY and PD that enter the brain. Pulse doses of intravenous CY are often used for NP-SLE7,8. Treated patients show considerable clinical and electrophysiological improvement of cerebral function37. Children with severe neuropsychiatric lupus also showed a favorable response to this treatment38. In a similar manner, CY treatment alleviated symptoms in lupus mouse models including reduced leukocyte (CD45) infiltration in MRL/lpr mice and prevention of behavioral changes such as floating in the forced swim test39.
Here we evaluated the effect of conventional CY/PD treatment on clinicopathological features of NP-SLE such as anxiety disorder and cognitive dysfunction. Our studies show that alterations in gliosis and extracellular matrix proteins tracked with behavioral changes in NZB/W mice, both of which were ameliorated by CY/PD treatment.
New Zealand Black and New Zealand White mice were from Jackson Laboratories (Bar Harbor, ME) and crossed in-house to obtain NZB/W F1 mice. Mice were maintained in 12 h light and dark cycles (lights off at 6:00 p.m., lights on at 6:00 a.m.) and a temperature-controlled environment (21 ± 1°C). All studies were approved by the Animal Care and Use Committee at the University of Chicago.
A total of 60 female mice were divided into three groups: 1) NZB/W mice treated from 22–44 weeks of age with CY/PD (each 3 mg/kg/d in saline) given once daily via intraperitoneal (i.p.) injection (n=20); 2) NZB/W mice treated identically, but without CY/PD (i.e., receiving saline vehicle only i.p.) (n=30); and, 3) NZW mice serving as controls (n=10).
Following behavioral testing at 44 weeks of age, animals were euthanized. For this, animals were first anesthetized with inhalational isoflurane. Animals were documented to be unresponsive to pain, prior to proceeding. Cardiac puncture was performed with a 21 gauge needle followed by cervical dislocation to ensure euthanasia prior to brain tissue harvest. These procedures are consistent with the Panel on Euthanasia of the American Veterinary Medical Association (https://grants.nih.gov/grants/olaw/Euthanasia2007.pdf).
Testing: All mice in a testing group were cage changed on the same day and no testing was performed until 1 day after a cage change. All behavioral testing was executed during the day, when the mice are normally active, with testing carried out in a behavioral testing room under normal light. To ensure the 5 min open field test was accurately measuring the activity of the mice, open field activity was measured over a 60 min period.
Preliminary workup: A series of preliminary observations40 of general health, home cage behaviors and neurological reflexes (eye blink, response to tail pinch and righting reflex) were first conducted for each mouse to avoid spurious false positives. All mice tested were groomed (the appearance of its fur and whiskers is noted), and moved around the cage normally.
Open-field analysis: Mice were assessed using an open-field activity monitor (Med Associates Inc., St Albans, VT, USA) for a period of 60 min. The testing chamber was wiped clean with water and then with ethanol between each test subject. The chambers were kept in a room used only for behavioral studies so that no movement or sound disturbs the behavior of the mice. Each subject was placed in the center of an open field apparatus. Measurements (calculated total distance traversed, number of movements, horizontal activity, vertical active and resting time and the beam–break counts for stereotyped behaviors) were recorded by accompanying software (Med Associates Inc, Activity Monitor, version 5.1) and calculated using Excel software (Microsoft, 2007). Ambulatory count and episodes were also recorded. Ambulatory count was defined as the number of beam breaks while the mouse is ambulating, while ambulatory episodes are the number of times the mouse begins ambulating (from a resting position). Data were collected over a 60-min period.
Maze: A circular maze having three chambers of 4, 8 and 12 inches was constructed. The mice were placed in the middle chamber. The time taken by the mice to escape from the maze was recorded.
Real-time quantitative (q) RT-PCR was performed on RNA from cerebral cortices dissected from NZW, NZB/W and CY/PD-treated NZB/W mice (n=10 each). RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and cleaned (RNeasy Mini Kit, Qiagen, Valencia, CA, USA). Total RNA (1 µg) from each sample was reverse transcribed with random hexamer primers using M-MuLV reverse transcriptase (Life Technologies). Ten ng of cDNA and gene-specific primers were added to SYBR Green PCR Master Mix (SYBR Green I Dye, AmpliTaq DNA polymerase, dNTPs with dUTP and optimal buffer components; Applied Biosystems, Foster City, CA, USA) and subjected to PCR amplification (one cycle at 50°C for 2 min, one cycle at 95°C for 10 min, and 40 cycles at 95°C for 15 s and 60°C for 1 min) in a TaqMan 5700 Sequence Detection System (Applied Biosystems). For each transcript, real-time PCR was conducted three times in duplicate using each of the RNA samples. The amplified transcripts were quantified with the comparative CT method using GAPDH RNA as the control (http://docs.appliedbiosystems.com/pebiodocs/04303859.pdf). The primers were designed using the Primer Express software (Applied Biosystems) based on the GenBank accession numbers; the sequences (5'−3') are as follows:
Sections of 6μm thickness were incubated with a blocking solution for 30 min, then with the primary antibodies overnight at 4°C. After washing in PBS, the sections were incubated in secondary fluorescence-conjugated antibodies (1:200) for 1 h at room temperature, washed in PBS and cover slips placed on them. Polyclonal antibodies used and their respective dilutions in PBS were: FITC-conjugated antibodies to mouse C3 (1:200, Cappel, 55500); rabbit anti-GFAP (1:200 Dako, CA, USA, Z0334), rabbit anti-collagen IV (1:60, Sigma, SAB4500369), anti-fibronectin (1:60, Sigma, F3648) followed by goat anti-rabbit-Alexa Fluor 594 (A-21207, 1:100), and 488 (A-11034, 1:100) respectively (Molecular Probes). Negative controls were generated by omitting either primary or secondary antibodies. Images were acquired with a Zeiss microscope. The sections were photographed maintaining the exposure time constant (Axiocam version 3.1; Zeiss).
Statistical analyses were performed using Minitab software (v. 17.1, State College, PA, USA). The number of control NZB/W mice included an estimated 40–60% mortality by 44 weeks and the positive effects of CY/PD treatment, with the resultant informative censoring of data41. Power analyses were done for one-way ANOVA with three levels, assuming α = 0.05 and β = 0.2 (power = 0.8). Sample sizes of 10 and 20 were sufficient to identify changes of 1.0 and 1.5 times the SD.
The data from each mouse in the study are presented graphically; group means are also depicted. Statistical significance was determined by one-way ANOVA; a P-value < 0.05 was used to reject the null hypothesis of no differences among the level means. Tukey’s method was then used for pairwise comparisons, with resultant P-values presented in the appropriate section.
The study was begun with 50 female NZB/W as well as 10 NZW control mice. At 22 weeks of age, 30 of NZB/W mice began treatment with CY and PD, which was administered during the full evolution of autoimmune disease, until 44 weeks when they were sacrificed. At 30 weeks the first untreated NZB/W mouse was found dead. Thereafter, there was progressive mortality, such that by the end of the study at 44 weeks only 15 NZB/W mice (50% of the starting number) remained. There were only two dead among the NZB/W that were treated (10% of the starting number) and no mortalities among the NZW controls (Figure 1).
A series of preliminary observations were first conducted prior to proceeding with testing31. These included assessments of general health, home cage behavior and neurological reflexes (eye blink, response to tail pinch, and righting reflex). All mice tested had appropriate grooming of fur and whiskers, and moved around the cage normally. The NZB/W mice had tail pinch reflex and their body weight remained the same.
Open field behavior in rodents is considered to be a fundamental index of their general behavior and inability to escape from a maze is considered as a sign of anxiety. As shown in Figure 2A, on average, control NZW mice traversed over 3,000 cm over 60 min. Untreated NZB/W mice had considerable hypoactivity compared to NZW control mice, which was largely prevented with CY/PD treatment (Figure 2A). Similar patterns were observed in ambulatory and stereotypic (i.e., animal rearing/vertical movement). The number of jumps did not differ between the groups (Supplementary Dataset 1). Control NZW mice escaped from the maze within 1–3 min, while untreated NZB/W mice demonstrated anxious/timid behavior by staying close to the walls of the maze, with all requiring more than 6 min to escape, including two animals that remained within the maze at 10 min (Figure 2B). Treatment with CY/PD reduced but did not fully reverse this phenomenon (Figure 2B).
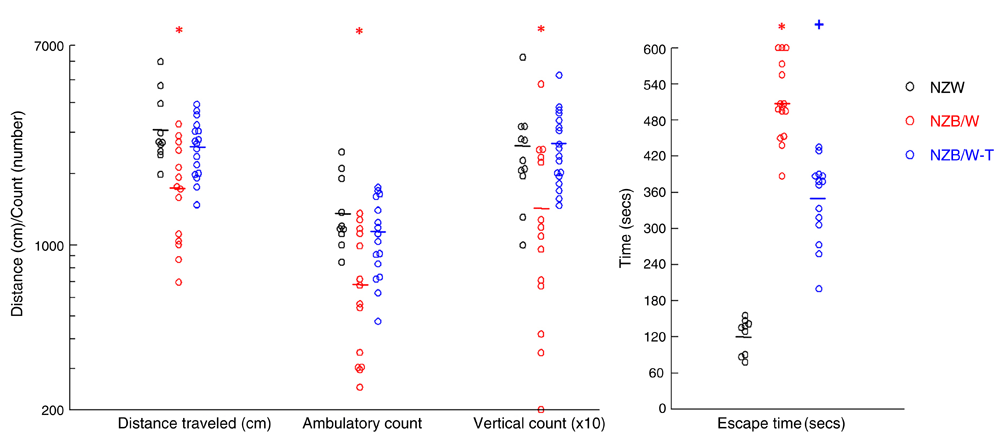
The ambulatory count, stereotypic count and average distance traveled by 44 week old NZW, and NZB/W mice treated CY/PD (NZB/W-T) or saline control were recorded for 60 min. Ambulatory episodes, vertical rearings and number of jumps were recorded for 60 min. The NZB/W mice covered significantly less distance and had significantly less number of vertical counts compared to their CY/PD treated counterparts. The NZB/W mice took longer to escape from the maze compared to the NZW controls, staying closer to the walls of the maze. Mice treated with CY/PD depicted less anxiety and escaped from the maze significantly faster than the untreated lupus mice. In all four measurements, the means were different in the three groups by ANOVA. *P < 0.05 vs. NZW and NZB/W-T. +P < 0.05 vs. NZW and untreated NZB/W.
| Jumps | ||
|---|---|---|
| NZW | NZB/W | NZB/W-T |
| 32 | 80 | 21 |
| 18 | 71 | 56 |
| 103 | 123 | 14 |
| 12 | 19 | 6 |
| 35 | 13 | 21 |
| 7 | 13 | 4 |
| 48 | 36 | 40 |
| 43 | 4 | 12 |
| 46 | 51 | 30 |
| 13 | 41 | 18 |
| 5 | 57 | |
| 4 | 14 | |
| 3 | 11 | |
| 24 | 13 | |
| 82 | ||
| 34 | ||
Upregulation of astrocytic intermediate filaments is a crucial step in astrocyte activation or astrocytosis. Therefore, we performed qPCR on cDNA derived from the brains of the mice in this study. As shown in Figure 3, the mRNA for GFAP, vimentin and syndecan 4 were all increased in NZB/W mouse brains. Treatment with CY/PD from 22 to 44 weeks of age almost completely prevented this upregulated expression of these genes (Figure 3).

RNA of mouse brains was reverse transcribed to cDNA and then subjected to real-time qPCR with gene-specific primers as described in Methods. Data are presented as gene expression relative to GAPDH. Each point represents data from NZW mice and NZB/W mice treated with either saline or CY and PD from 22 to 44 weeks of age. In all three measurements, the means were different in the three groups by ANOVA. *P < 0.05 vs. NZW and NZB/W-T.
The upregulated expression of GFAP mRNA in brains of NZB/W mice suggested the presence of astrogliosis (reactive astrocytosis). We therefore examined GFAP protein expression by IF microscopy. In NZW mice, there was low level expression of GFAP in the cortex (Figure 4A). In NZB/W mice, many GFAP-positive activated glia were observed in the cortex (Figure 4B), as well as the hippocampus (not shown). The number of GFAP-positive glia was reduced qualitatively by CY/PD treatment which tended to match the changes observed at the mRNA level for this protein.
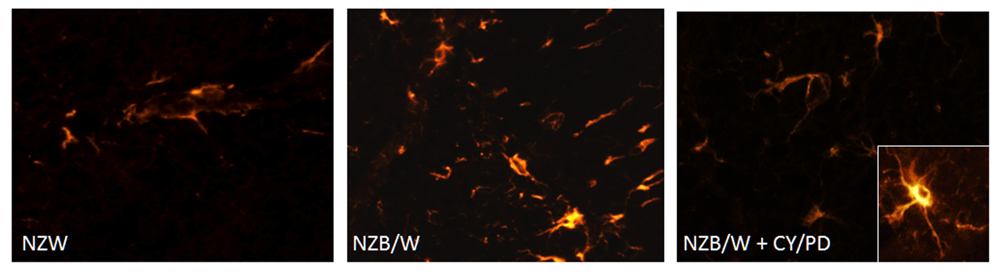
Representative sections from NZW, untreated NZB/W and CY/PD-treated NZB/W brains were stained for GFAP. Inset, is an astrocyte at 60x under oil. Note the more intense GFAP staining, thicker, shorter processes and swollen soma of astrocytes in the NZB/W sections compared with the controls and treated mice. CY/PD treatment significantly reduced the number of reactive GFAP-expressing astroglia in these brains.
Since syndecan 4 associates with fibronectin and collagen IV, we determined their expression in the brains of NZB/W mice and it followed the same pattern of expression as the others, with and without treatment. Fibronectin (Figure 5A) and collagen IV (Figure 5B) were localized around the microvasculature. CY/PD treatment prevented the increase in extracellular matrix protein expression in lupus brains.
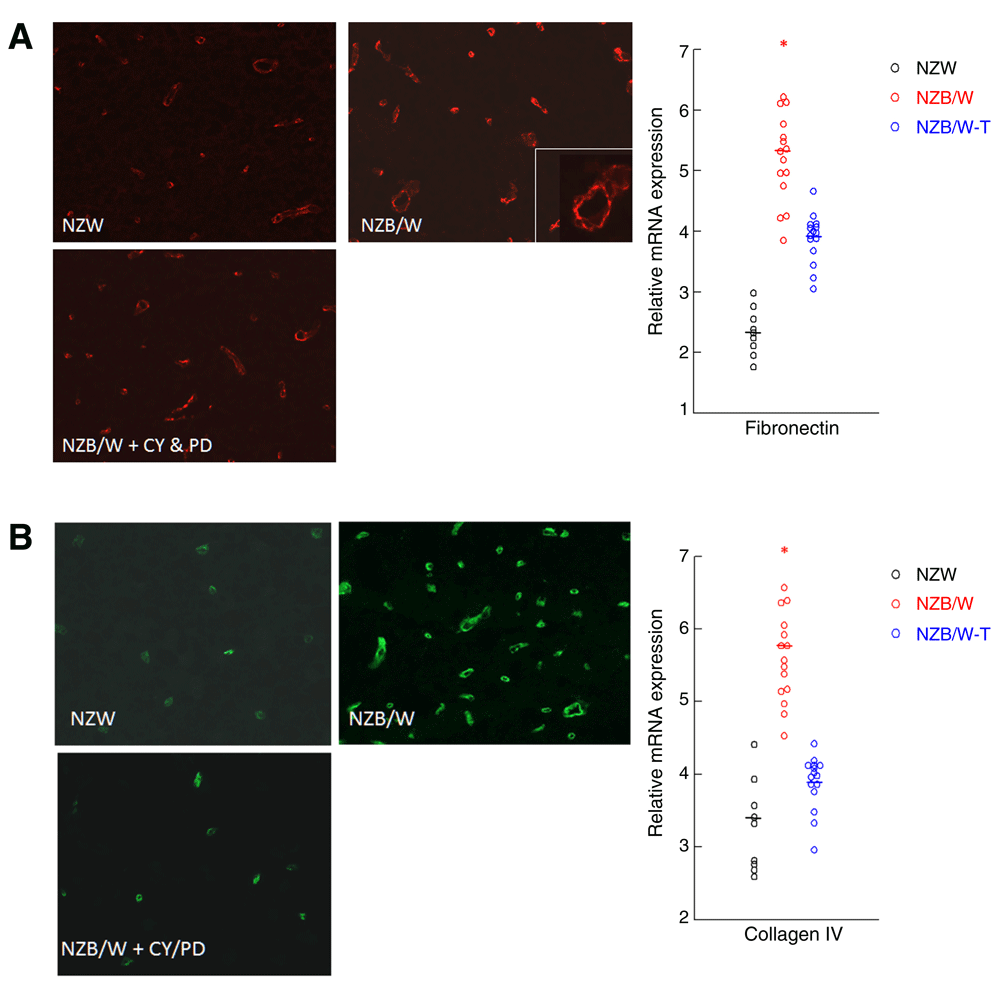
Accumulation of fibronectin (A) and collagen IV (B) occurs in NZB/W lupus mouse brains which is reduced by CY/PD treatment. Representative cryosections were immunostained with rabbit anti-mouse fibronectin and detected using Alexa 594 labeled anti-rabbit antibody (A) and rabbit anti-mouse collagen and detected using Alexa 488 labeled anti-rabbit antibody (B). Fibronectin and collagen IV was localized mainly around the microvasculature. RNA of mouse brains was reverse transcribed to cDNA and then subjected to real-time qPCR with primers for fibronectin and collagen IV as described in Methods. Data are presented as gene expression relative to GAPDH. Each point represents data from NZW mice and NZB/W mice treated with either saline or CY and PD from 22 to 44 weeks of age. CY and PD treatment reduced the expression of these extracellular matrix molecules in these brains to levels observed in control NZW brains. In both measurements, the means were different in the three groups by ANOVA. *P < 0.05 vs. NZW and NZB/W-T.
SLE is a complex, autoimmune disease, and considerable work has been done in the field. Yet, the mechanism underlying the CNS pathology, with its tight regulation and specialized microenvironment, is not completely understood. It is a complex, systemic disease that might be mediated by the synergistic action of many factors. As expected in the setting of an autoimmune disease, IgG deposits were significantly increased in NZB/W lupus mice compared to the NZW controls, which was prevented by CY and PD treatment. Using the quantitative real time PCR technique, we observed increased expression of GFAP, vimentin and syndecan 4 genes in the cortex, which was prevented by CY and PD treatment.
Astrocytes play a major role in regulating immune responses within the CNS and express major histocompatibility complex molecules required for antigen presenting cellular activity47. During neurodegeneration, astrocytes are activated and release both proinflammatory cytokines and chemokines48,49. Our results give insight into the potential contributions of intrinsic astroglial cellular hyper-responsiveness in the development of NP-SLE. Recent studies have shown that GFAP levels in SLE patients with CNS manifestations were 3-fold higher than those patients without CNS involvement, which was prevented by CY treatment. Furthermore, the GFAP level in these patients significantly correlated with MRI abnormalities50. A significant positive correlation was observed between anti-GFAP serum antibodies and NP manifestations35.
Although we cannot exclude (a) a possible relation between health deterioration and the behavioral abnormalities and (b) the exposure of brain to systemic toxins in the context of loss of BBB integrity, we believe these studies are important as many of the changes were also observed in preclinical mice of 6 weeks of age and changes persisted after alleviation of systemic symptoms. The NZB/W mice display more anxiety behavior, less activity, and less exploratory behavior than non-autoimmune female NZW mice, prior to and during disease emergence at 6 and 12 weeks of age, respectively11,12,51. The behavioral alterations observed prior to clinical disease suggest the participation of a genetic component to these differences. Concomitant with our findings of increased expression of astrogliosis and alteration in extracellular matrix proteins, the NZB/W mice showed significantly less activity than the NZW mice, based on the open-field behavior. Furthermore, they also demonstrated increased anxiety. These changes in behavior were prevented by treatment with CY/PD. These findings were similar to the alleviation of neuropsychiatric behavior observed in SLE patients treated with CY/PD37.
Finally, this is the first attempt to understand the effect of CY/PD treatment in experimental NP-SLE, in a comprehensive manner. The treatment significantly alleviated both molecular and behavioral alterations in these mice, similar to humans. Future investigations elucidating the precise signaling mechanisms by which astrocyte activation occurs, prevent regeneration of neurons and contribute to the pathology observed in brain, in SLE, will reveal a critical location for therapeutic intervention in NP-SLE. In addition, GFAP in the cerebrospinal fluid could be used as indicators of brain damage and should be used as a follow-up tool in these patients.
F1000Research: Dataset 1. Raw data for Figure 2, 10.5256/f1000research.6568.d4971442
F1000Research: Dataset 2. Raw data for Supplementary Figure S1, 10.5256/f1000research.6568.d4971743
F1000Research: Dataset 3. Raw data for Figure 3, 10.5256/f1000research.6568.d4971544
F1000Research: Dataset 4. Raw data for Figure 5, 10.5256/f1000research.6568.d4971645
F1000Research: Dataset 5. Behavioural data, 10.5256/f1000research.6568.d4971846
JJA and AJ performed the studies. JJA and RJQ planned and designed the experiments and analyzed data. JJA, AJ, and RJQ wrote the manuscript.
This work was supported by National Institutes of Health grants R01DK041873 and R01DK055357, and by the Arthur M. Morris Endowed Chair to RJQ.
I confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 22 Jan 18 |
||
|
Version 1 23 Jun 15 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)