Introduction
“It is now well established that some micro-organisms can, under certain conditions, be deprived of all visible signs of life and yet these organisms are not dead, for, when their original conditions are restored, they can return to normal life and activity.”1
“Bacterial populations in both batch and continuous culture are much more heterogeneous than is normally assumed, and such cultures may consist of several types of subpopulations simultaneously differing in viability, activity and integrity of the cells.”2
Consider a typical axenic flask or broth culture of bacteria (Figure 1), arguably the staple of modern laboratory microbiology. We seed a suitable growth medium with an appropriate inoculum of cells known to be capable of replicating in that growth medium. After a lag phase the number of culturable cells (the ‘viable count’3,4, as judged by plate counts of the number of colony-forming units observable on the same medium solidified by agar or a similar material) is observed to increase, typically exponentially, for a number of generations (the growth phase or exponential phase). Apart from the changes in nutrient concentration, and for non-synchronised cultures, it is generally taken that cells pass smoothly through their cell cycles en route to doubling their numbers by binary fission. The population distribution of organisms in different parts of their cell cycle during the exponential phase is thereby unchanged and thus in a steady state (from which the cell cycle parameters can even be inferred5). In time this increase in cell numbers ceases, usually because of the exhaustion of a nutrient in a closed system, or sometimes in part or whole because of the build-up of toxins. Again, after a further period, the viable or colony count decreases (often to quite low levels if such starvation is carried out for extended periods). Inoculation of a new broth culture with a similar number of viable cells from this culture usually provides a simple repeat of the previous culture6, and in the absence of mutation may reasonably be anticipated, for organisms proliferating asexually, to be played out indefinitely.
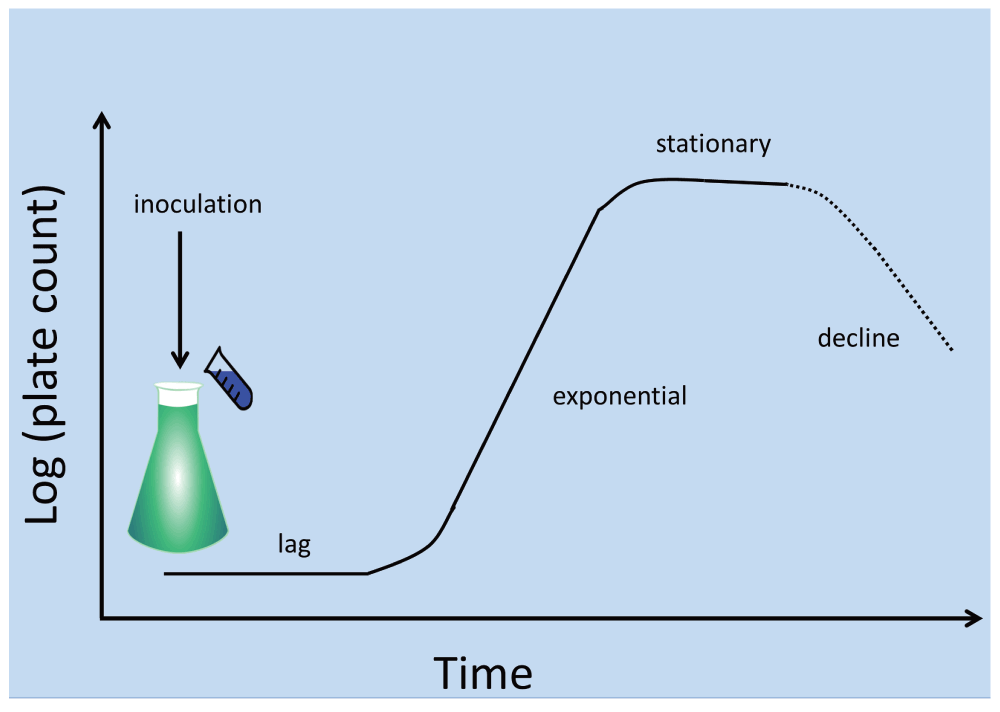
Figure 1. A typical laboratory bacterial culture.
After the end of stationary phase the viable count decreases over time, but very rarely to precisely zero. Some authors recognise an extended “period of prolonged decrease”825 during which some of the survivors undergo significant dynamics, and in which mutants are selected. Our interest here is largely in cells that have not mutated.
The development of continuous7, nutrient-limited (‘chemostat’8) or feedback-controlled (‘turbidostat’9–11) cultures was and is entirely consistent with this view of steady-state microbial doubling via homogeneous cell cycles that are common, within statistical fluctuations, to each cell. The same is true for cultures undergoing serial transfer (where there is slightly more of a focus on selection for genotypic variants that grow faster – see e.g. 12–14).
There should be nothing controversial in the above passage, but in fact it hides a variety of assumptions that themselves conceal a considerable feast of very interesting physiology. The chief one here is that – given that all cells in the culture are genetically homogeneous and see the same ‘environment’, and modulo where they are in their cell cycles – all such cells are indeed supposed to represent a single population (as per Figure 2). If they do not, and as we shall see they never do15–18, we are dealing with differentiated systems. It turns out that a particular subset of typical cell cultures – a phenotypically dormant or non-growing sub-population, occurring even in non-sporulating bacteria2 – is widespread to the point of ubiquity. This leads to an exceptionally important biology with significant consequences both for our understanding of microorganisms and our ability to harness and domesticate them. Although the relevant literatures rarely cite each other or overlap, it is clear that similar phenomena are common to bacterial behaviour in the natural environment, the laboratory, and in a variety of samples of clinical interest. This theory or hypothesis that we develop here comes about from the synthesis19 of a large amount of data, and is summarised in Figure 3 and Figure 4.
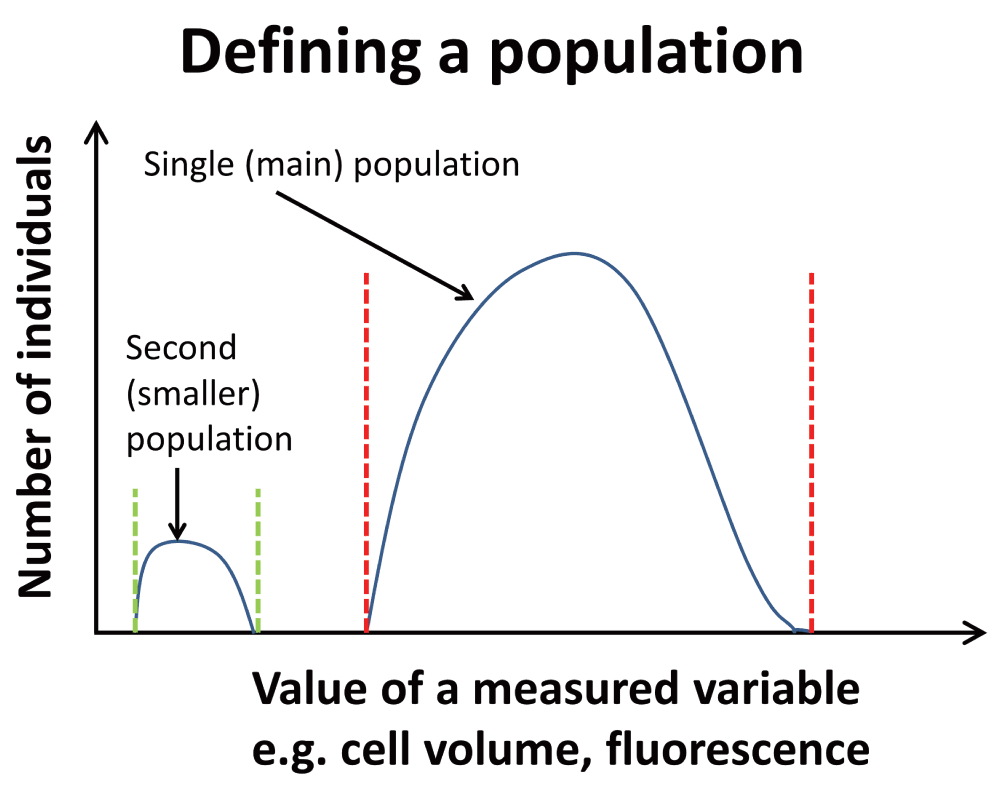
Figure 2. To clarify the general concept of a population as used here, a population of individuals involves those who share certain properties (between stated values).
One main population is shown. A second, smaller population is also shown; these might represent dormant cells.
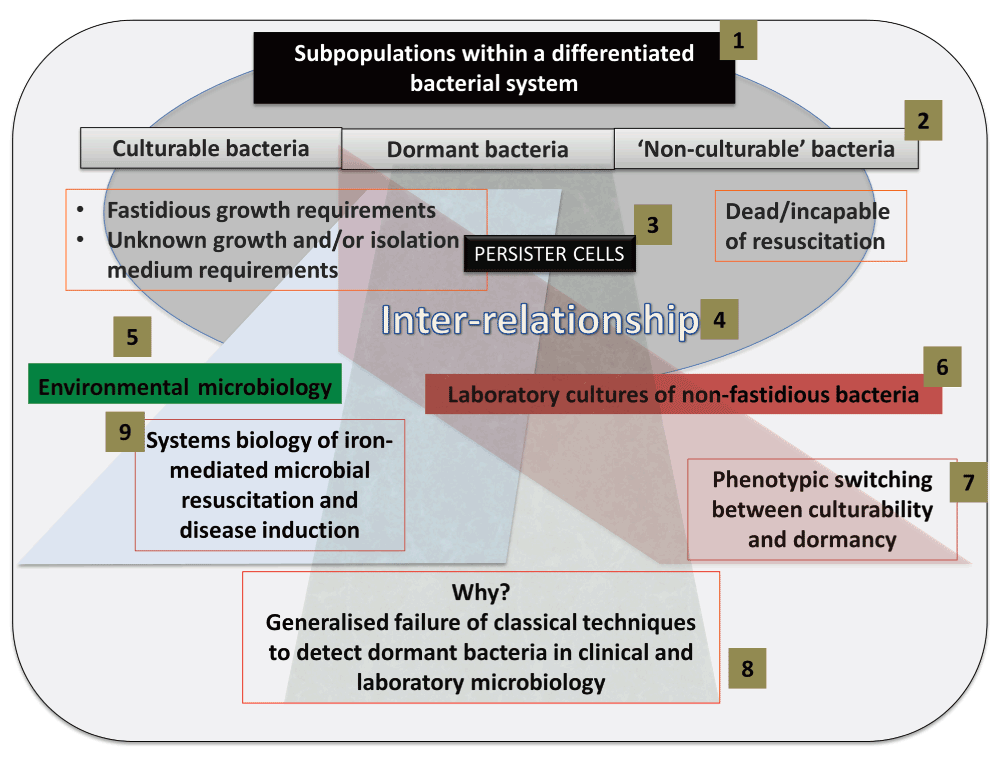
Figure 3. Infographic summary of the review.
(1) A bacterial system contains distinct subpopulations, that we classify as culturable, dormant and ‘non-culturable’ (2). Specific attention is given to persister cells (3), and the inter-relationship (4) between the subpopulations. Subpopulations within environmental biology are discussed (5), followed by subpopulations within laboratory cultures (6). Particular emphasis is placed on phenotypic switching between the culturable and dormant subpopulation of laboratory cultures (7). Generalized detection techniques typically fail to detect dormant cells, and we review the various reasons for this failure and discuss alternatives (8). Resuscitation of and endotoxin production by such dormant cells underpins many diseases not normally seen as having a microbial component.
Figure 4. Summary of the review in the form of a ‘mind map’826 of the article.
Phenotypic differentiation to dormancy – some early indications
While dormancy and resuscitation of rotifers had been observed by Leeuwenhoek himself in 17021, some of the earliest modern indications for a physiologically significant phenotypic differentiation of microbial cultures came in the 1940s. In a conceptually simple experiment (illustrated in Figure 5), Bigger20 exposed staphylococcal cultures to concentrations of penicillin that would normally be sufficient to kill them completely (and they did kill all but 1 in a million). However, these (10-6) survivors, that Bigger20 and McDermott21 (and many modern commentators have) referred to as ‘persisters’, were not genetic mutations selected for resistance to penicillin, since when they were inoculated into fresh broth they were just as susceptible as were those in the first culture. Bigger recognised (correctly) that the only explanation that made any kind of sense was that despite being exposed to nominally the same conditions, these cells were operationally dormant (even if metabolically active22,23) and thus phenotypically resistant to the penicillin (that anyway kills only dividing cells24,25). Similarly, Luria and Latarjet26 noted that approximately 1% of the cells in a culture of Escherichia coli displayed a phenotypic resistance to normally sterilising doses of ultraviolet irradiation. Many similar experiments since (e.g. 27–29), discussed in more detail below, have recapitulated this basic phenomenon. (We note here that high-frequency antigenic ‘phase’ variation can occur due e.g. to changes in microsatellite DNA30; detailed discussions of such genotypic changes31, including those that can affect the extent of dormancy in persistent bacteria32, are outwith the scope of the present, purely phenotypic analyses.)
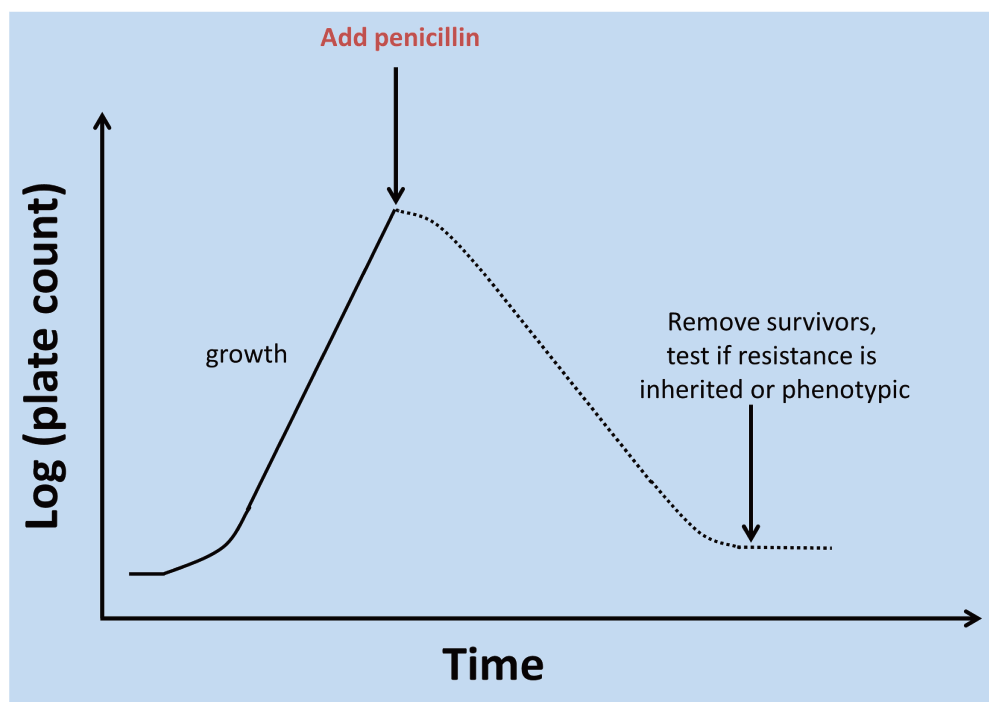
Figure 5. Assessment of phenotypic differentiation of a dormant subpopulation via antibiotic challenge.
This kind of protocol can be used to determine if the resistant subpopulation has accumulated genetic mutations that encoded resistance or whether, as focused on here, the resistance is purely phenotypic. A detailed analysis of the shape of the time-survivor curves may also be informative827.
Dormancy as an operational property
For the avoidance of doubt, and in accordance with Keilin’s description with which we opened, we shall define dormancy as:
“a reversible state of {often} low metabolic activity, in which cells can persist for extended periods without division; we shall see that this often corresponds to a state in which cells are not 'alive' in the sense of being able to form a colony when plated on a suitable solid medium, but one in which they are not 'dead' in that when conditions are more favourable they can revert to a state of 'aliveness' as so defined”2.
We thus stress33 the recognition that dormancy is not solely an innate property of a bacterial cell; it is a property assessed by one or more experiments, so whether a cell appears to be dormant depends on both the cell and the experiment used to assess that dormancy. (This principle shares a similar philosophical foundation to the independence from any specific experiment, or otherwise, of the perceived state of objects within the quantum theory33–35.) As do Postgate3,4,36 and Barer37–41, we take the hallmark of a viable or living bacterial cell to be its ability to replicate or its ‘culturability’. This means that we cannot tell via culturability that a cell is alive, only (after a cell division) that it was alive33,42. Dormant cells – even if ‘not immediately culturable’ – must by definition be resuscitable to form culturable cells. Although the term ‘nonculturable’ is quite commonly used to describe not-immediately-culturable cells it is best avoided, as we cannot try every possible combination43 of incubation conditions that might serve to resuscitate a cell in a sample. ‘Non-cultured’, ‘as-yet-uncultured’ or ‘operationally nonculturable’ are better terms. Culturable, (operationally) non-culturable and (operationally) dormant bacteria in the differentiated bacterial (cellular) system can therefore be seen as distinct subpopulations of the system, and culturable and dormant bacteria as reversible states of the same population. The relationships between such subpopulations of the bacteria within a differentiated cellular system are shown in Figure 6.
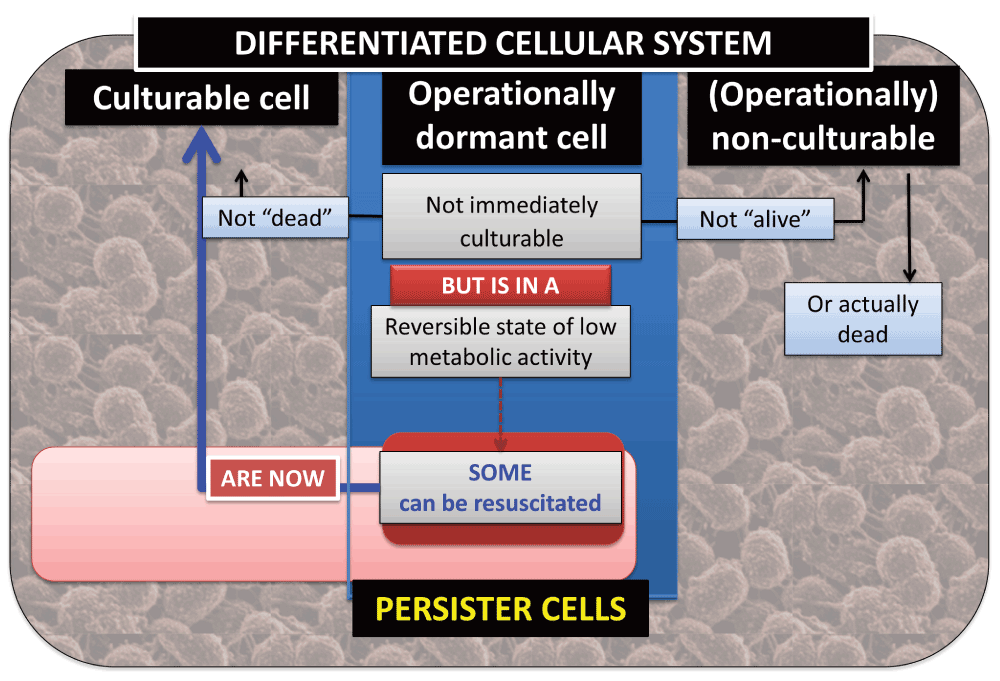
Figure 6. The relationships between culturable, dormant and operationally non-culturable bacteria within a differentiated cellular system.
On methods for detecting microbial presence, ‘viability’ and culturability
Given our operational definition of dormancy as including reversible culturability, we note that different kinds of assays for the presence or activity of bacteria necessarily reflect cells in different kinds of physiological states (and can thereby be used to discriminate them). Thus direct counts with stains such as acridine orange (a list of these and other methods is given in Table 1 of 33) do not determine culturability, only presence or activity. Similarly, macromolecular sequencing methods such as those based on rDNA and its amplification (e.g. 44–49) almost certainly reflect mainly dormant cells plus any actively dividing ones (in that ‘naked’ DNA is usually degraded fairly rapidly in serum or the environment). The difference between culturable counts and total sequence-based counts probably provides one of the best methods for detecting and enumerating dormant cells when they cannot yet be brought back into culture. It is particularly noteworthy (and see also 50 and below) that the amount of prokaryotic DNA in whole blood exceeds by 10–100-fold that detectable in serum51, implying adsorption onto or sequestration within blood cells.
We shall return to clinical and laboratory microbiology later, but it is to environmental microbiology that we now turn to discuss the culturability of typical microbes. While the same general truths undoubtedly pertain in viruses (e.g. 52,53), and in yeasts, fungi, archaea, mycoplasmas and other unicellular organisms, our focus will be on prokaryotes.
Bacterial culturability and dormancy in environmental microbiology
It has long been known that the number of bacteria observable microscopically exceeds, typically 100-fold, those that can readily be grown axenically in standard isolation media (i.e. to proliferate in liquid culture or to form colonies on solid media). The latter has been referred to as ‘the great plate count anomaly’54, and has been amply confirmed by more modern, culture-independent sequencing methods. A selection of papers and reviews serve to document both the numerical anomaly and the much greater biodiversity detectable by sequencing (e.g. 55–73). It is thus useful to discriminate (1) bacteria that have been cultured, that are typically available in culture collections, and whose growth requirements are known, from (2) bacteria that may be recognised as novel via macromolecular sequencing (typically of ribosomal DNA68,74–77) but that have not yet been cultured and whose growth requirements may not yet even be known. Much (sequencing) evidence indicates that the bulk of the ‘missing microbes’ or ‘dark matter’78,79 in natural ecosystems falls into this second category80, and that ‘single cell’ methods may be required to culture them81.
There are at least four general reasons of principle why these organisms have not yet been cultured. We consider each in turn (although more than one may contribute in individual cases).
Not-yet-cultured bacteria may have more-or-less fastidious growth requirements
It is an elementary observation in microbiology, and the basis for selective isolation media, that not all bacteria grow on all media and in all conditions. Leaving aside truly syntrophic bacteria (that for thermodynamic or unknown nutritional reasons require another organism for growth (e.g. 82–88)), some organisms may have quite fastidious growth requirements. A number of bacteria determined as causative of disease, whose role had originally been inferred only through microscopic observation, were later cultured and could be shown to fulfil Koch’s postulates. These include Helicobacter pylori89,90 (with an unusually high requirement for urea to fuel its alkalinogenic urease activity91) and Legionella pneumophila92–95 (with an unusually high requirement for cysteine). Note that even the supposedly rich LB medium96 (Lysogeny Broth, often erroneously called Luria-Bertani medium, see http://schaechter.asmblog.org/schaechter/2009/11/the-limitations-of-lb-medium.html) is not in fact a particularly rich medium97–99. An especially nice example100,101 is provided by Tropheryma whipplei, the causative organism of Whipple’s disease102,103. It resisted attempts (over many decades) to bring it into axenic culture until systematic genome sequencing104,105 showed its requirements for a variety of common amino acids that it was unable to synthesise itself, the provision of which permitted its growth. The MetaGrowth database106 is now available for similar purposes. Another good example is Coxiella burnetii, the causative agent of Q fever, for which a genome-derived growth medium (‘acidified citrate cysteine medium’) permitting axenic culture has now been developed107,108. Other examples are given by Stewart109 and by Singh and colleagues100, and include marine bacteria of the highly common SAR11 clade71,110,111. Of course these kinds of phenomena are not absolute; much evidence indicates that host stress hormones may act as growth or virulence factors for a variety of Gram-negative organisms, representing a kind of ‘microbial endocrinology’ (e.g. 112–114).
Not-yet-cultured bacteria may even be killed by our isolation media
Organisms in nature are often living in low-nutrient conditions115–119. It is thus reasonable (and unsurprising) that the isolation of microbes from starved, oligotrophic environments benefits from the use of low-nutrient conditions63,109,120–122; some manifest this ‘starvation’ through their size, as ‘ultramicrobacteria’ (see e.g. 123–129). In a similar vein, taking cells from low-nutrient natural environments directly onto, say, a highly aerobic agar plate may produce stresses that effectively kill them, so that afterwards they would not even grow on the kinds of media (as in the previous section) that would support their growth. Thus, Tanaka and colleagues130 showed interactions between phosphate and agar when autoclaved together that led to the production of compounds inimical to bacterial growth. Gellan may be a better solidifying agent here82. However, we recognise that it may be hard to discriminate cells that we kill in the act of trying to isolate and grow them from ‘already dead’ bacteria.
Not-yet-cultured bacteria may simply be dead and thus incapable of resuscitation
While this possibility certainly exists, and is included for completeness, it is actually the least likely for a number of conceptual and empirical reasons. The first is that if an organism is present in a particular environment it must have been able to grow and divide in it at some point in the more or less recent past, even if the result of such growth was its utilisation of a finite amount of necessary nutrients or growth factors whose exhaustion caused replication to cease. (Interestingly, in soil it seems that sequestration, rather than complete exhaustion, of nutrients is the more significant phenomenon131–133.) Secondly, it is highly unlikely that evolution could select for unicellular organisms that cannot replicate. Thirdly, environmental organisms can be shown to metabolise even when they cannot be shown to divide (e.g. in the ‘Direct Viable Count’ method134 and in any number of other tests that detect metabolic activity33,135). And finally, as we shall see in the next section, careful methods of resuscitation/cultivation do indeed allow a very significant fraction of organisms that can be isolated from a variety of environments (e.g. the gut136–139) to be resuscitated and to grow very effectively.
Not-yet-cultured bacteria are mainly dormant and thus resuscitable
As indicated in the introduction, it is now well established that even laboratory cultures, that from a macroscopic point of view are growing exponentially, contain subpopulations of non-growing cells. These cells are dormant by definition, because they may later be resuscitated and grow. It is easy to ascribe an evolutionary advantage of this culture differentiation from the perspective of the benefits of having a sub-population that by not growing is more resistant to environmental stresses (e.g. 140–142). Indeed, this general kind of phenotypic differentiation strategy, in which the variance in reproductive rate is traded off at the expense of the mean, has been referred to as bet hedging66,142–152 and is actually adaptive153,154. An important point here153 is that in many natural environments, asexually reproducing organisms such as bacteria are likely to be (spatially) close to their ancestors and descendants, such that inclusive fitness theory155,156 implies that it is entirely reasonable for them to behave altruistically, e.g. by ‘bet hedging’. This is also discussed further below.
It is also reasonable that in isolated (closed) natural environments, nutrients and thus sources of energy must be exhausted at some point, and thus for simple energetic reasons multiplication becomes impossible and a dormant state likely (if later resuscitation proves it to be so). Similarly, it is likely that in the absence of energy, nutrients and/or signalling molecules, and based on more ecological or community considerations (e.g. 157–159), it is necessary to add any or each of them to ‘prime’ bacteria to resuscitate. This has indeed been shown58,159–163, including for sources of energy164,165, iron-acquiring compounds166 (siderophores167–169), cell wall muropeptides170, and various signalling molecules171,172 (especially pheromones153,154,173,174) that exist in natural environments58,159,175. We note too that ‘kick starting’ dormant cells may require the synthesis of transporters necessary for the uptake of all kinds of molecules176–179. Overall, the idea that most bacteria that may be observed in the natural environment are ‘unculturable’ is incorrect.
Finally here, and though this is obvious it is well worth rehearsing, the simple fact that we can store non-growing microbes under desiccated or frozen conditions or as agar ‘stabs’ in culture collections for extended periods means that most microbes are certainly well adapted to entering and leaving dormancy.
Pheromonal proteins
A related and unexpected discovery came from analyses of starved laboratory cultures of the actinobacterium Micrococcus luteus, in which almost all cells lost culturability2,180–182. However, they were not dead but dormant, as they could be resuscitated by using a combination of weak nutrient media and a signalling molecule found in spent culture supernatants183–188. The original studies used flow cytometry to discriminate the physiological state of individual cells189–193 (see also 194,195). By using another ‘single cell’ assay based on dilution to extinction (that avoids artefacts connected with the regrowth of ‘initially viable’ bacteria33), we were able to purify the signalling molecule. It turned out to be a protein, named Rpf (for ‘resuscitation-promoting factor’)196. In M. luteus there is only one homologue197, and the gene (product) is essential for both resuscitation and multiplication196,198. Rpf contains a highly conserved 70 amino acid ‘Rpf domain’ and is widely (and probably ubiquitously) distributed throughout the actinobacteria199–202, but with examples elsewhere203,204. Most organisms that have a homologue have more than one. Thus M. tuberculosis has five homologues205–207. Rpfs can have peptidoglycanase and muralytic activity208–213 and known crystal structures are consistent with this214–219. These activities can certainly account for at least some220 of the resuscitation-promoting properties. As an extracellular protein that may be required for growth, and with a high level of immunogenicity221, it is obviously an excellent candidate target for inclusion in appropriate vaccines against pathogenic actinobacteria196,208,222–229. It is also more directly of potential utility in stimulating bacterial communication and resuscitation in a variety of cultures in both samples taken from Nature230–240 and in the laboratory241–254.
Culturability, dormancy and persistence in laboratory cultures of non-fastidious bacteria
Having established the frequency of occurrence of microbial dormancy in the natural environment, it is of interest to understand better the mechanisms by which microbes might effect this dormancy and potential resuscitation. Unsurprisingly, microbiologists have turned to E. coli, and considerable progress has been made23,255–262.
The starting position is as in Figure 1 and Figure 6, to the effect that at any given moment in a typical culture a small fraction of the population is dormant. Since clearly the same fraction cannot (or is wise not to) remain in dormancy indefinitely in the presence of suitable nutrients that permit the growth of its siblings, we must invoke at least one mechanism that can cause the bacteria to ‘oscillate’ between growing and dormant states. Many simple gene expression network topologies admit this behaviour145,263–267, including a simple feedback loop with delay268,269, and we note that even whole cultures can exhibit oscillations and deterministic chaos270. While flow cytometric observations (e.g. 192,271) show that even ‘homogeneous’ laboratory cultures show highly heterogeneous distributions in cellular volume (not just between X and 2X) and expression profiles (and see 272), our particular focus will be on ‘binary’ or ‘bistable’ systems in which individual cells either are or are not operationally culturable.
Experimentally, it is also common to assess the phenotypic ability of subpopulations of cells to tolerate normally inhibitory concentrations of bactericidal drugs273,274, this being a marker for that fraction of cells that is dormant at the stage in question. Note that the persistence phenotype is not induced by the drugs258. Changes or transitions in the state of a particular cell in a population between the various phenotypic states is a phenomenon that may be (and is commonly) referred to as ‘phenotypic switching’.
‘Phenotypic switching’ in experimental laboratory cultures
A particularly well-developed example of this ‘bet hedging’ or phenotypic switching between physiologically dormant and growing states may be observed in laboratory cultures of organisms such as E. coli demonstrating ‘persistence’147,150,151,275–281. In general, any scheme in which both a first gene product inhibits cellular proliferation and in which this first gene product may be titrated out potently282 by a second gene product that thereby undoes the inhibition of proliferation, can have the effect of phenotypically switching cells between growth and dormancy. This seems to be precisely what is going on, and such pairs of gene products have been referred to (somewhat misleadingly283) as toxin-antitoxin (TA) pairs283–290. One such involves the well-known pp(p)Gpp metabolic system that can serve to inhibit DNA gyrase23,291–294, and points to the fact that in these circumstances, persisters may be quite metabolically active22,23,292,295, even if transiently incapable of reproduction. Another phenotype switching mechanism, underlying colony phenotype switching, comes from metabolic bifurcations driven by the levels of a particular metabolite296.
Any mechanisms that permit cells to communicate with each other can amplify switching effects by cell synchronisation, and by definition such ‘social’ signals act as pheromones, whose apparent ‘altruism’ can be explained on the basis of kin selection theory153. There is considerable interest, largely outwith our scope here, in these evolutionary aspects (e.g. 297–304). Such systems are commonly, but far too broadly relative to the term’s origin305, referred to as ‘quorum-sensing’. However, they do offer opportunities for limiting bacterial virulence (e.g. 306–313).
Classical clinical microbiology of culturable organisms
Until relatively recently, almost all of clinical microbiology314,315 was based on rather classical methods of plate counting316, coupled to assessment of antibiotic sensitivity. Various means of automated blood culture that assess metabolism exist (although they require typically 48–72h to show a ‘positive’)317. Positive tests, often implicitly involving culture (and not just metabolism) within the assay, would be followed by other tests seeking to identify the organisms detected, nowadays typically by nucleic acid sequence-based methods49,318–321. However, these and other tests for the presence of antigens or even antibodies322 cannot speak to the question of culturability (and of course antigens such as lipopolysaccharide (LPS) are shed by dying cells).
The existence of bacterial DNA in even ‘healthy’ blood has long been known323, and since naked DNA would be degraded and living cells would soon kill the host, the (seemingly) obvious conclusion that the prokaryotic DNA must reflect dormant cells seems neither to have been drawn nor acted upon.
Some well-established cases of dormancy in clinical microbiology
The idea that (typically intracellular) dormancy is a major component in some infectious diseases (including in the absence of antibiotics that may serve to light up ‘persisters’) is of course well-established, and the main purpose of this brief section is simply to remind readers of this. Such a reminder serves as a prelude to a longer discussion of the very many clinical circumstances where we consider that the role of dormant microbes is not widely appreciated, and where they are not really considered to involve a communicable or microbial component at all. Thus Table 1 shows a few organisms (and references) for which we consider that most readers would regard the idea of and evidence for dormancy as more or less uncontroversial. We do not include disease-causing infectious agents where they are better known for their ability to persist in the natural environment. Organisms such as Legionella pneumophila that represent significant public health issues, fall into this category, and Legionella and other persisters (in environments such as water system biofilms) are indeed well known (e.g. 324–328), although they too have special adaptations to an intracellular lifestyle (e.g. 329).
Table 1. Some bacterial infections for which an intracellular, reversibly non-replicating, persistent or dormant state is well established as part of the cells’ lifestyle.
Examples are given for both low- and high-GC Gram positives, as well as a number of Gram-negative organisms.
| Organism | Comments | Selected
References |
|---|
| Bartonella spp. | Persists inside erythrocytes | 330–333 |
| Brucella spp. | Environmental and intracellular persistence and immune evasion | 334–337 |
| Listeria monocytogenes | Well-established low-GC Gram-positive intracellular saprophyte
and non-sporulating persister | 338,339 |
| Mycobacterium tuberculosis | The ‘classical’ dormant bacterium, a high-GC Gram-positive;
probably one third of humans carry it in a dormant state | 340–348 |
| Salmonella typhimurium | Gram-negative; non-replicating forms common in macrophages
and elsewhere | 349–352 |
| Staphylococcus aureus | Low-GC Gram-positive; can escape antibiotics by hiding inside
various phagocytes | 353–356 |
Generalised failure of classical techniques to detect dormant bacteria in clinical microbiology
As noted above for environmental microbiology, dormant bacteria can represent as much as 99% of the organisms that may be observed microscopically or by macromolecular sequencing, but classically (and by definition) they are not enumerated by culture-based methods that determine ‘immediate culturability’33. Such culture-based methods are also widely used in clinical microbiology. However, if we were to plate out 100 μL of a culture containing 200 bacteria.mL-1, of which 99% were dormant at any instant, we would expect (based on a Poisson distribution) to see fewer than 1 propagule or colony-forming unit per sample. We have noted above that it can be determined by sequencing that many of the non-cultured environmental organisms largely differ from those in standard culture collections. Certainly the examples given above in clinical microbiology, such as Tropheryma whipplei, were both observed microscopically and were sequenced prior to being brought into axenic culture.
The PCR method is exquisitely sensitive (down to one cell or propagule per sample), and we note that contamination artefacts from the PCR reagents represent a real issue that must always be checked (e.g. 357–361), albeit this is no less true of blood cultures362. We have rehearsed elsewhere50 five classes of argument that collectively make it implausible that these are all contamination artefacts; probably the most persuasive is simply the sheer number of prokaryotic DNA molecules that can be measured in blood and serum (e.g. 363–365). While some of the most recent nucleic acid sequencing methods (e.g. 366–371) do operate on single molecules, the analysis of prokaryotes usually used a broad-range PCR step to amplify small-subunit rDNA to assess their presence, whether in environmental62,68,75,372 or clinical370,373–385 samples. Using this, and while these methods alone cannot tell whether they were operationally dormant or dead, a very considerable number of studies have been performed in which ‘culture-negative’ clinical samples showed the presence of prokaryotes (at least as judged by sequence-based methods). This has some profound consequences.
Broad-range PCR methods indicate the widespread presence of prokaryotic DNA in culture-negative clinical samples
While PCR-based methods have long been used to assess the species involved in culture-positive samples386, e.g. from blood, our interest here is in samples that are culture-negative387 that may yet (and indeed likely do) contain dormant cells. Among the first such indications of this was the study by Relman’s group323, who showed that the blood of even healthy controls contained significant amounts of prokaryotic DNA. Table 2 lists some studies in which broad-range PCR has been used to amplify and detect prokaryotic rDNA in culture-negative samples.
Table 2. Some examples of blood culture-negative but PCR-positive systems, implying the presence of dormant bacteria.
Note that we have sought to exclude examples where anaerobic bacteria could be detected by PCR but not cultured simply because cultures were not anaerobic, and also cases (e.g. 388,389) where high antibiotic concentrations might have prevented culture.
| Aims | Culture-negative but PCR-positive | References |
|---|
| Assessment of endocarditis | 6 out of 29 | 390 |
| Development of broad-range PCR | 71 out of 382 | 386 |
Development of broad-range PCR;
limit of detection 5000 cfu.mL-1 | 10 out of 103 | 391 |
| Improved broad-range PCR method | 20 out of 24 | 44 |
| Review | Many examples | 392 |
| Interstitial cystitis | 14 out of 14 | 393 |
| Endocarditis | 270 (36.5%) of 740 | 394 (and see 395) |
| Endophthalmitis | 116 out of 116 (selected) | 396 |
| General study | 18 out of 394 (271 also
culture-positive, PCR-positive) | 397 |
| Bacteraemia in intensive care | 48 out of 197
45 out of 94 | 398
399 |
| Sepsis/SIRS | 29 out of 59
38 out of 72 culture-positive
14.6% vs 10.3% (no antibiotics)
123 vs 95 | 400
401
402
403 |
| Osteoarticular samples | 141 out of 1667 | 404 |
| Review | Many examples | 405 |
| Various, including antibiotic-treated | 34 out of 240 | 406 |
| Meningitis | 26 out of 274
19 out of 21 | 407
408 |
| Orthopaedic samples | 9% out of 125 | 378 |
| Thoracic empyaema | 14 out of 22 | 409 |
| Trauma | 28 out of 35 | 410 |
In environmental microbiology, as mentioned above, there were many early indications (as observed microscopically or flow cytometrically) for the presence of bacteria that did not (or not easily) prove resuscitable or culturable. In a similar vein, many studies have shown microscopically observable organisms in culture-negative but disease-positive samples. This is true both for diseases considered to be due to microbial pathogens and, in fact, for many others normally considered non-communicable50.
Microscopically observable and potentially dormant bacteria in clinical disease
Microscopic observations in tissues have been a major part of the discovery process by which certain bacteria were indeed identified as the cause of various diseases. Billings411, Price412, Domingue393,413–415, Mattman416, Ewald417 and Onwuamaegbu and colleagues418 review the extensive and largely forgotten early literature. Domingue and Schlegel419 also mentioned that they could recover culturable bacteria, probably mainly from L forms (see 50,416,420), from lysates of normal and diseased blood. It was to be assumed that these cells were not replicating at significant rates in the blood itself. However, we can find no evidence that this was ever followed up. Our own work421,422, summarised in 50, showed that both bacillary and coccoid cells could be found attached to and within the erythrocytes of patients with Parkinson’s disease and Alzheimer’s disease, at rather greater concentrations than in samples taken from nominally healthy controls.
In a similar way, our preliminary data show that bacteria are visible in plasma, as well as in whole blood smears in various inflammatory conditions. Here we show bacteria in platelet-rich plasma (PRP) taken from a patient with systemic lupus erythematosus and smeared onto a glass cover slip (Figure 7A and Figure 7B). We also show the same from patients with hereditary hemochromatosis (Figure 7C) and type 2 diabetes (Figure 7D). We also noted microbiota associated with erythrocytes in thromboembolic ischemic stroke (Figure 8A and Figure 8B). (Our microscopy methods are as published previously (e.g. 422–431), but fuller publications will appear elsewhere.) The ultramicroscopic evidence that these are indeed small bacteria and not say, cellular debris or microparticles (see 432) is presently mainly morphological, though we note the considerable evidence for the presence of bacterial DNA in blood (see previous sections and e.g. 51,323,433).
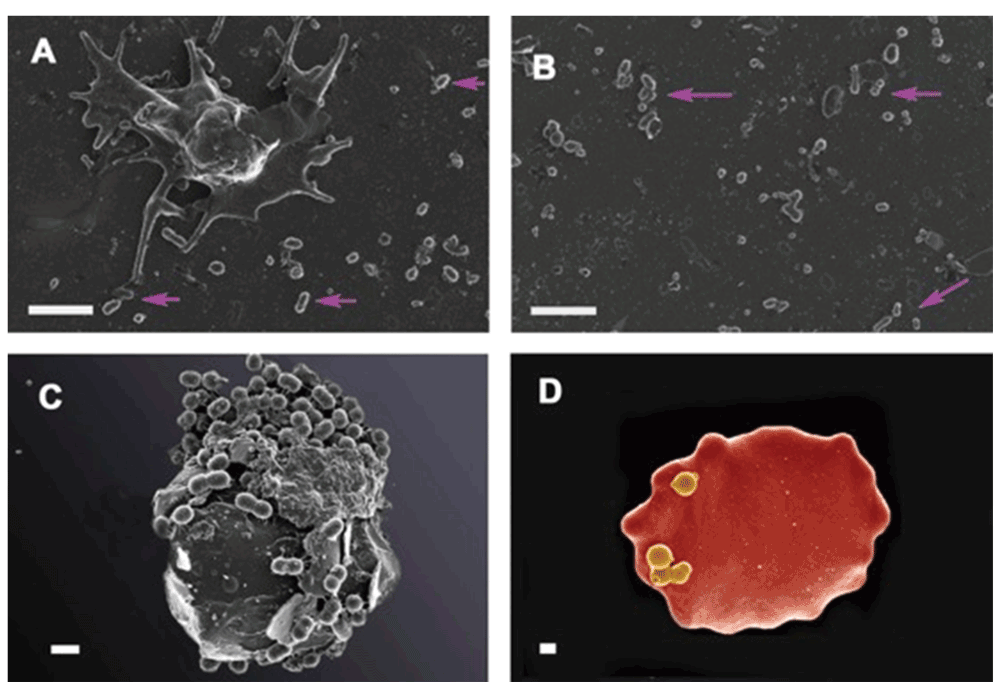
Figure 7.
A and B) Platelet rich plasma (PRP) from a patient with systemic lupus erythematosus (SLE). A) Platelet with bacteria visible in the surrounding smear (pink arrows); B) areas in smear with bacteria (pink arrows); C) Erythrocyte with associated bacteria from patient with confirmed hereditary hemochromatosis; D) Erythrocytes with bacteria from patients with diagnosed type II diabetes. A–C Scale bar: 1 μm and D 400 nm.
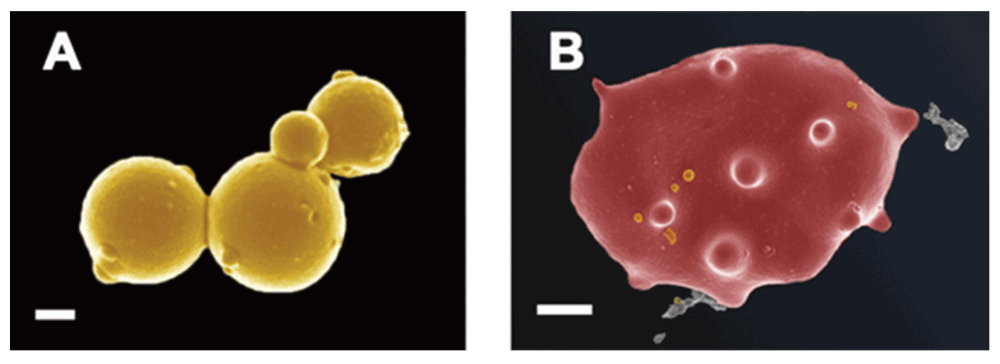
Figure 8.
Bacteria in whole blood from a patient with thromboembolic ischemic stroke A) Microbiota in whole blood; scale bar: 200 nm. B) Erythrocyte with bacteria; scale bar: 1 μm.
It is worth rehearsing the very great significance of this. With erythrocytes being present at some 5×109.mL-1 in human blood, even if only one erythrocyte in a thousand harboured just a single dormant bacterium (that would be hard to detect microscopically, but see 433–437), the dormant bacterial load would still be 5,106.mL-1. This is both far from negligible, and serves to exclude the (always potentially worrisome) claim that ‘it is all contaminants’.
A culturable blood microbiome
A recent and highly significant paper by Damgaard and colleagues438 bears discussion. These workers note438 that while bacterial growth can normally be elicited during sterility testing in vitro from fewer than 1 in a 1000 blood units439–441, transfusion-transmitted infections occur with a very much higher frequency (more like 10–12%442,443, or even more444), and are responsible for a high fraction of transfusion-associated deaths445–447. Although it was acknowledged that venepuncture-associated contamination or an effect of transfusion in suppressing the immune system might contribute, it was also recognised438 that one means by which to account for this would be that ‘normal blood’, and in particular its erythrocyte components, might also contain infectious agents that might be able to grow post-transfusion. Indeed, these authors found438 that under anaerobic conditions a small number of colony-forming units (ca 4–5.mL-1) could be recovered by direct plating from fully 62% of blood units, with ‘controls’ producing an average of just 1 cfu.mL-1. More of the bacteria were associated with red blood cells than with plasma, and rDNA was used to identify them. These data are entirely consistent with the idea that dormant bacteria are present in the blood of even ‘normal’ individuals (note that periodontitis was not a criterion for donor exclusion here438), that they are probably lurking in or on erythrocytes448,449, and that they can be resuscitated and grow under the correct conditions.
Evidence for a microbial component in a very large variety of ‘non-communicable’ diseases
We have surveyed the literature for evidence in which a microbial component has indeed been observed to be an accompaniment of, and probably a major contributory factor to, a variety of (typically inflammatory) diseases that are normally considered ‘non-communicable’. Rarely has the physiological state of these microbes been considered, but since it would be obvious if they were growing, it is most likely that they are indeed dormant. Table 3 summarises these highly extensive associations. While some are just associations, and we could have extended this table considerably, some studies (e.g. 450) contain very detailed aetiological arguments that leave little room for doubt. Overall, the sheer size of the Table does strongly indicate the commonality of many of the microbially based mechanisms underpinning or accompanying various autoimmune and inflammatory diseases. In conditions such as atherosclerosis, transient ischemic attacks (TIAs), and stroke, it is very easy to conceive how resuscitating bacteria might serve to block the flow of blood, for instance. At all events, our main point here is that the evidence for a microbial contribution to many diseases supposedly lacking a microbial component is both multi-factorial and very considerable. Indeed, the purpose of a synthetic review such as this is to provide such pointers for more detailed studies in individual cases. Our specific interest is with the chief mechanisms by which these supposedly dormant bacteria might resuscitate and act as triggers of disease.
Table 3. Evidence for infectious agents in non-communicable diseases.
We purposely largely confine ourselves to bacteria here, but include the occasional parasite, fungus, mycoplasma and virus. While obesity is usually seen as a cause of other diseases, rather than a disease itself, we note the influence of endotoxaemia on obesity451–456. We note too the extensive evidence for the role of LPS in inflammation457–459, and the experimental models (e.g. for Parkinson’s460) where it can induce disease directly. We do not much discuss diseases such as Crohn’s disease where the extensive uncertainty over the extent of involvement of mycobacteria (e.g. 461–463) needs no extra rehearsal (albeit it serves to illustrate the difficulties of identifying the role of hard-to-cultivate bacteria in chronic diseases). Further, while similar phenomena may be observed in a variety of cancers (e.g. 464–469), for reasons of space we have determined that this must be the subject of a separate work.
| Disease | Effect of bacterial
involvement | Class of bacteria | Nature of the evidence | Selected
References |
|---|
| AUTOIMMUNE DISEASES |
|---|
| Ankylosing spondylitis | | Klebsiella pneumoniae | Antibodies | 470–473 |
| Multiple sclerosis | Blood brain barrier permeability
and oligodendrocyte cell
death in the absence of an
adaptive immune filtrate
correlate with the mechanistic
action of Epsilon toxin (ETX). | Clostridium perfringens type B,
an epsilon toxin-secreting bacillus | Immunoreactivity to ETX, fecal
culture and PCR analysis,
lysogenic bacteriophage
footprint analysis (to exclude
the possibility of laboratory
contamination), sequencing of
the patient-derived ETX gene | 474 |
| Chlamydia (Chlamydophila)
pneumoniae | PCR, Serology | 475–481 |
Rheumatoid arthritis
(RA)/osteoarthritis/
reactive arthritis | Mostly antigens against these
infections | Porphyromonas gingivalis | Anaerobic cultures (from
subgingival samples), PCR,
ELISA | 482–486 |
| Proteus mirabilis, Escherichia coli | ELISA and other evidence | 450,487–495 |
| Epstein-Barr virus cytomegalovirus | PCR, ELISA, in situ hybridization,
immunohistochemistry | 496–499 |
Mycoplasma (arthritidis mitogen,
hominis and fermentans) | PCR, Western Blot | 500–502 |
| Staphylococcus aureus | Microbiology reports from
patient records | 503,504 |
| Salmonella
Shigella
Yersinia
Campylobacter
Clostridium difficile | | 505 |
| Propionibacterium acnes | Culture | 506 |
Unusual case of inflammatory
monoarthritis and subsequent
diagnosis of RA | Chlamydia trachomatis | Tissue culture inoculation
Role of antibiotics | 507
508 |
Systemic Lupus
Erythematosus | | Cell wall-deficient form | Microscopy | 509 |
| Hypocomplementaemia and
infection with encapsulated
bacteria; patients are very
susceptible to infections | Streptococcus pneumonia,
Haemophilus influenza,
Mycobacterium tuberculosis,
Listeria monocytogenes,
Klebsiella pneumonia,
Staphylococcus aureus;
Cryptococcus neoformans,
Aspergillus fumigatus | Blood & tissue culture, patient
records | 510–514 |
| Vasculitis | Various reviews | Possibly mainly viral, but bacteria
include Staphylococcus aureus,
Treponema pallidum,
Rickettsiaceae, Borrelia
burgdorferi, M. tuberculosis. | | 515–521 |
| CARDIOVASCULAR DISEASES | 365,522,523 |
|---|
| Atherosclerosis | | Aggregatibacter
actinomycetemcomitans | | 524 |
| Chlamydia (Chlamydophila)
pneumoniae | Antibiotics, Antigens, PCR | 525–529 |
| Helicobacter cinaedi | | 530 |
| Helicobacter pylori | | 527 |
| Porphyromonas gingivalis | PCR | 531–536 |
| Prevotella intermedia | PCR | 532 |
| Streptococcus pneumoniae | Inoculated animals | 537 |
| Toxoplasma gondii | | 538 |
| Treponema denticola | PCR | 532 |
| Endocarditis | | Many cell-wall-deficient forms | Microscopy
PCR | 539
See Table 2 |
| | | Benefit of antibiotic prophylaxis | 540 |
Hereditary
haemochromatosis | | Chryseomonas, Veillonella,
Streptococcus | qPCR | 541 |
| Gemella haemolysans | Blood culture (Gram stain,
catalase activity and
biochemical characteristics) | 542 |
| Listeria monocytogenes | | 543,544 |
| Plesiomonas shigelloides | Blood culture; API20E system | 545 |
| Vibrio vulnificus | | 546,547 |
| Vibrio cholerae | Blood culture; PASCO and
API20E | 548 |
| Yersinia enterocolitica | Microbial cultures, serotype
O:3, serotype 9 | 549–552 |
| Yersinia pseudotuberculosis | Mobility test and API | 553,554 |
| Hypertension | Strong positive association
between periodontal infection
and prevalent hypertension | Periodontal infection with
A. actinomycetemcomitans,
P. gingivalis, T. forsythia, and
T. denticola | DNA-DNA hybridization | 555,556 |
| Myocardial infarction | Association between dental
chronic inflammatory diseases
and the occurrence of acute
myocardial infarction was
studied | Chronic dental infection
correlated positively with MI | | 557–559 |
Correlation between infection
and MI | Chlamydia pneumoniae,
Helicobacter pylori | ELISA to IgG; anti-infectives | 560,561 |
| Association study | Enterobacteria & influenza-like
illness | Immunohistochemistry | 562 |
Virus infections fall outside
the scope of this review, but
the relevance of this study
necessitates mentioning this
here | Influenza was associated with an
increase in MI-associated deaths | Poissonian regression models
to study the relationship
between influenza and MI | 563 |
In mice – microlesions in
myocardium as a result of
pneumolysin toxin | Streptococcus pneumoniae | Immunofluorescence imaging | 564 |
| Stroke | | 565–574 |
| 84 different species detected in
77 patients | | 575,576 |
| Community-acquired bacteremia | Population-based cohort study | 577 |
Observational cross-sectional
study | Bacterial endocarditis
(Organisms found included
S. pneumoniae, N. meningitides
and other) | Culture of cerebrospinal fluid | 578 |
| Borrelia burgdorferi | ELISA | 579 |
| TIA | Brucella spp. | Brucella agglutination and
Coombs’ tests in blood | 580 |
| Chlamydia pneumoniae | Serology | 581–583 |
| Haemophilus influenzae | Multivariate time series
analysis to assess an
association between
infections and stroke using the
established ‘3h-algorithm’ | 584 |
| Mycobacterium tuberculosis | Cox proportional hazard
regressions | 585 |
| Mycoplasma pneumoniae | Association between MP
infection and risk of ischemic
stroke; ELISA; serology | 586–588 |
| Neisseria meningitidis | Latex agglutination test and
counterimmunoelectrophoresis | 589 |
| Staphylococcus aureus | Prospective observational
cohort study; retrospective
review; | 590,591 |
| Streptococcus bovis | Blood culture | 592 |
| Streptococcus mutans | PCR | 593 |
| Streptococcus pneumonia | Cox proportional hazard model | 594 |
| Streptococcus viridans | Blood culture | 595 |
| Neurosyphillis also present | Treponema pallidum | Serology and Treponema
pallidum haem agglutination
test; rapid plasma reagin test,
and fluorescent treponemal
antibody-absorption test | 596,597 |
Vascular disease
(aneurysmal
and lesions and
atherosclerotic
plaques) | | Numerous ‘uncultivable’ bacterial
species found in atheromas | | 598 |
| DERMATOLOGICAL DISEASES |
|---|
| Psoriasis | | Streptococcus haemolyticus
group A, Staphylococcus
aureus, Haemophilus influenzae,
Klebsiella oxytoca, Moraxella
catarrhalis, Escherichia coli | Culture from nasal/pharyngeal
swab | 599 |
| Escherichia coli | | 600 |
| Streptococcus pyogenes,
Staphylococcus aureus | | 601–603 |
| ENDOCRINE DISEASES |
|---|
| Diabetes | | 604,605 |
| Blood | Pseudomonads,
Stenotrophomonas maltophilia
and Ps. aeruginoas | PCR and antibodies | 606 |
| Type 1 | Urinary tract infection | E. coli, Candida albicans,
enterovirus | Urine and blood culture | 607–609 |
| | Various proteobacteria | PCR | 610 |
| | Decreased bacteroidetes | | 611 |
| Type 2 | | Systemic antibiotics improved
diabetes control | Measured as a reduction
in glycated hemoglobin
or reduction in insulin
requirements | 612 |
| | Many Gram-positives | qPCR | 613 |
| NEUROLOGICAL DISORDERS | 614–616 |
|---|
| Alzheimer’s Disease | | 617,618 |
LPS will activate innate
immune system in CNS and
initiate pro-inflammatory
cascades. | Porphyromonas gingivalis | Immunolabeling and
immunoblotting of brain tissue
for the presence of LPS from
P. gingivalis | 619 |
| Chlamydia pneumoniae | Immunohistochemistry,
Statistical correlation of a
meta-analysis | 620–633 |
| Spirochetal bacteria |
| Helicobacter pylori | Histology, direct experiment | 634–636 |
| | Actinomyces naeslundii | Antibodies | 637 |
Amyotrophic Lateral
Sclerosis | | Mycoplasma infections
(M. fermentas, M. genitalium,
M. penetrans, M. fermentans,
M. hominis, M. pneumoniae),
Chlamydia pneumoniae, Borrelia
burgdorferi | PCR, serology, microscopic observation | 416,638–640 |
Autism spectrum
disorders | | Mycoplasmal infections
(M. fermentas, M. genitalium,
M. penetrans, M. fermentans,
M. hominis, M. pneumonia) | PCR | 641 |
Chlamydia pneumoniae
(co-infection with mycoplasma
and human herpes virus-6), or
wall-less bacteria | PCR | 642,643 |
Maternal viral infection in first
trimester and maternal bacterial
infection in second trimester were
found to be associated with ASD | Cox proportional hazards
regression | 644 |
| Chronic depression | | Numerous Gram-negatives
from gut, e.g. Hafnia alvei,
Pseudomonas aeruginosa,
Morganella morganii,
Pseudomonas putida, Citrobacter
koseri, Klebsiella pneumoniae | | 645 |
| Parkinson’s Disease | | Helicobacter pylori | 13C urea breath test, odd ratios
for the association between
treatment for HP and risk of PD
using logistic regression | 646–649 |
| Toxoplasma gondii | Serology, ELISA | 650 |
| | Helicobacter suis | DNA evidence | 651 |
| Schizophrenia | A correlation between contact
with house cats in early life
and the development of
schizophrenia exist | Toxoplasma gondii and Herpes
simplex virus type 2 | | 652–656 |
| Prenatal exposure to bacterial
infection in the first trimester
increased risk of schizophrenia in
the offspring | Prospective association study | 657 |
| | Chlamydia trachomatis | antibodies | 658,659 |
| RESPIRATORY DISEASES |
|---|
| Asthma | Review | | | 660 |
| | Branhamella catarrhalis,
Haemophilus influenzae,
Streptococcus pneumonia | | 661 |
| Increased mast cell numbers
in airways | Atypical bacteria
Mycoplasma pneumoniae and
Chlamydia pneumoniae | | 662 |
A significant association
exists between bacterial
infections and acute wheezy
episodes in young children,
independent of viral infection | Streptococcus pneumoniae,
Haemophilus influenzae,
Moraxella catarrhalis | | 663,664 |
| Lower airway infection | Haemophilus influenzae,
Streptococcus pneumoniae | | 665 |
Chronic Obstructive
Pulmonary Disease
(COPD) | | Haemophilus influenzae,
Streptococcus pneumoniae,
Moraxella catarrhalis,
Staphylococcus aureus,
Pseudomonas aeruginosa,
Enterobacter spp. | | 666–668 |
| OTHER INFLAMMATORY CONDITIONS |
|---|
| Preeclampsia | Acute atherosis | Tannerella forsythensis,
Porphyromonas gingivalis,
Actinobacillus
actinomycetemcomitans,
Prevotella intermedia,
Fusobacterium nucleatum
Treponema denticola | PCR | 669 |
| Significantly lowered risk
following antibiotic treatment | | | 670 |
Significant association with
periodontal disease and UTI | | | 45,671–674 |
| Chlamydia pneumonia | ELISA and qPCR of genomic
DNA | 675 (but cf.
676) |
| Chlamydia trachomatis | Serology | 677 |
| Helicobacter pylori | Serology | 678,679 |
Chronic fatigue
syndrome | LPS a culprit | Hafnia alvei, Pseudomonas
aeruginosa, Morganella morganii,
Proteus mirabilis, Pseudomonas
putida, Citrobacter koseri,
Klebsiella pneumoniae | Serology | 680–683 |
| Mycoplasmal infections
(M. pneumonia, M. fermentans,
M. honinis, M. penetrans),
Chlamydia pneumonia, Human
herpes virus-6 | PCR | 684 |
| | Various enterbacteria and others | IgG | 685 |
Vitamin D receptor
(VDR) dysregulation | Evade immune destruction
by invading nucleated cells
where they persist in the
cytoplasm. From here they
down-regulated the VDR | Cell wall deficient bacteria | | 686 |
| | Multiple organisms, including
mycrobacteria, Borrelia. | | 685 |
Antiphospholipid
syndrome | | S. aureus cross-reacting
antibodies | | 687 |
Various viral and bacterial
triggers | | 688–690 |
| Toxoplasma | Anti-Toxoplasma antibodies | 691 |
Sudden Infant Death
Syndrome | Review | S. aureus most common | Seasonality, bacteriology | 692–694 |
| | | Inflammatory markers | 695,696 |
| | | Toxaemic shock indicators in
serum | 697,698 |
Other Inflammatory
Bowel Diseases | Many examples of dysbiosis
of gut microbiota | | | 699–707 |
| Sarcoidosis | | | P. acnes antibodies and
antigens | 708–710 |
| Migraine | | | H. pylori | 711,712 |
Relation between iron dysregulation, sepsis and other comorbidities
Many of the diseases in Table 3 are precisely those inflammatory diseases that we have listed before as coupled to iron dysregulation167,168,429,432,713. A consequence of our analysis is that iron dysregulation and sepsis (as judged either by genuine infection by culturable bacteria or their inflammatory products such as LPS) should be associated causally with these various other diseases.
This leads to a variety of predictions and postdictions that we rehearse. A purposely simple (and simplistic) indication of a plausible chain of events (for which each step is underpinned by substantial evidence) is given in Figure 9, both in general terms (for unspecified diseases) and for a couple of steps to type 2 diabetes. Figure 9 aims specifically to highlight the relationship between the ability of available iron to stimulate bacterial growth and the potential disease sequelae thereof.
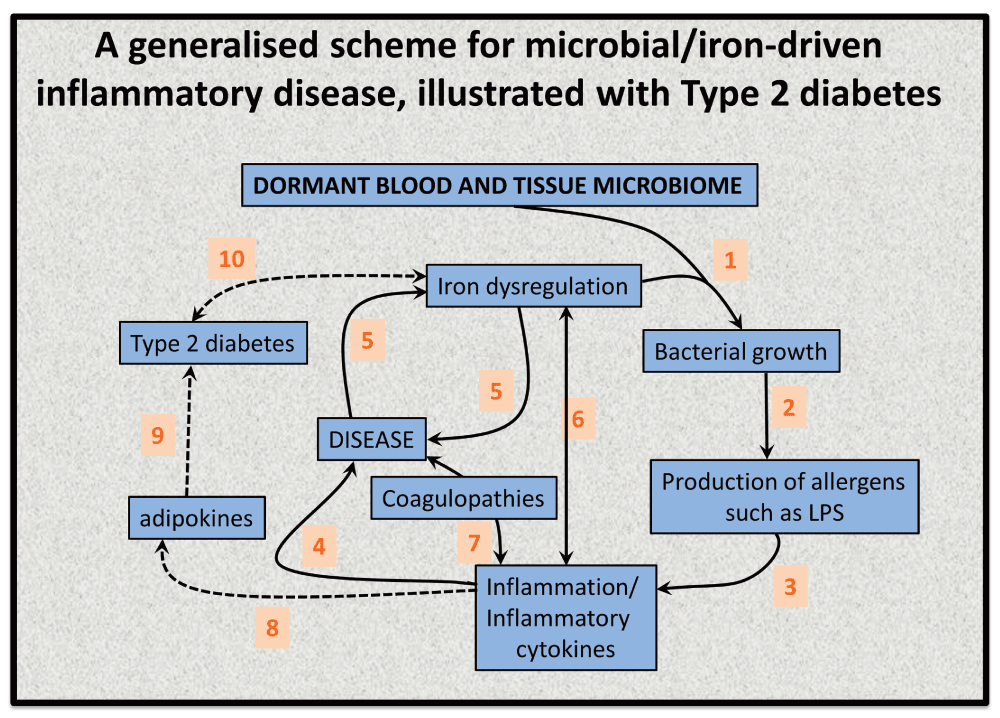
Figure 9. An elementary systems biology model of how iron dysregulation can stimulate dormant bacterial growth that can in turn lead to antigen production (e.g. of LPS) that can then trigger inflammation leading to cell death168 and to a variety of diseases.
While it is recognised that this simple diagram is very far from capturing the richness of these phenomena, there is abundant evidence for each of these steps, but sample references for the numbered interactions are (1)828–831 (especially including the release of free iron from ferritin432), (2)832–834, (3)268,453,455,835–842, (4)456,713,843–846, (5)167,168,432 (6)847,848, (7)849–855 (8)856 (9)857–859 (10)860,861.
Iron and sepsis
First of all, it is well established that free iron may be raised in sepsis and related conditions714–722, as may serum ferritin723–727 (that has mainly dumped its iron432). We have here argued that this is likely to be a significant contributor to the relationship between overt or cryptic infection and the many iron-related inflammatory diseases discussed here and elsewhere167,168,432,713. Note that patients suffering from iron overload diseases such as hereditary haemochromatosis are especially susceptible to infection (see e.g. 728–730 and Table 3). Certainly the idea that iron-related metabolism and siderophores are virulence factors (e.g. 731–743) is established unequivocally. In many diseases (e.g. lupus744,745 or type 1 diabetes746) it is considered that patients with the disease are more prone to sepsis, but we suggest here that (as with stroke561,565,566,568–570,747–755) it may more likely be the converse that is truer: patients suffering from latent infections are in fact more prone to acquiring, having, or exacerbating the state of these other conditions, in a vicious cycle (see Figure 9).
Role of iron chelation in preventing sepsis
This was discussed at considerable length previously168, and that discussion is not repeated here (though a few more recent and pertinent references include756–759). However, while (shockingly, given the evidence) it does not even appear in the guidelines760, there is considerable evidence168 that iron chelation slows, inhibits or overcomes sepsis. On this basis, iron chelation may be a suitable alternative to antibiotics in preventing multiple inflammatory diseases (and such chelation may be nutritional rather than pharmacological in nature, e.g. 167). However, it is clear that we also need to learn to kill ‘dormant’ bacteria, and this usually requires that they are growing.
Utility of antibiotics in treating non-communicable diseases
It is well established that the re-use of protein motifs in natural (and directed761) evolution means that most drugs, especially the more lipophilic ones, are promiscuous in the sense that they bind to multiple targets177,762 (on average six known ones for marketed drugs763). This said (and while we are very far from wishing to encourage the unnecessary use of antibiotics), the prediction here is that appropriate antibiotics will prove to have clinical benefit in diseases commonly seen as non-communicable. This is certainly known to be the case for a number of autoimmune diseases764 such as rheumatoid arthritis765–770, multiple sclerosis771–777 and psoriasis778–780. Vaccination may prove equally effective781,782.
Concluding comments: on the systems properties of dormancy and virulence
We have here brought together some of the relevant elements of environmental, laboratory, and clinical microbiology. We have argued that while their languages may differ (e.g. ‘dormancy’ vs ‘persistence’), very similar phenomena have been observed in each of these spheres (plausibly underlying a commonality of mechanism). Certainly the ability to culture microbes, and not merely to observe them (whether microscopically or via their macromolecular sequences or chemical products), remains an important goal of basic microbiology. This is likely to have significant payoffs in bioprospecting (e.g. 163,783). However, we are sure that improved methods of detecting and identifying these dormant bacteria, whether this is done via chemical imaging, through macromolecular amplification and/or sequencing, or through resuscitation and culturing, will have a major role to play in increasing the awareness of their existence and importance.
Clearly dormant, persistent bacteria are likely to be relatively avirulent when they are in such dormant states, and able to bypass the attentions of the innate immune system (albeit the production of superantigens by at least some microorganisms784,785 may be what triggers autoimmune diseases). This ‘stealth’ antigenicity is probably why they have been largely unnoticed by us too786, and their routine estimation via molecular methods787 seems highly desirable. Indeed, virulence varies widely between individual strains (e.g. 788,789). Modern molecular microbiology places much emphasis on the virulence of the pathogen, with concepts such as ‘pathogenicity islands’790–795, ‘virulence genes’796,797, and the ‘virulome’798 being commonplace. However, if dormant microbes resuscitate (or are to be resuscitated) in vivo we shall need to pay much more attention to the environmental triggers that cause this to happen than we probably have so far799 (given that the pathogen genotype is fixed800,801). In other words, virulence, like dormancy, is a phenotypic as well as a genotypic property. We remain largely ignorant of the means by which an optimal immune system has been selected for (or against) by longer-term evolution on the basis of microbial exposures in early life, and how this may have changed with more recent changes in human lifestyle802–805. Nor do we understand how such microbes might enter and exit blood cells (and see 50,330,806–810) (albeit the known endosymbiotic origins811,812 of eukaryotic organelles must have presaged such mechanisms). Similarly, we do not yet know what may cause these dormant microbes to resuscitate (and/or to exit their intracellular niches). However, the potential for iron-associated replication and (e.g.) LPS production and shedding does provide a very straightforward explanation for the continuing low- or medium-grade inflammation characteristic of the many inflammatory diseases we have considered here and elsewhere167,168,429,432,713 (Figure 9).
One approach to Science is based on varying independently something considered a cause and observing its predicted effects (e.g. 178,813,814). To assess causality in microbiology it is usual (e.g. 792,815–817) to invoke what are (variously818) referred to the Henle-Koch or Koch’s postulates. These are based on the nature and presence, but not the physiological state, of an agent that might be believed to ‘cause’ (or at least contribute to) an infectious disease. Consequently, dormancy poses something of a challenge to the full completion of the required tests. Indeed a number of authors417,792,818–821 have recognised that these tests may need revision in the light of the ability to identify disease-causing microbes by sequence alone. We suspect that a key element here will be the ability to resuscitate dormant organisms in vivo and to see the effects of that on clinical disease.
As phrased by Silvers822, “Several of our contributors showed how discoveries and insights could emerge with what seemed great promise, and yet be pushed aside, discarded, and forgotten – only to re-emerge once again, sometimes many years later, and become, in their new formulation, accepted as important”. In this sense, and as presaged in the opening quotation1, it seems that ideas, as well as bacteria, can remain dormant for extended periods823,824.
Author contributions
This review originated as part of a discussion between the corresponding authors, who have a funded collaboration as outlined under ‘grant information’, and was partly written during a visit of EP and MP to Manchester. All authors contributed to the writing of the manuscript and have agreed to its final content.
Competing interests
No competing interests were disclosed.
Grant information
We thank the Biotechnology and Biological Sciences Research Council (grant BB/L025752/1) as well as the National Research Foundation (NRF) of South Africa for supporting this collaboration. This is also a contribution from the Manchester Centre for Synthetic Biology of Fine and Speciality Chemicals (SYNBIOCHEM) (BBSRC grant BB/M017702/1).
Faculty Opinions recommendedReferences
- 1.
Keilin D:
The problem of anabiosis or latent life: history and current concept.
Proc R Soc Lond B Biol Sci.
1959; 150(939): 149–91. PubMed Abstract
| Publisher Full Text
- 2.
Kaprelyants AS, Gottschal JC, Kell DB:
Dormancy in non-sporulating bacteria.
FEMS Microbiol Rev.
1993; 104(3–4): 271–86. Publisher Full Text
- 3.
PostGate JR:
Viability measurements and the survival of microbes under minimum stress.
Adv Microb Physiol.
1967; 1: 1–23. Publisher Full Text
- 4.
PostGate JR:
Viable counts and viability.
Meth Microbiol.
1969; 1: 611–28. Publisher Full Text
- 5.
Bugeja VC, Saunders PT, Bazin MJ:
Estimating the mode of growth of individual microbial cells from cell volume distributions.
Biosystems.
1985; 18(1): 47–63. PubMed Abstract
| Publisher Full Text
- 6.
Kell DB, Sonnleitner B:
GMP - Good Modelling Practice: an essential component of good manufacturing practice.
Trends Biotechnol.
1995; 13(11): 481–92. Publisher Full Text
- 7.
Pirt SJ:
Principles of microbe and cell cultivation. London: Wiley. 1975; 260–268. Reference Source
- 8.
Tempest DW:
The continuous cultivation of microorganisms. I. Theory of the chemostat. In: Norris JR, Ribbons DW, editors.
Methods in Microbiology.
1970; 2: 259–276. Publisher Full Text
- 9.
Munson RJ:
Turbidostats. In: Norris JR, Ribbons DW, editors. Methods in Microbiology. Academic Press; 1970; 2: 349–76. Publisher Full Text
- 10.
Watson TG:
The Present Status and Future Prospects of the Turbidostat.
J Appl Chem Biotechnol.
1972; 22(2): 229–43. Publisher Full Text
- 11.
Markx GH, Davey CL, Kell DB, et al.:
The permittistat: a novel type of turbidostat.
J Gen Microbiol.
1991; 137(4): 735–43. Publisher Full Text
- 12.
Cooper VS, Bennett AF, Lenski RE:
Evolution of thermal dependence of growth rate of Escherichia coli populations during 20,000 generations in a constant environment.
Evolution.
2001; 55(5): 889–96. PubMed Abstract
| Publisher Full Text
- 13.
Conrad TM, Lewis NE, Palsson BØ:
Microbial laboratory evolution in the era of genome-scale science.
Mol Syst Biol.
2011; 7(1): 509. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 14.
Lennen RM, Herrgård MJ:
Combinatorial strategies for improving multiple-stress resistance in industrially relevant Escherichia coli strains.
Appl Environ Microbiol.
2014; 80(19): 6223–42. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 15.
Koch AL:
The variability and individuality of the bacterium. In Neidhardt FC, Low KB, Magasanik B, Schaechter M, Umbarger HE, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington: American Society for Microbiology. 1987: 1606–14.
- 16.
Avery SV:
Microbial cell individuality and the underlying sources of heterogeneity.
Nat Rev Microbiol.
2006; 4(8): 577–87. PubMed Abstract
| Publisher Full Text
- 17.
Davidson CJ, Surette MG:
Individuality in bacteria.
Annu Rev Genet.
2008; 42: 253–68. PubMed Abstract
| Publisher Full Text
- 18.
Ackermann M:
Microbial individuality in the natural environment.
ISME J.
2013; 7(3): 465–7. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 19.
Kell DB:
Publishing: Reviews turn facts into understanding.
Nature.
2012; 490(7418): 37. PubMed Abstract
| Publisher Full Text
- 20.
Bigger JW:
Treatment of staphylococcal infections with penicillin - by intermittent sterilisation.
Lancet.
1944; 244(6320): 497–500. Publisher Full Text
- 21.
McDermott W:
Microbial persistence.
Yale J Biol Med.
1958; 30(4): 257–91. PubMed Abstract
| Free Full Text
- 22.
Orman MA, Brynildsen MP:
Dormancy is not necessary or sufficient for bacterial persistence.
Antimicrob Agents Chemother.
2013; 57(7): 3230–9. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 23.
Amato SM, Fazen CH, Henry TC, et al.:
The role of metabolism in bacterial persistence.
Front Microbiol.
2014; 5: 70. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 24.
Tuomanen E, Cozens R, Tosch W, et al.:
The rate of killing of Escherichia coli by beta-lactam antibiotics is strictly proportional to the rate of bacterial growth.
J Gen Microbiol.
1986; 132(5): 1297–304. PubMed Abstract
| Publisher Full Text
- 25.
Roostalu J, Jõers A, Luidalepp H, et al.:
Cell division in Escherichia coli cultures monitored at single cell resolution.
BMC Microbiol.
2008; 8: 68. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 26.
Luria SE, Latarjet R:
Ultraviolet irradiation of bacteriophage during intracellular growth.
J Bacteriol.
1947; 53(2): 149–63. PubMed Abstract
| Free Full Text
- 27.
Wiuff C, Zappala RM, Regoes RR, et al.:
Phenotypic tolerance: antibiotic enrichment of noninherited resistance in bacterial populations.
Antimicrob Agents Chemother.
2005; 49(4): 1483–94. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 28.
Cohen NR, Lobritz MA, Collins JJ:
Microbial persistence and the road to drug resistance.
Cell Host Microbe.
2013; 13(6): 632–42. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 29.
Levin BR, Concepción-Acevedo J, Udekwu KI:
Persistence: a copacetic and parsimonious hypothesis for the existence of non-inherited resistance to antibiotics.
Curr Opin Microbiol.
2014; 21: 18–21. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 30.
De Bolle X, Bayliss CD, Field D, et al.:
The length of a tetranucleotide repeat tract in Haemophilus influenzae determines the phase variation rate of a gene with homology to type III DNA methyltransferases.
Mol Microbiol.
2000; 35(1): 211–22. PubMed Abstract
| Publisher Full Text
- 31.
Wisniewski-Dyé F, Vial L:
Phase and antigenic variation mediated by genome modifications.
Antonie Van Leeuwenhoek.
2008; 94(4): 493–515. PubMed Abstract
| Publisher Full Text
- 32.
Girgis HS, Harris K, Tavazoie S:
Large mutational target size for rapid emergence of bacterial persistence.
Proc Natl Acad Sci U S A.
2012; 109(31): 12740–5. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 33.
Kell DB, Kaprelyants AS, Weichart DH, et al.:
Viability and activity in readily culturable bacteria: a review and discussion of the practical issues.
Antonie van Leeuwenhoek.
1998; 73(2): 169–87. PubMed Abstract
| Publisher Full Text
- 34.
Primas H:
Chemistry, Quantum Mechanics and Reductionism. Berlin: Springer. 1981.
- 35.
Gribbin JR:
In search of Schrödinger's cat: quantum physics and reality. London: Bantam Books, 1985.
- 36.
Postgate JR:
Death in microbes and macrobes. In: Gray TRG, Postgate JR, editors. In The Survival of Vegetative Microbes. Cambridge: Cambridge University Press, 1976: 1–19.
- 37.
Barer MR, Gribbon LT, Harwood CR, et al.:
The viable but non-culturable hypothesis and medical bacteriology.
Rev Med Microbiol.
1993; 4(4): 183–91. Publisher Full Text
- 38.
Barer MR:
Viable but non-culturable and dormant bacteria: time to resolve an oxymoron and a misnomer?
J Med Microbiol.
1997; 46(8): 629–31. Publisher Full Text
- 39.
Barer MR, Kaprelyants AS, Weichart DH, et al.:
Microbial stress and culturability: conceptual and operational domains.
Microbiology.
1998; 144(8): 2009–10. Publisher Full Text
- 40.
Barer MR, Harwood CR:
Bacterial viability and culturability.
Adv Microb Physiol.
1999; 41: 93–137. PubMed Abstract
| Publisher Full Text
- 41.
Barer MR, Bogosian G:
The viable but nonculturable concept, bacteria in urine samples, and Occam's razor.
J Clin Microbiol.
2004; 42(11): 5434. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 42.
Bogosian G, Bourneuf EV:
A matter of bacterial life and death.
EMBO Rep.
2001; 2(9): 770–4. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 43.
Kell DB:
Scientific discovery as a combinatorial optimisation problem: how best to navigate the landscape of possible experiments?
Bioessays.
2012; 34(3): 236–44. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 44.
Cherkaoui A, Emonet S, Ceroni D, et al.:
Development and validation of a modified broad-range 16S rDNA PCR for diagnostic purposes in clinical microbiology.
J Microbiol Methods.
2009; 79(2): 227–31. PubMed Abstract
| Publisher Full Text
- 45.
Parahitiyawa NB, Jin LJ, Leung WK, et al.:
Microbiology of odontogenic bacteremia: beyond endocarditis.
Clin Microbiol Rev.
2009; 22(1): 46–64. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 46.
Tlaskalová-Hogenová H, Štěpánková R, Kozáková H, et al.:
The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases.
Cell Mol Immunol.
2011; 8(2): 110–20. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 47.
Lundberg DS, Yourstone S, Mieczkowski P, et al.:
Practical innovations for high-throughput amplicon sequencing.
Nat Methods.
2013; 10(10): 999–1002. PubMed Abstract
| Publisher Full Text
- 48.
Bacconi A, Richmond GS, Baroldi MA, et al.:
Improved sensitivity for molecular detection of bacterial and Candida infections in blood.
J Clin Microbiol.
2014; 52(9): 3164–74. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 49.
Valencia-Shelton F, Loeffelholz M:
Nonculture techniques for the detection of bacteremia and fungemia.
Future Microbiol.
2014; 9(4): 543–59. PubMed Abstract
| Publisher Full Text
- 50.
Potgieter M, Bester J, Kell DB, et al.:
The dormant blood microbiome in chronic, inflammatory diseases.
FEMS Microbiol Rev.
2015. PubMed Abstract
| Publisher Full Text
- 51.
Gaibani P, Mariconti M, Bua G, et al.:
Development of a broad-range 23S rDNA real-time PCR assay for the detection and quantification of pathogenic bacteria in human whole blood and plasma specimens.
Biomed Res Int.
2013; 2013: 264651. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 52.
Itzhaki RF, Wozniak MA:
Herpes simplex virus type 1 in Alzheimer's disease: the enemy within.
J Alzheimers Dis.
2008; 13(4): 393–405. PubMed Abstract
- 53.
Itzhaki RF:
Herpes simplex virus type 1 and Alzheimer’s disease: increasing evidence for a major role of the virus.
Front Aging Neurosci.
2014; 6: 202. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 54.
Staley JT, Konopka A:
Measurement of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats.
Annu Rev Microbiol.
1985; 39: 321–46. PubMed Abstract
| Publisher Full Text
- 55.
Mason CA, Hamer G, Bryers JD:
The death and lysis of microorganisms in environmental processes.
FEMS Microbiol Rev.
1986; 2(4): 373–401. Publisher Full Text
- 56.
Eilers H, Pernthaler J, Glockner FO, et al.:
Culturability and in situ abundance of pelagic bacteria from the North Sea.
Appl Environ Microbiol.
2000; 66(7): 3044–51. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 57.
Hugenholtz P:
Exploring prokaryotic diversity in the genomic era.
Genome Biol.
2002; 3(2): reviews0003.1–reviews0003.8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 58.
Keller M, Zengler K:
Tapping into microbial diversity.
Nat Rev Microbiol.
2004; 2(2): 141–50. PubMed Abstract
| Publisher Full Text
- 59.
Fierer N, Jackson RB:
The diversity and biogeography of soil bacterial communities.
Proc Natl Acad Sci U S A.
2006; 103(3): 626–31. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 60.
Kimura N:
Metagenomics: access to unculturable microbes in the environment.
Microbes Env.
2006; 21(4): 201–15. Publisher Full Text
- 61.
Tuffin M, Anderson D, Heath C, et al.:
Metagenomic gene discovery: how far have we moved into novel sequence space?
Biotechnol J.
2009; 4(12): 1671–83. PubMed Abstract
| Publisher Full Text
- 62.
Logares R, Haverkamp TH, Kumar S, et al.:
Environmental microbiology through the lens of high-throughput DNA sequencing: synopsis of current platforms and bioinformatics approaches.
J Microbiol Methods.
2012; 91(1): 106–13. PubMed Abstract
| Publisher Full Text
- 63.
Pham VHT, Kim J:
Cultivation of unculturable soil bacteria.
Trends Biotechnol.
2012; 30(9): 475–84. PubMed Abstract
| Publisher Full Text
- 64.
Epstein SS:
The phenomenon of microbial uncultivability.
Curr Opin Microbiol.
2013; 16(5): 636–42. PubMed Abstract
| Publisher Full Text
- 65.
Amann RI, Ludwig W, Schleifer KH, et al.:
Phylogenetic identification and in situ detection of individual microbial cells without cultivation.
Microbiol Rev.
1995; 59(1): 143–69. PubMed Abstract
| Free Full Text
- 66.
Jones SE, Lennon JT:
Dormancy contributes to the maintenance of microbial diversity.
Proc Natl Acad Sci U S A.
2010; 107(13): 5881–6. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 67.
Lennon JT, Jones SE:
Microbial seed banks: the ecological and evolutionary implications of dormancy.
Nat Rev Microbiol.
2011; 9(2): 119–30. PubMed Abstract
| Publisher Full Text
- 68.
Caporaso JG, Lauber CL, Walters WA, et al.:
Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms.
ISME J.
2012; 6(8): 1621–4. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 69.
Langille MG, Zaneveld J, Caporaso JG, et al.:
Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences.
Nat Biotechnol.
2013; 31(9): 814–21. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 70.
Narihiro T, Kamagata Y:
Cultivating yet-to-be cultivated microbes: the challenge continues.
Microbes Environ.
2013; 28(2): 163–5. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 71.
Yarza P, Yilmaz P, Pruesse E, et al.:
Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences.
Nat Rev Microbiol.
2014; 12(9): 635–45. PubMed Abstract
| Publisher Full Text
- 72.
Aanderud ZT, Jones SE, Fierer N, et al.:
Resuscitation of the rare biosphere contributes to pulses of ecosystem activity.
Front Microbiol.
2015; 6: 24. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 73.
Wang G, Jagadamma S, Mayes MA, et al.:
Microbial dormancy improves development and experimental validation of ecosystem model.
ISME J.
2015; 9(1): 226–37. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 74.
Yarza P, Richter M, Peplies J, et al.:
The All-Species Living Tree project: a 16S rRNA-based phylogenetic tree of all sequenced type strains.
Syst Appl Microbiol.
2008; 31(4): 241–50. PubMed Abstract
| Publisher Full Text
- 75.
Caporaso JG, Lauber CL, Walters WA, et al.:
Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample.
Proc Natl Acad Sci U S A.
2011; 108(Suppl 1): 4516–22. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 76.
Quast C, Pruesse E, Yilmaz P, et al.:
The SILVA ribosomal RNA gene database project: improved data processing and web-based tools.
Nucleic Acids Res.
2013; 41(Database issue): D590–6. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 77.
Yilmaz P, Parfrey LW, Yarza P, et al.:
The SILVA and "All-species Living Tree Project (LTP)" taxonomic frameworks.
Nucleic Acids Res.
2014; 42(Database issue): D643–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 78.
Rinke C, Schwientek P, Sczyrba A, et al.:
Insights into the phylogeny and coding potential of microbial dark matter.
Nature.
2013; 499(7459): 431–7. PubMed Abstract
| Publisher Full Text
- 79.
Lok C:
Mining the microbial dark matter.
Nature.
2015; 522(7556): 270–3. PubMed Abstract
| Publisher Full Text
- 80.
Fodor AA, DeSantis TZ, Wylie KM, et al.:
The "most wanted" taxa from the human microbiome for whole genome sequencing.
PLoS One.
2012; 7(7): e41294. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 81.
Wilson MC, Mori T, Ruckert C, et al.:
An environmental bacterial taxon with a large and distinct metabolic repertoire.
Nature.
2014; 506(7486): 58–62. PubMed Abstract
| Publisher Full Text
- 82.
Kamagata Y, Tamaki H:
Cultivation of uncultured fastidious microbes.
Microbes Environ.
2005; 20(2): 85–91. Publisher Full Text
- 83.
McInerney MJ, Struchtemeyer CG, Sieber J, et al.:
Physiology, ecology, phylogeny, and genomics of microorganisms capable of syntrophic metabolism.
Ann N Y Acad Sci.
2008; 1125: 58–72. PubMed Abstract
| Publisher Full Text
- 84.
McInerney MJ, Sieber JR, Gunsalus RP:
Syntrophy in anaerobic global carbon cycles.
Curr Opin Biotechnol.
2009; 20(6): 623–32. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 85.
Orphan VJ:
Methods for unveiling cryptic microbial partnerships in nature.
Curr Opin Microbiol.
2009; 12(3): 231–7. PubMed Abstract
| Publisher Full Text
- 86.
Peters BM, Jabra-Rizk MA, O'May GA, et al.:
Polymicrobial interactions: impact on pathogenesis and human disease.
Clin Microbiol Rev.
2012; 25(1): 193–213. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 87.
Sieber JR, McInerney MJ, Gunsalus RP, et al.:
Genomic insights into syntrophy: the paradigm for anaerobic metabolic cooperation.
Annu Rev Microbiol.
2012; 66: 429–52. PubMed Abstract
| Publisher Full Text
- 88.
Murray JL, Connell JL, Stacy A, et al.:
Mechanisms of synergy in polymicrobial infections.
J Microbiol.
2014; 52(3): 188–99. PubMed Abstract
| Publisher Full Text
- 89.
Decross AJ, Marshall BJ:
The role of Helicobacter pylori in acid-peptic disease.
Amer J Med Sci.
1993; 306(6): 381–92. PubMed Abstract
- 90.
Marshall B:
Helicobacter pylori--a Nobel pursuit?
Can J Gastroenterol.
2008; 22(11): 895–6. PubMed Abstract
| Free Full Text
- 91.
Montecucco C, Rappuoli R:
Living dangerously: how Helicobacter pylori survives in the human stomach.
Nat Rev Mol Cell Biol.
2001; 2(6): 457–66. PubMed Abstract
| Publisher Full Text
- 92.
Meyer RD:
Legionella infections - a review of 5 years of research.
Rev Infect Dis.
1983; 5(2): 258–78. PubMed Abstract
- 93.
Barker J, Farrell ID, Hutchison JG, et al.:
Factors affecting growth of Legionella pneumophila in liquid media.
J Med Microbiol.
1986; 22(2): 97–100. PubMed Abstract
| Publisher Full Text
- 94.
Molinari J:
Legionella and human disease: Part 1: A path of scientific and community discovery.
Compend Contin Educ Dent.
1997; 18(6): 556–9. PubMed Abstract
- 95.
Saito A, Rolfe RD, Edelstein PH, et al.:
Comparison of liquid growth media for Legionella pneumophila.
J Clin Microbiol.
1981; 14(6): 623–7. PubMed Abstract
| Free Full Text
- 96.
Bertani G:
Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli.
J Bacteriol.
1951; 62(3): 293–300. PubMed Abstract
| Free Full Text
- 97.
Wang CH, Koch AL:
Constancy of growth on simple and complex media.
J Bacteriol.
1978; 136(3): 969–75. PubMed Abstract
| Free Full Text
- 98.
Payne JW, Gilvarg C:
Size restriction on peptide utilization in Escherichia coli.
J Biol Chem.
1968; 243(23): 6291–9. PubMed Abstract
- 99.
Sezonov G, Joseleau-Petit D, D'Ari R:
Escherichia coli physiology in Luria-Bertani broth.
J Bacteriol.
2007; 189(23): 8746–9. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 100.
Singh S, Eldin C, Kowalczewska M, et al.:
Axenic culture of fastidious and intracellular bacteria.
Trends Microbiol.
2013; 21(2): 92–9. PubMed Abstract
| Publisher Full Text
- 101.
Lagier JC, Edouard S, Pagnier I, et al.:
Current and past strategies for bacterial culture in clinical microbiology.
Clin Microbiol Rev.
2015; 28(1): 208–36. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 102.
Maiwald M, Schuhmacher F, Ditton HJ, et al.:
Environmental occurrence of the Whipple's disease bacterium (Tropheryma whippelii).
Appl Environ Microbiol.
1998; 64(2): 760–2. PubMed Abstract
| Free Full Text
- 103.
Maiwald M, Relman DA:
Whipple's disease and Tropheryma whippelii: secrets slowly revealed.
Clin Infect Dis.
2001; 32(3): 457–63. PubMed Abstract
| Publisher Full Text
- 104.
Bentley SD, Maiwald M, Murphy LD, et al.:
Sequencing and analysis of the genome of the Whipple's disease bacterium Tropheryma whipplei.
Lancet.
2003; 361(9358): 637–44. PubMed Abstract
| Publisher Full Text
- 105.
Renesto P, Crapoulet N, Ogata H, et al.:
Genome-based design of a cell-free culture medium for Tropheryma whipplei.
Lancet.
2003; 362(9382): 447–9. PubMed Abstract
| Publisher Full Text
- 106.
Ogata H, Claverie JM:
Metagrowth: a new resource for the building of metabolic hypotheses in microbiology.
Nucleic Acids Res.
2005; 33(Database issue): D321–D4. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 107.
Omsland A, Cockrell DC, Howe D:
Host cell-free growth of the Q fever bacterium Coxiella burnetii.
Proc Natl Acad Sci U S A.
2009; 106(11): 4430–4. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 108.
Omsland A:
Axenic growth of Coxiella burnetii.
Adv Exp Med Biol.
2012; 984: 215–29. PubMed Abstract
| Publisher Full Text
- 109.
Stewart EJ:
Growing unculturable bacteria.
J Bacteriol.
2012; 194(16): 4151–60. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 110.
Rappé MS, Connon SA, Vergin KL, et al.:
Cultivation of the ubiquitous SAR11 marine bacterioplankton clade.
Nature.
2002; 418(6898): 630–3. PubMed Abstract
| Publisher Full Text
- 111.
Rappé MS, Giovannoni SJ:
The uncultured microbial majority.
Annu Rev Microbiol.
2003; 57: 369–94. PubMed Abstract
| Publisher Full Text
- 112.
Freestone PP, Lyte M:
Microbial endocrinology: experimental design issues in the study of interkingdom signalling in infectious disease.
Adv Appl Microbiol.
2008; 64: 75–105. PubMed Abstract
| Publisher Full Text
- 113.
Freestone PP, Sandrini SM, Haigh RD, et al.:
Microbial endocrinology: how stress influences susceptibility to infection.
Trends Microbiol.
2008; 16(2): 55–64. PubMed Abstract
| Publisher Full Text
- 114.
Lyte M:
Microbial endocrinology and the microbiota-gut-brain axis.
Adv Exp Med Biol.
2014; 817: 3–24. PubMed Abstract
| Publisher Full Text
- 115.
Koch AL:
The adaptive responses of Escherichia coli to a feast and famine existence.
Adv Microb Physiol.
1971; 6: 147–217. PubMed Abstract
| Publisher Full Text
- 116.
Poindexter J:
Oligotrophy: fast and famine existence.
Adv Microbial Ecology.
1981; 5: 63–89. Reference Source
- 117.
Poindexter JS:
Bacterial responses to nutrient limitation.
Symp Soc Gen Microbiol.
1987; 41: 283–317.
- 118.
Zinn M, Witholt B, Egli T:
Dual nutrient limited growth: models, experimental observations, and applications.
J Biotechnol.
2004; 113(1–3): 263–79. PubMed Abstract
| Publisher Full Text
- 119.
Egli T:
How to live at very low substrate concentration.
Water Res.
2010; 44(17): 4826–37. PubMed Abstract
| Publisher Full Text
- 120.
Olsen RA, Bakken LR:
Viability of soil bacteria: Optimization of plate-counting technique and comparison between total counts and plate counts within different size groups.
Microb Ecol.
1987; 13(1): 59–74. PubMed Abstract
| Publisher Full Text
- 121.
Vartoukian SR, Palmer RM, Wade WG:
Strategies for culture of 'unculturable' bacteria.
FEMS Microbiol Lett.
2010; 309(1): 1–7. PubMed Abstract
| Publisher Full Text
- 122.
Dedysh SN:
Cultivating uncultured bacteria from northern wetlands: knowledge gained and remaining gaps.
Front Microbiol.
2011; 2: 184. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 123.
MacDonell MT, Hood MA:
Isolation and characterization of ultramicrobacteria from a gulf coast estuary.
Appl Environ Microbiol.
1982; 43(3): 566–71. PubMed Abstract
| Free Full Text
- 124.
Schut F, de Vries EJ, Gottschal JC, et al.:
Isolation of Typical Marine Bacteria by Dilution Culture: Growth, Maintenance, and Characteristics of Isolates under Laboratory Conditions.
Appl Environ Microbiol.
1993; 59(7): 2150–60. PubMed Abstract
| Free Full Text
- 125.
Schut F, Gottschal JC, Prins RA, et al.:
Isolation and characterisation of the marine ultramicrobacterium Sphingomonas sp. strain RB2256.
FEMS Microbiol Rev.
1997; 20(3–4): 363–9. Publisher Full Text
- 126.
Lysak LV, Lapygina EV, Konova IA, et al.:
Quantity and taxonomic composition of ultramicrobacteria in soils.
Microbiology.
2010; 79(3): 408–12. Publisher Full Text
- 127.
Sahin N, Gonzalez JM, Iizuka T, et al.:
Characterization of two aerobic ultramicrobacteria isolated from urban soil and a description of Oxalicibacterium solurbis sp. nov.
FEMS Microbiol Lett.
2010; 307(1): 25–9. PubMed Abstract
| Publisher Full Text
- 128.
Soina VS, Lysak LA, Konova IA, et al.:
Study of ultramicrobacteria (Nanoforms) in soils and subsoil deposits by electron microscopy.
Eurasian Soil Sci.
2012; 45(11): 1048–56. Publisher Full Text
- 129.
Duda VI, Suzina NE, Polivtseva VN, et al.:
Ultramicrobacteria: Formation of the concept and contribution of ultramicrobacteria to biology.
Mikrobiologiia.
2012; 81(4): 415–27. PubMed Abstract
| Publisher Full Text
- 130.
Tanaka T, Kawasaki K, Daimon S, et al.:
A hidden pitfall in the preparation of agar media undermines microorganism cultivability.
Appl Environ Microbiol.
2014; 80(24): 7659–66. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 131.
Dungait JA, Cardenas LM, Blackwell MS, et al.:
Advances in the understanding of nutrient dynamics and management in UK agriculture.
Sci Total Environ.
2012; 434: 39–50. PubMed Abstract
| Publisher Full Text
- 132.
Schmidt MW, Torn MS, Abiven S, et al.:
Persistence of soil organic matter as an ecosystem property.
Nature.
2011; 478(7367): 49–56. PubMed Abstract
| Publisher Full Text
- 133.
Kell DB:
Large-scale sequestration of atmospheric carbon via plant roots in natural and agricultural ecosystems: why and how.
Philos Trans R Soc Lond B Biol Sci.
2012; 367(1595): 1589–97. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 134.
Kogure K, Simidu U, Taga N:
A tentative direct microscopic method for counting living marine bacteria.
Can J Microbiol.
1979; 25(3): 415–20. PubMed Abstract
| Publisher Full Text
- 135.
Choi JW, Sherr EB, Sherr BF:
Relation between presence-absence of a visible nucleoid and metabolic activity in bacterioplankton cells.
Limnol Oceanogr.
1996; 41(6): 1161–8. Publisher Full Text
- 136.
Goodman AL, Kallstrom G, Faith JJ, et al.:
Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice.
Proc Natl Acad Sci U S A.
2011; 108(15): 6252–7. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 137.
Allen-Vercoe E:
Bringing the gut microbiota into focus through microbial culture: recent progress and future perspective.
Curr Opin Microbiol.
2013; 16(5): 625–9. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 138.
Walker AW, Duncan SH, Louis P, et al.:
Phylogeny, culturing, and metagenomics of the human gut microbiota.
Trends Microbiol.
2014; 22(5): 267–74. PubMed Abstract
| Publisher Full Text
- 139.
Lagier JC, Hugon P, Khelaifia S, et al.:
The rebirth of culture in microbiology through the example of culturomics to study human gut microbiota.
Clin Microbiol Rev.
2015; 28(1): 237–64. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 140.
Booth IR:
Stress and the single cell: intrapopulation diversity is a mechanism to ensure survival upon exposure to stress.
Int J Food Microbiol.
2002; 78(1–2): 19–30. PubMed Abstract
| Publisher Full Text
- 141.
Bishop AL, Rab FA, Sumner ER, et al.:
Phenotypic heterogeneity can enhance rare-cell survival in 'stress-sensitive' yeast populations.
Mol Microbiol.
2007; 63(2): 507–20. PubMed Abstract
| Publisher Full Text
- 142.
Holland SL, Reader T, Dyer PS, et al.:
Phenotypic heterogeneity is a selected trait in natural yeast populations subject to environmental stress.
Environ Microbiol.
2014; 16(6): 1729–40. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 143.
Slatkin M:
Hedging one's evolutionary bets.
Nature.
1974; 250(5469): 704–5. Publisher Full Text
- 144.
Philippi T, Seger J:
Hedging one's evolutionary bets, revisited.
Trends Ecol Evol.
1989; 4(2): 41–4. PubMed Abstract
| Publisher Full Text
- 145.
Veening JW, Smits WK, Kuipers OP:
Bistability, epigenetics, and bet-hedging in bacteria.
Annu Rev Microbiol.
2008; 62: 193–210. PubMed Abstract
| Publisher Full Text
- 146.
Beaumont HJ, Gallie J, Kost C, et al.:
Experimental evolution of bet hedging.
Nature.
2009; 462(7269): 90–3. PubMed Abstract
| Publisher Full Text
- 147.
Balaban NQ:
Persistence: mechanisms for triggering and enhancing phenotypic variability.
Curr Opin Genet Dev.
2011; 21(6): 768–75. PubMed Abstract
| Publisher Full Text
- 148.
Libby E, Rainey PB:
Exclusion rules, bottlenecks and the evolution of stochastic phenotype switching.
Proc Biol Sci.
2011; 278(1724): 3574–83. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 149.
Mora T, Bai F, Che YS, et al.:
Non-genetic individuality in Escherichia coli motor switching.
Phys Biol.
2011; 8(2): 024001. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 150.
Fudenberg D, Imhof LA:
Phenotype switching and mutations in random environments.
Bull Math Biol.
2012; 74(2): 399–421. PubMed Abstract
| Publisher Full Text
- 151.
Carja O, Liberman U, Feldman MW:
The evolution of phenotypic switching in subdivided populations.
Genetics.
2014; 196(4): 1185–97. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 152.
Stepanyan K, Wenseleers T, Duéñez-Guzmán EA, et al.:
Fitness trade-offs explain low levels of persister cells in the opportunistic pathogen Pseudomonas aeruginosa.
Mol ecol.
2015; 24(7): 1572–83. PubMed Abstract
| Publisher Full Text
- 153.
Kell DB, Kaprelyants AS, Grafen A:
Pheromones, social behaviour and the functions of secondary metabolism in bacteria.
Trends Ecol Evol.
1995; 10: 126–9. PubMed Abstract
| Publisher Full Text
- 154.
Mukamolova GV, Kaprelyants AS, Kell DB, et al.:
Adoption of the transiently non-culturable state--a bacterial survival strategy?
Adv Microb Physiol.
2003; 47: 65–129. PubMed Abstract
| Publisher Full Text
- 155.
Hamilton WD:
The evolution of altruistic behaviour.
Amer Nat.
1963; 97(896): 354–6. Reference Source
- 156.
Hamilton WD:
The genetical evolution of social behaviour, I and II.
J Theoret Biol.
1964; 7: 1–52.
- 157.
Morris JJ, Kirkegaard R, Szul MJ, et al.:
Facilitation of robust growth of Prochlorococcus colonies and dilute liquid cultures by "helper" heterotrophic bacteria.
Appl Environ Microbiol.
2008; 74(14): 4530–4. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 158.
Puspita ID, Kamagata Y, Tanaka M, et al.:
Are Uncultivated Bacteria Really Uncultivable?
Microbes Environ.
2012; 27(4): 356–66. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 159.
Nichols D, Cahoon N, Trakhtenberg EM, et al.:
Use of ichip for high-throughput in situ cultivation of "uncultivable" microbial species.
Appl Environ Microbiol.
2010; 76(8): 2445–50. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 160.
Zengler K, Toledo G, Rappe M, et al.:
Cultivating the uncultured.
Proc Natl Acad Sci U S A.
2002; 99(24): 15681–6. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 161.
Zengler K:
Central role of the cell in microbial ecology.
Microbiol Mol Biol Rev.
2009; 73(4): 712–29. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 162.
Ma L, Kim J, Hatzenpichler R, et al.:
Gene-targeted microfluidic cultivation validated by isolation of a gut bacterium listed in Human Microbiome Project's Most Wanted taxa.
Proc Natl Acad Sci U S A.
2014; 111(27): 9768–73. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 163.
Ling LL, Schneider T, Peoples AJ, et al.:
A new antibiotic kills pathogens without detectable resistance.
Nature.
2015; 517(7535): 455–9. PubMed Abstract
| Publisher Full Text
- 164.
Allison KR, Brynildsen MP, Collins JJ:
Metabolite-enabled eradication of bacterial persisters by aminoglycosides.
Nature.
2011; 473(7346): 216–20. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 165.
Allison KR, Brynildsen MP, Collins JJ:
Heterogeneous bacterial persisters and engineering approaches to eliminate them.
Curr Opin Microbiol.
2011; 14(5): 593–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 166.
D'Onofrio A, Crawford JM, Stewart EJ, et al.:
Siderophores from neighboring organisms promote the growth of uncultured bacteria.
Chem Biol.
2010; 17(3): 254–64. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 167.
Kell DB:
Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases.
BMC Med Genomics.
2009; 2: 2. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 168.
Kell DB:
Towards a unifying, systems biology understanding of large-scale cellular death and destruction caused by poorly liganded iron: Parkinson’s, Huntington’s, Alzheimer’s, prions, bactericides, chemical toxicology and others as examples.
Arch Toxicol.
2010; 84(11): 825–89. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 169.
Hider RC, Kong X:
Chemistry and biology of siderophores.
Nat Prod Rep.
2010; 27(5): 637–57. PubMed Abstract
| Publisher Full Text
- 170.
Dworkin J, Shah IM:
Exit from dormancy in microbial organisms.
Nat Rev Microbiol.
2010; 8(12): 890–6. PubMed Abstract
| Publisher Full Text
- 171.
Stevenson BS, Eichorst SA, Wertz JT, et al.:
New strategies for cultivation and detection of previously uncultured microbes.
Appl Environ Microbiol.
2004; 70(8): 4748–55. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 172.
Nichols D, Lewis K, Orjala J, et al.:
Short peptide induces an "uncultivable" microorganism to grow in vitro.
Appl Environ Microbiol.
2008; 74(15): 4889–97. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 173.
Stephens K:
Pheromones among the procaryotes.
Crit Rev Microbiol.
1986; 13(4): 309–34. PubMed Abstract
| Publisher Full Text
- 174.
Kaprelyants AS, Kell DB:
Do bacteria need to communicate with each other for growth?
Trends Microbiol.
1996; 4(6): 237–42. PubMed Abstract
| Publisher Full Text
- 175.
Lewis K, Epstein S, D'Onofrio A, et al.:
Uncultured microorganisms as a source of secondary metabolites.
J Antibiot (Tokyo).
2010; 63(8): 468–76. PubMed Abstract
| Publisher Full Text
- 176.
Dobson PD, Kell DB:
Carrier-mediated cellular uptake of pharmaceutical drugs: an exception or the rule?
Nat Rev Drug Disc.
2008; 7(3): 205–20. PubMed Abstract
| Publisher Full Text
- 177.
Kell DB, Dobson PD, Bilsland E, et al.:
The promiscuous binding of pharmaceutical drugs and their transporter-mediated uptake into cells: what we (need to) know and how we can do so.
Drug Disc Today.
2013; 18(5–6): 218–39. PubMed Abstract
| Publisher Full Text
- 178.
Kell DB, Oliver SG:
How drugs get into cells: tested and testable predictions to help discriminate between transporter-mediated uptake and lipoidal bilayer diffusion.
Front Pharmacol.
2014; 5: 231. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 179.
Kell DB, Swainston N, Pir P, et al.:
Membrane transporter engineering in industrial biotechnology and whole cell biocatalysis.
Trends Biotechnol.
2015; 33(4): 237–46. PubMed Abstract
| Publisher Full Text
- 180.
Kaprelyants AS, Kell DB:
Rapid assessment of bacterial viability and vitality by rhodamine 123 and flow cytometry.
J Appl Bacteriol.
1992; 72(5): 410–22. Publisher Full Text
- 181.
Kaprelyants AS, Kell DB:
The use of 5-Cyano-2,3-ditolyl tetrazolium chloride and flow cytometry for the visualisation of respiratory activity in individual cells of Micrococcus luteus.
J Microbiol Meth.
1993; 17(2): 115–22. Publisher Full Text
- 182.
Kaprelyants AS, Kell DB:
Dormancy in Stationary-Phase Cultures of Micrococcus luteus: Flow Cytometric Analysis of Starvation and Resuscitation.
Appl Environ Microbiol.
1993; 59(10): 3187–96. PubMed Abstract
| Free Full Text
- 183.
Kaprelyants AS, Mukamolova GV, Kell DB:
Estimation of dormant Micrococcus luteus cells by penicillin lysis and by resuscitation in cell-free spent culture medium at high dilution.
FEMS Microbiol Lett.
1994; 115(2–3): 347–52. Publisher Full Text
- 184.
Kaprelyants AS, Mukamolova GV, Davey HM, et al.:
Quantitative Analysis of the Physiological Heterogeneity within Starved Cultures of Micrococcus luteus by Flow Cytometry and Cell Sorting.
Appl Environ Microbiol.
1996; 62(4): 1311–6. PubMed Abstract
| Free Full Text
- 185.
Kell DB, Mukamolova GV, Finan CL, et al.:
Resuscitation of 'uncultured' microorganisms. In: Bull AT, editor. Microbial diversity and bioprospecting.
Washington, DC: American Society for Microbiology.
2003: 100–8. Reference Source
- 186.
Mukamolova GV, Yanopolskaya ND, Kell DB, et al.:
On resuscitation from the dormant state of Micrococcus luteus.
Antonie van Leeuwenhoek.
1998; 73(2): 237–43. PubMed Abstract
| Publisher Full Text
- 187.
Votyakova TV, Kaprelyants AS, Kell DB:
Influence of Viable Cells on the Resuscitation of Dormant Cells in Micrococcus luteus Cultures Held in an Extended Stationary Phase: the Population Effect.
Appl Env Microbiol.
1994; 60(9): 3284–91. PubMed Abstract
| Free Full Text
- 188.
Votyakova TV, Mukamolova GV, ShteinMargolina VA, et al.:
Research on the heterogeneity of a Micrococcus luteus culture during an extended stationary phase: Subpopulation separation and characterization.
Microbiology (Russia).
1998; 67(1): 71–7. Reference Source
- 189.
Kell DB, Ryder HM, Kaprelyants AS, et al.:
Quantifying heterogeneity: Flow cytometry of bacterial cultures.
Antonie van Leeuwenhoek.
1991; 60(3–4): 145–58. PubMed Abstract
| Publisher Full Text
- 190.
Davey HM, Kaprelyants AS, Kell DB:
Flow Cytometric Analysis, using Rhodamine 123, of Micrococcus luteus at Low Growth Rate in Chemostat Culture. In: Lloyd D, editor. Flow cytometry in Microbiology.
London: Springer-Verlag.
1993: 83–93. Publisher Full Text
- 191.
Davey HM, Kell DB, Weichart DH, et al.:
Estimation of microbial viability using flow cytometry.
Current Protoc Cytom.
2004; Chapter 11: Unit 11.3. PubMed Abstract
| Publisher Full Text
- 192.
Davey HM, Kell DB:
Flow cytometry and cell sorting of heterogeneous microbial populations: the importance of single-cell analyses.
Microbiol Rev.
1996; 60(4): 641–96. PubMed Abstract
| Free Full Text
- 193.
Davey HM, Kaprelyants AS, Weichart DH, et al.:
Approaches to the estimation of microbial viability using flow cytometry. In: Robinson JP, editor.
Current Protocols in Cytometry: Volume 11 Microbial Cytometry. New York: Wiley,
1999; 11.3.1–11.3.20. Reference Source
- 194.
Sachidanandham R, Gin KY:
Flow cytometric analysis of prolonged stress-dependent heterogeneity in bacterial cells.
FEMS Microbiol Lett.
2009; 290(2): 143–8. PubMed Abstract
| Publisher Full Text
- 195.
Sachidanandham R, Yew-Hoong Gin K:
A dormancy state in nonspore-forming bacteria.
Appl Microbiol Biotechnol.
2009; 81(5): 927–41. PubMed Abstract
| Publisher Full Text
- 196.
Mukamolova GV, Kaprelyants AS, Young DI, et al.:
A bacterial cytokine.
Proc Natl Acad Sci U S A.
1998; 95: 8916–21. PubMed Abstract
| Free Full Text
- 197.
Young M, Artsatbanov V, Beller HR, et al.:
Genome sequence of the Fleming strain of Micrococcus luteus, a simple free-living actinobacterium.
J Bacteriol.
2010; 192(3): 841–60. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 198.
Mukamolova GV, Turapov OA, Kazarian K, et al.:
The rpf gene of Micrococcus luteus encodes an essential secreted growth factor.
Mol Microbiol.
2002; 46(3): 611–21. PubMed Abstract
| Publisher Full Text
- 199.
Kaprelyants AS, Mukamolova GV, Kormer SS, et al.:
Intercellular signalling and the multiplication of prokaryotes: bacterial cytokines.
Symp Soc Gen Microbiol.
1999; 57: 33–69. Reference Source
- 200.
Schroeckh V, Martin K:
Resuscitation-promoting factors: distribution among actinobacteria, synthesis during life-cycle and biological activity.
Antonie Van Leeuwenhoek.
2006; 89(3–4): 359–65. PubMed Abstract
| Publisher Full Text
- 201.
Koltunov V, Greenblatt CL, Goncharenko AV, et al.:
Structural changes and cellular localization of resuscitation-promoting factor in environmental isolates of Micrococcus luteus.
Microb Ecol.
2010; 59(2): 296–310. PubMed Abstract
| Publisher Full Text
- 202.
Gupta RK, Srivastava R:
Resuscitation promoting factors: a family of microbial proteins in survival and resuscitation of dormant mycobacteria.
Indian J Microbiol.
2012; 52(2): 114–21. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 203.
Ravagnani A, Finan CL, Young M:
A novel firmicute protein family related to the actinobacterial resuscitation-promoting factors by non-orthologous domain displacement.
BMC Genomics.
2005; 6(1): 39. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 204.
Commichau FM, Halbedel S:
The resuscitation promotion concept extends to firmicutes.
Microbiology.
2013; 159(Pt 7): 1298–300. PubMed Abstract
| Publisher Full Text
- 205.
Mukamolova GV, Turapov OA, Young DI, et al.:
A family of autocrine growth factors in Mycobacterium tuberculosis.
Mol Microbiol.
2002; 46(3): 623–35. PubMed Abstract
| Publisher Full Text
- 206.
Downing KJ, Betts JC, Young DI, et al.:
Global expression profiling of strains harbouring null mutations reveals that the five rpf-like genes of Mycobacterium tuberculosis show functional redundancy.
Tuberculosis (Edinb).
2004; 84(3–4): 167–79. PubMed Abstract
| Publisher Full Text
- 207.
Downing KJ, Mischenko VV, Shleeva MO, et al.:
Mutants of Mycobacterium tuberculosis lacking three of the five rpf-like genes are defective for growth in vivo and for resuscitation in vitro.
Infect Immun.
2005; 73(5): 3038–43. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 208.
Yeremeev VV, Kondratieva TK, Rubakova EI, et al.:
Proteins of the Rpf family: immune cell reactivity and vaccination efficacy against tuberculosis in mice.
Infect Immun.
2003; 71(8): 4789–94. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 209.
Keep NH, Ward JM, Cohen-Gonsaud M, et al.:
Wake up! Peptidoglycan lysis and bacterial non-growth states.
Trends Microbiol.
2006; 14(6): 271–6. PubMed Abstract
| Publisher Full Text
- 210.
Mukamolova GV, Murzin AG, Salina EG, et al.:
Muralytic activity of Micrococcus luteus Rpf and its relationship to physiological activity in promoting bacterial growth and resuscitation.
Mol Microbiol.
2006; 59(1): 84–98. PubMed Abstract
| Publisher Full Text
- 211.
Telkov MV, Demina GR, Voloshin SA, et al.:
Proteins of the Rpf (resuscitation promoting factor) family are peptidoglycan hydrolases.
Biochemistry (Mosc).
2006; 71(4): 414–22. PubMed Abstract
| Publisher Full Text
- 212.
Kana BD, Mizrahi V:
Resuscitation-promoting factors as lytic enzymes for bacterial growth and signaling.
FEMS Immunol Med Microbiol.
2010; 58(1): 39–50. PubMed Abstract
| Publisher Full Text
- 213.
Sexton DL, St-Onge RJ, Haiser HJ, et al.:
Resuscitation-promoting factors are cell wall lytic enzymes with important roles in the germination and growth of Streptomyces coelicolor.
J Bacteriol.
2015; 197(5): 848–60. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 214.
Cohen-Gonsaud M, Keep NH, Davies AP, et al.:
Resuscitation-promoting factors possess a lysozyme-like domain.
Trends Biochem Sci.
2004; 29(1): 7–10. PubMed Abstract
| Publisher Full Text
- 215.
Cohen-Gonsaud M, Barthe P, Bagnéris C, et al.:
The structure of a resuscitation-promoting factor domain from Mycobacterium tuberculosis shows homology to lysozymes.
Nat Struct Mol Biol.
2005; 12(3): 270–3. PubMed Abstract
| Publisher Full Text
- 216.
Ruggiero A, Tizzano B, Pedone E, et al.:
Crystal structure of the resuscitation-promoting factor DeltaDUFRpfB from M. tuberculosis.
J Mol Biol.
2009; 385(1): 153–62. PubMed Abstract
| Publisher Full Text
- 217.
Ruggiero A, Squeglia F, Pirone L, et al.:
Expression, purification, crystallization and preliminary X-ray crystallographic analysis of a major fragment of the resuscitation-promoting factor RpfB from Mycobacterium tuberculosis.
Acta Crystallogr Sect F Struct Biol Cryst Commun.
2011; 67(Pt 1): 164–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 218.
Mavrici D, Prigozhin DM, Alber T:
Mycobacterium tuberculosis RpfE crystal structure reveals a positively charged catalytic cleft.
Protein Sci.
2014; 23(4): 481–7. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 219.
Chauviac FX, Robertson G, Quay DH, et al.:
The RpfC (Rv1884) atomic structure shows high structural conservation within the resuscitation-promoting factor catalytic domain.
Acta Crystallogr F Struct Biol Commun.
2014; 70(Pt 8): 1022–6. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 220.
Wivagg CN, Hung DT:
Resuscitation-promoting factors are required for β-lactam tolerance and the permeability barrier in Mycobacterium tuberculosis.
Antimicrob Agents Chemother.
2012; 56(3): 1591–4. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 221.
Zvi A, Ariel N, Fulkerson J, et al.:
Whole genome identification of Mycobacterium tuberculosis vaccine candidates by comprehensive data mining and bioinformatic analyses.
BMC Med Genomics.
2008; 1: 18. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 222.
Russell-Goldman E, Xu J, Wang X, et al.:
A Mycobacterium tuberculosis Rpf double-knockout strain exhibits profound defects in reactivation from chronic tuberculosis and innate immunity phenotypes.
Infect Immun.
2008; 76(9): 4269–81. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 223.
Fan A, Jian W, Shi C, et al.:
Production and characterization of monoclonal antibody against Mycobacterium tuberculosis RpfB domain.
Hybridoma (Larchmt).
2010; 29(4): 327–32. PubMed Abstract
| Publisher Full Text
- 224.
Romano M, Aryan E, Korf H, et al.:
Potential of Mycobacterium tuberculosis resuscitation-promoting factors as antigens in novel tuberculosis sub-unit vaccines.
Microbes Infect.
2012; 14(1): 86–95. PubMed Abstract
| Publisher Full Text
- 225.
Kondratieva T, Rubakova E, Kana BD, et al.:
Mycobacterium tuberculosis attenuated by multiple deletions of rpf genes effectively protects mice against TB infection.
Tuberculosis (Edinb).
2011; 91(3): 219–23. PubMed Abstract
| Publisher Full Text
- 226.
Riaño F, Arroyo L, París S, et al.:
T cell responses to DosR and Rpf proteins in actively and latently infected individuals from Colombia.
Tuberculosis (Edinb).
2012; 92(2): 148–59. PubMed Abstract
| Publisher Full Text
- 227.
Kim JS, Kim WS, Choi HG, et al.:
Mycobacterium tuberculosis RpfB drives Th1-type T cell immunity via a TLR4-dependent activation of dendritic cells.
J Leukocyte Biol.
2013; 94(4): 733–49. PubMed Abstract
| Publisher Full Text
- 228.
Lee J, Kim J, Lee J, et al.:
DNA immunization of Mycobacterium tuberculosis resuscitation-promoting factor B elicits polyfunctional CD8+ T cell responses.
Clin Exp Vaccine Res.
2014; 3(2): 235–43. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 229.
Zhao S, Song X, Zhao Y, et al.:
Protective and therapeutic effects of the resuscitation-promoting factor domain and its mutants against Mycobacterium tuberculosis in mice.
Pathog Dis.
2015; 73(3): pii: ftu025. PubMed Abstract
| Publisher Full Text
- 230.
Davies AP, Dhillon AP, Young M, et al.:
Resuscitation-promoting factors are expressed in Mycobacterium tuberculosis-infected human tissue.
Tuberculosis (Edinb).
2008; 88(5): 462–8. PubMed Abstract
| Publisher Full Text
- 231.
Kesavan AK, Brooks M, Tufariello J, et al.:
Tuberculosis genes expressed during persistence and reactivation in the resistant rabbit model.
Tuberculosis (Edinb).
2009; 89(1): 17–21. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 232.
Ding L, Yokota A:
Curvibacter fontana sp. nov., a microaerobic bacteria isolated from well water.
Gen Appl Microbiol.
2010; 56(3): 267–71. PubMed Abstract
| Publisher Full Text
- 233.
Mukamolova GV, Turapov O, Malkin J, et al.:
Resuscitation-promoting factors reveal an occult population of tubercle Bacilli in Sputum.
Am J Respir Crit Care Med.
2010; 181(2): 174–80. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 234.
Commandeur S, van Meijgaarden KE, Lin MY, et al.:
Identification of human T-cell responses to Mycobacterium tuberculosis resuscitation-promoting factors in long-ferm latently infected individuals.
Clin Vaccine Immunol.
2011; 18(4): 676–83. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 235.
Dewi Puspita I, Uehara M, Katayama T, et al.:
Resuscitation promoting factor (Rpf) from Tomitella biformata AHU 1821T promotes growth and resuscitates non-dividing cells.
Microbes Environ.
2013; 28(1): 58–64. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 236.
Su X, Shen H, Yao X, et al.:
A novel approach to stimulate the biphenyl-degrading potential of bacterial community from PCBs-contaminated soil of e-waste recycling sites.
Bioresour Technol.
2013; 146: 27–34. PubMed Abstract
| Publisher Full Text
- 237.
Turapov O, Glenn S, Kana B, et al.:
The in vivo environment accelerates generation of resuscitation-promoting factor-dependent mycobacteria.
Am J Respir Crit Care Med.
2014; 190(12): 1455–7. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 238.
Shleeva M, Kondratieva T, Rubakova E, et al.:
Reactivation of dormant “non-culturable” Mycobacterium tuberculosis developed in vitro after injection in mice: both the dormancy depth and host genetics influence the outcome.
Microb Pathog.
2015; 78: 63–6. PubMed Abstract
| Publisher Full Text
- 239.
Su XM, Liu YD, Hashmi MZ, et al.:
Culture-dependent and culture-independent characterization of potentially functional biphenyl-degrading bacterial community in response to extracellular organic matter from Micrococcus luteus.
Microb Biotechnol.
2015; 8(3): 569–78. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 240.
Su X, Zhang Q, Hu J:
Enhanced degradation of biphenyl from PCB-contaminated sediments: the impact of extracellular organic matter from Micrococcus luteus.
Appl Microbiol Biotechnol.
2015; 99(4): 1989–2000. PubMed Abstract
| Publisher Full Text
- 241.
Shleeva MO, Bagramyan K, Telkov MV, et al.:
Formation and resuscitation of “non-culturable” cells of Rhodococcus rhodochrous and Mycobacterium tuberculosis in prolonged stationary phase.
Microbiology.
2002; 148(5): 1581–91. PubMed Abstract
- 242.
Shleeva MO, Mukamolova GV, Telkov MV:
Formation of nonculturable Mycobacterium tuberculosis and their regeneration.
Mikrobiologiia.
2003; 72(1): 76–83. PubMed Abstract
- 243.
Zhu W, Plikaytis BB, Shinnick TM:
Resuscitation factors from mycobacteria: homologs of Micrococcus luteus proteins.
Tuberculosis (Edinb).
2003; 83(4): 261–9. PubMed Abstract
| Publisher Full Text
- 244.
Hartmann M, Barsch A, Niehaus K, et al.:
The glycosylated cell surface protein Rpf2, containing a resuscitation-promoting factor motif, is involved in intercellular communication of Corynebacterium glutamicum.
Arch Microbiol.
2004; 182(4): 299–312. PubMed Abstract
| Publisher Full Text
- 245.
Shleeva M, Mukamolova GV, Young M, et al.:
Formation of ‘non-culturable’ cells of Mycobacterium smegmatis in stationary phase in response to growth under suboptimal conditions and their Rpf-mediated resuscitation.
Microbiology.
2004; 150(6): 1687–97. PubMed Abstract
| Publisher Full Text
- 246.
Keep NH, Ward JM, Robertson G, et al.:
Bacterial resuscitation factors: revival of viable but non-culturable bacteria.
Cell Mol Life Sci.
2006; 63(22): 2555–9. PubMed Abstract
| Publisher Full Text
- 247.
Tufariello JM, Mi K, Xu J, et al.:
Deletion of the Mycobacterium tuberculosis resuscitation-promoting factor Rv1009 gene results in delayed reactivation from chronic tuberculosis.
Infect Immun.
2006; 74(5): 2985–95. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 248.
Panutdaporn N, Kawamoto K, Asakura H, et al.:
Resuscitation of the viable but non-culturable state of Salmonella enterica serovar Oranienburg by recombinant resuscitation-promoting factor derived from Salmonella typhimurium strain LT2.
Int J Food Microbiol.
2006; 106(3): 241–7. PubMed Abstract
| Publisher Full Text
- 249.
Biketov S, Potapov V, Ganina E, et al.:
The role of resuscitation promoting factors in pathogenesis and reactivation of Mycobacterium tuberculosis during intra-peritoneal infection in mice.
BMC Infect Dis.
2007; 7: 146. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 250.
Gao H, Bai Y, Xue Y, et al.:
Expression, purification, and characterization of soluble RpfD with high bioactivity as a recombinant protein in Mycobacterium vaccae.
Protein Expr Purif.
2007; 55(1): 112–8. PubMed Abstract
| Publisher Full Text
- 251.
Hett EC, Chao MC, Steyn AJ, et al.:
A partner for the resuscitation-promoting factors of Mycobacterium tuberculosis.
Mol Microbiol.
2007; 66(3): 658–68. PubMed Abstract
| Publisher Full Text
- 252.
Kana BD, Gordhan BG, Downing KJ, et al.:
The resuscitation-promoting factors of Mycobacterium tuberculosis are required for virulence and resuscitation from dormancy but are collectively dispensable for growth in vitro.
Mol Microbiol.
2008; 67(3): 672–84. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 253.
Kana BD, Mizrahi V, Gordhan BG:
Depletion of resuscitation-promoting factors has limited impact on the drug susceptibility of Mycobacterium tuberculosis.
J Antimicrob Chemother.
2010; 65(8): 1583–5. PubMed Abstract
| Publisher Full Text
- 254.
Pinto D, São-José C, Santos MA, et al.:
Characterization of two resuscitation promoting factors of Listeria monocytogenes.
Microbiology.
2013; 159(Pt 7): 1390–401. PubMed Abstract
| Publisher Full Text
- 255.
Nyström T:
Nonculturable bacteria: programmed survival forms or cells at death’s door?
Bioessays.
2003; 25(3): 204–11. PubMed Abstract
| Publisher Full Text
- 256.
Keren I, Shah D, Spoering A, et al.:
Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli.
J Bacteriol.
2004; 186(24): 8172–80. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 257.
Shah D, Zhang Z, Khodursky A, et al.:
Persisters: a distinct physiological state of E. coli.
BMC Microbiol.
2006; 6: 53. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 258.
Keren I, Kaldalu N, Spoering A:
Persister cells and tolerance to antimicrobials.
FEMS Microbiol Lett.
2004; 230(1): 13–8. PubMed Abstract
| Publisher Full Text
- 259.
Tsilibaris V, Maenhaut-Michel G, Mine N, et al.:
What is the benefit to Escherichia coli of having multiple toxin-antitoxin systems in its genome?
J Bacteriol.
2007; 189(17): 6101–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 260.
Jõers A, Kaldalu N, Tenson T:
The frequency of persisters in Escherichia coli reflects the kinetics of awakening from dormancy.
J Bacteriol.
2010; 192(13): 3379–84. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 261.
Luidalepp H, Jõers A, Kaldalu N, et al.:
Age of inoculum strongly influences persister frequency and can mask effects of mutations implicated in altered persistence.
J Bacteriol.
2011; 193(14): 3598–605. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 262.
Kester JC, Fortune SM:
Persisters and beyond: mechanisms of phenotypic drug resistance and drug tolerance in bacteria.
Crit Rev Biochem Mol Biol.
2014; 49(2): 91–101. PubMed Abstract
| Publisher Full Text
- 263.
Tyson JJ, Chen KC, Novak B:
Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell.
Curr Opin Cell Biol.
2003; 15(2): 221–31. PubMed Abstract
| Publisher Full Text
- 264.
Kussell E, Kishony R, Balaban NQ, et al.:
Bacterial persistence: a model of survival in changing environments.
Genetics.
2005; 169(4): 1807–14. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 265.
Dubnau D, Losick R:
Bistability in bacteria.
Mol Microbiol.
2006; 61(3): 564–72. PubMed Abstract
| Publisher Full Text
- 266.
Smits WK, Kuipers OP, Veening JW:
Phenotypic variation in bacteria: the role of feedback regulation.
Nat Rev Microbiol.
2006; 4(4): 259–71. PubMed Abstract
| Publisher Full Text
- 267.
Casadesús J, Low DA:
Programmed heterogeneity: epigenetic mechanisms in bacteria.
J Biol Chem.
2013; 288(20): 13929–35. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 268.
Nelson DE, Ihekwaba AE, Elliott M, et al.:
Oscillations in NF-kappaB signalling control the dynamics of gene expression.
Science.
2004; 306(5696): 704–8. PubMed Abstract
| Publisher Full Text
- 269.
Kell DB:
Theodor Bücher Lecture. Metabolomics, modelling and machine learning in systems biology - towards an understanding of the languages of cells. Delivered on 3 July 2005 at the 30th FEBS Congress and the 9th IUBMB conference in Budapest.
FEBS J.
2006; 273(5): 873–94. PubMed Abstract
| Publisher Full Text
- 270.
Davey HM, Davey CL, Woodward AM, et al.:
Oscillatory, stochastic and chaotic growth rate fluctuations in permittistatically controlled yeast cultures.
Biosystems.
1996; 39(1): 43–61. PubMed Abstract
| Publisher Full Text
- 271.
Ghaemmaghami S, Huh WK, Bower K, et al.:
Global analysis of protein expression in yeast.
Nature.
2003; 425(6959): 737–41. PubMed Abstract
| Publisher Full Text
- 272.
Raser JM, O’Shea EK:
Noise in gene expression: origins, consequences, and control.
Science.
2005; 309(5743): 2010–3. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 273.
Cogan NG, Brown J, Darres K, et al.:
Optimal control strategies for disinfection of bacterial populations with persister and susceptible dynamics.
Antimicrob Agents Chemother.
2012; 56(9): 4816–26. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 274.
Orman MA, Brynildsen MP:
Establishment of a method to rapidly assay bacterial persister metabolism.
Antimicrob Agents Chemother.
2013; 57(9): 4398–409. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 275.
Balaban NQ, Merrin J, Chait R, et al.:
Bacterial persistence as a phenotypic switch.
Science.
2004; 305(5690): 1622–5. PubMed Abstract
| Publisher Full Text
- 276.
Gefen O, Balaban NQ:
The importance of being persistent: heterogeneity of bacterial populations under antibiotic stress.
FEMS Microbiol Rev.
2009; 33(4): 704–17. PubMed Abstract
| Publisher Full Text
- 277.
Lewis K:
Persister cells.
Annu Rev Microbiol.
2010; 64: 357–72. PubMed Abstract
| Publisher Full Text
- 278.
Rainey PB, Beaumont HJ, Ferguson GC, et al.:
The evolutionary emergence of stochastic phenotype switching in bacteria.
Microb Cell Fact.
2011; 10(Suppl 1): S14. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 279.
Balaban NQ, Gerdes K, Lewis K, et al.:
A problem of persistence: still more questions than answers?
Nat Rev Microbiol.
2013; 11(8): 587–91. PubMed Abstract
| Publisher Full Text
- 280.
Zhang Y:
Persisters, persistent infections and the Yin-Yang model.
Emerg Microbes Infec.
2014; 3(1):e3. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 281.
Putrinš M, Kogermann K, Lukk E, et al.:
Phenotypic heterogeneity enables uropathogenic Escherichia coli to evade killing by antibiotics and serum complement.
Infect Immun.
2015; 83(3): 1056–67. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 282.
Rotem E, Loinger A, Ronin I, et al.:
Regulation of phenotypic variability by a threshold-based mechanism underlies bacterial persistence.
Proc Natl Acad Sci U S A.
2010; 107(28): 12541–6. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 283.
Lewis K:
Persister cells and the riddle of biofilm survival.
Biochemistry (Mosc).
2005; 70(2): 267–74. PubMed Abstract
| Publisher Full Text
- 284.
Vázquez-Laslop N, Lee H, Neyfakh AA:
Increased persistence in Escherichia coli caused by controlled expression of toxins or other unrelated proteins.
J Bacteriol.
2006; 188(10): 3494–7. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 285.
Fozo EM, Makarova KS, Shabalina SA, et al.:
Abundance of type I toxin-antitoxin systems in bacteria: searches for new candidates and discovery of novel families.
Nucleic Acids Res.
2010; 38(11): 3743–59. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 286.
Yamaguchi Y, Park JH, Inouye M, et al.:
Toxin-antitoxin systems in bacteria and archaea.
Annu Rev Genet.
2011; 45: 61–79. PubMed Abstract
| Publisher Full Text
- 287.
Yamaguchi Y, Inouye M:
Regulation of growth and death in Escherichia coli by toxin-antitoxin systems.
Nat Rev Microbiol.
2011; 9(11): 779–90. PubMed Abstract
| Publisher Full Text
- 288.
Gerdes K, Maisonneuve E:
Bacterial persistence and toxin-antitoxin loci.
Annu Rev Microbiol.
2012; 66: 103–23. PubMed Abstract
| Publisher Full Text
- 289.
Kint CI, Verstraeten N, Fauvart M, et al.:
New-found fundamentals of bacterial persistence.
Trends Microbiol.
2012; 20(12): 577–85. PubMed Abstract
| Publisher Full Text
- 290.
Leung V, Lévesque CM:
A stress-inducible quorum-sensing peptide mediates the formation of persister cells with noninherited multidrug tolerance.
J Bacteriol.
2012; 194(9): 2265–74. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 291.
Nguyen D, Joshi-Datar A, Lepine F, et al.:
Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria.
Science.
2011; 334(6058): 982–6. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 292.
Amato SM, Orman MA, Brynildsen MP:
Metabolic control of persister formation in Escherichia coli.
Mol Cell.
2013; 50(4): 475–87. PubMed Abstract
| Publisher Full Text
- 293.
Amato SM, Brynildsen MP:
Nutrient transitions are a source of persisters in Escherichia coli biofilms.
PLoS One.
2014; 9(3): e93110. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 294.
Hauryliuk V, Atkinson GC, Murakami KS, et al.:
Recent functional insights into the role of (p)ppGpp in bacterial physiology.
Nat Rev Microbiol.
2015; 13(5): 298–309. PubMed Abstract
| Publisher Full Text
- 295.
Gefen O, Gabay C, Mumcuoglu M, et al.:
Single-cell protein induction dynamics reveals a period of vulnerability to antibiotics in persister bacteria.
Proc Natl Acad Sci U S A.
2008; 105(16): 6145–9. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 296.
Gallie J, Libby E, Bertels F, et al.:
Bistability in a metabolic network underpins the de novo evolution of colony switching in Pseudomonas fluorescens.
PLoS Biol.
2015; 13(3): e1002109. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 297.
West SA, Buckling A:
Cooperation, virulence and siderophore production in bacterial parasites.
Proc Biol Sci.
2003; 270(1510): 37–44. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 298.
Diggle SP, Griffin AS, Campbell GS, et al.:
Cooperation and conflict in quorum-sensing bacterial populations.
Nature.
2007; 450(7168): 411–4. PubMed Abstract
| Publisher Full Text
- 299.
Harrison F, Buckling A:
Cooperative production of siderophores by Pseudomonas aeruginosa.
Front Biosci (Landmark Ed).
2009; 14: 4113–26. PubMed Abstract
| Publisher Full Text
- 300.
Harrison F, Buckling A:
Siderophore production and biofilm formation as linked social traits.
ISME J.
2009; 3(5): 632–4. PubMed Abstract
| Publisher Full Text
- 301.
Reuven P, Eldar A:
Macromotives and microbehaviors: the social dimension of bacterial phenotypic variability.
Curr Opin Genet Dev.
2011; 21(6): 759–67. PubMed Abstract
| Publisher Full Text
- 302.
Schuster M, Sexton DJ, Diggle SP, et al.:
Acyl-homoserine lactone quorum sensing: from evolution to application.
Annu Rev Microbiol.
2013; 67: 43–63. PubMed Abstract
| Publisher Full Text
- 303.
Cornforth DM, Popat R, McNally L, et al.:
Combinatorial quorum sensing allows bacteria to resolve their social and physical environment.
Proc Natl Acad Sci U S A.
2014; 111(11): 4280–4. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 304.
Popat R, Cornforth DM, McNally L, et al.:
Collective sensing and collective responses in quorum-sensing bacteria.
J R Soc Interface.
2015; 12(103): pii: 20140882. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 305.
Fuqua WC, Winans SC, Greenberg EP:
Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators.
J Bacteriol.
1994; 176(2): 269–75. PubMed Abstract
| Free Full Text
- 306.
Lowery CA, Salzameda NT, Sawada D, et al.:
Medicinal chemistry as a conduit for the modulation of quorum sensing.
J Med Chem.
2010; 53(21): 7467–89. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 307.
Galloway WR, Hodgkinson JT, Bowden S, et al.:
Applications of small molecule activators and inhibitors of quorum sensing in Gram-negative bacteria.
Trends Microbiol.
2012; 20(9): 449–58. PubMed Abstract
| Publisher Full Text
- 308.
Kaprelyants AS, Mukamolova GV, Ruggiero A, et al.:
Resuscitation-promoting factors (Rpf): in search of inhibitors.
Protein Pept Lett.
2012; 19(10): 1026–34. PubMed Abstract
| Publisher Full Text
- 309.
Rutherford ST, Bassler BL:
Bacterial quorum sensing: its role in virulence and possibilities for its control.
Cold Spring Harb Perspect Med.
2012; 2(11): pii: a012427. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 310.
Wang Y, Ma S:
Small molecules modulating AHL-based quorum sensing to attenuate bacteria virulence and biofilms as promising antimicrobial drugs.
Curr Med Chem.
2014; 21(3): 296–311. PubMed Abstract
| Publisher Full Text
- 311.
LaSarre B, Federle MJ:
Exploiting quorum sensing to confuse bacterial pathogens.
Microbiol Mol Biol Rev.
2013; 77(1): 73–111. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 312.
Kalia VC:
Quorum sensing inhibitors: an overview.
Biotechnol Adv.
2013; 31(2): 224–45. PubMed Abstract
| Publisher Full Text
- 313.
Kalia VC, Wood TK, Kumar P:
Evolution of resistance to quorum-sensing inhibitors.
Microb Ecol.
2014; 68(1): 13–23. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 314.
Tille PM:
Bailey & Scott's Diagnostic Microbiology. St Louis: Elsevier Mosby.
2014. Reference Source
- 315.
Bennett JE, Dolin R, Blaser MJ:
Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, 8th Edition. Philadelphia: Saunders Elsevier.
2015. Reference Source
- 316.
Murray PR:
The clinician and the microbiology laboratory. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, 8th Edition. Philadelphia: Saunders Elsevier.
2015: 191–223. Reference Source
- 317.
Petti CA, Weinstein MP, Carroll KC:
Systems for detection and identification of bacteria and yeasts. In: Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW, editors. Manual of Clinical Microbiology. 10th Edition. Washington: American Society of Microbiology.
2011: 15–26. Publisher Full Text
- 318.
Nolte FS, Caliendo AM:
Molecular microbiology. In: Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW, editors. Manual of Clinical Microbiology. 10th Edition. Washington: American Society of Microbiology.
2011: 27–59. Publisher Full Text
- 319.
Persing DH, Tenover FC, Tang YW, et al.:
Molecular Microbiology: Diagnostic Principles and Practice. 2nd Ed. Washinton, DC: American Society for Microbiology. 2011. Publisher Full Text
- 320.
Zumla A, Gant V, Bates M, et al.:
Rapid diagnostics urgently needed for killer infections.
Lancet Respir Med.
2013; 1(4): 284–5. PubMed Abstract
| Publisher Full Text
- 321.
Zumla A, Al-Tawfiq JA, Enne VI, et al.:
Rapid point of care diagnostic tests for viral and bacterial respiratory tract infections--needs, advances, and future prospects.
Lancet Infect Dis.
2014; 14(11): 1123–35. PubMed Abstract
| Publisher Full Text
- 322.
Carpenter AB:
Immunoassays for the diagnosis of infectious diseases. In: Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW, editors. Manual of Clinical Microbiology. 10th Edition. Washington: American Society of Microbiology.
2011: 60–72. Publisher Full Text
- 323.
Nikkari S, McLaughlin IJ, Bi W, et al.:
Does blood of healthy subjects contain bacterial ribosomal DNA?
J Clin Microbiol.
2001; 39(5): 1956–9. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 324.
Garcia-Nuñez M, Sopena N, Ragull S, et al.:
Persistence of Legionella in hospital water supplies and nosocomial Legionnaires' disease.
FEMS Immunol Med Microbiol.
2008; 52(2): 202–6. PubMed Abstract
| Publisher Full Text
- 325.
Declerck P:
Biofilms: the environmental playground of Legionella pneumophila.
Environ Microbiol.
2010; 12(3): 557–66. PubMed Abstract
| Publisher Full Text
- 326.
Wang H, Masters S, Hong Y, et al.:
Effect of disinfectant, water age, and pipe material on occurrence and persistence of Legionella, mycobacteria, Pseudomonas aeruginosa, and two amoebas.
Environ Sci Technol.
2012; 46(21): 11566–74. PubMed Abstract
| Publisher Full Text
- 327.
Abdel-Nour M, Duncan C, Low DE, et al.:
Biofilms: the stronghold of Legionella pneumophila.
Int J Mol Sci.
2013; 14(11): 21660–75. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 328.
Hellberg RS, Chu E:
Effects of climate change on the persistence and dispersal of foodborne bacterial pathogens in the outdoor environment: A review.
Crit Rev Microbiol.
2015; 1–25. PubMed Abstract
| Publisher Full Text
- 329.
Khweek AA, Amer A:
Replication of Legionella Pneumophila in Human Cells: Why are We Susceptible?
Front Microbiol.
2010; 1: 133. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 330.
Dehio C:
Bartonella interactions with endothelial cells and erythrocytes.
Trends Microbiol.
2001; 9(6): 279–85. PubMed Abstract
| Publisher Full Text
- 331.
Dehio C:
Molecular and cellular basis of Bartonella pathogenesis.
Annu Rev Microbiol.
2004; 58: 365–90. PubMed Abstract
| Publisher Full Text
- 332.
Harms A, Dehio C:
Intruders below the radar: molecular pathogenesis of Bartonella spp.
Clin Microbiol Rev.
2012; 25(1): 42–78. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 333.
Pulliainen AT, Dehio C:
Persistence of Bartonella spp. stealth pathogens: from subclinical infections to vasoproliferative tumor formation.
FEMS Microbiol Rev.
2012; 36(3): 563–99. PubMed Abstract
| Publisher Full Text
- 334.
Roop RM, Gaines JM, Anderson ES, et al.:
Survival of the fittest: how Brucella strains adapt to their intracellular niche in the host.
Med Microbiol Immunol.
2009; 198(4): 221–38. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 335.
Atluri VL, Xavier MN, de Jong MF, et al.:
Interactions of the human pathogenic Brucella species with their hosts.
Annu Rev Microbiol.
2011; 65: 523–41. PubMed Abstract
| Publisher Full Text
- 336.
Martirosyan A, Moreno E, Gorvel JP:
An evolutionary strategy for a stealthy intracellular Brucella pathogen.
Immunol Rev.
2011; 240(1): 211–34. PubMed Abstract
| Publisher Full Text
- 337.
von Bargen K, Gorvel JP, Salcedo SP:
Internal affairs: investigating the Brucella intracellular lifestyle.
FEMS Microbiol Rev.
2012; 36(3): 533–62. PubMed Abstract
| Publisher Full Text
- 338.
Lungu B, Ricke SC, Johnson MG:
Growth, survival, proliferation and pathogenesis of Listeria monocytogenes under low oxygen or anaerobic conditions: a review.
Anaerobe.
2009; 15(1–2): 7–17. PubMed Abstract
| Publisher Full Text
- 339.
Xayarath B, Freitag NE:
Optimizing the balance between host and environmental survival skills: lessons learned from Listeria monocytogenes.
Future Microbiol.
2012; 7(7): 839–52. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 340.
Barry CE, Boshoff HI, Dartois V, et al.:
The spectrum of latent tuberculosis: rethinking the biology and intervention strategies.
Nat Rev Microbiol.
2009; 7(12): 845–55. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 341.
Gengenbacher M, Kaufmann SH:
Mycobacterium tuberculosis: success through dormancy.
FEMS Microbiol Rev.
2012; 36(3): 514–32. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 342.
Cambier CJ, Falkow S, Ramakrishnan L:
Host evasion and exploitation schemes of Mycobacterium tuberculosis.
Cell.
2014; 159(7): 1497–509. PubMed Abstract
| Publisher Full Text
- 343.
Kondratieva T, Azhikina T, Nikonenko B, et al.:
Latent tuberculosis infection: what we know about its genetic control?
Tuberculosis (Edinb).
2014; 94(5): 462–8. PubMed Abstract
| Publisher Full Text
- 344.
Monin L, Khader SA:
Chemokines in tuberculosis: the good, the bad and the ugly.
Semin Immunol.
2014; 26(6): 552–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 345.
Orme IM, Basaraba RJ:
The formation of the granuloma in tuberculosis infection.
Semin Immunol.
2014; 26(6): 601–9. PubMed Abstract
| Publisher Full Text
- 346.
Barry C:
Infectious disease. More than just bugs in spit.
Science.
2015; 348(6235): 633–4. PubMed Abstract
| Publisher Full Text
- 347.
Bumann D:
Heterogeneous host-pathogen encounters: act locally, think globally.
Cell Host Microbe.
2015; 17(1): 13–9. PubMed Abstract
| Publisher Full Text
- 348.
Guirado E, Mbawuike U, Keiser TL, et al.:
Characterization of host and microbial determinants in individuals with latent tuberculosis infection using a human granuloma model.
MBio.
2015; 6(1): e02537–14. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 349.
Gonzalez-Escobedo G, Gunn JS:
Gallbladder epithelium as a niche for chronic Salmonella carriage.
Infect Immun.
2013; 81(8): 2920–30. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 350.
Claudi B, Spröte P, Chirkova A, et al.:
Phenotypic variation of Salmonella in host tissues delays eradication by antimicrobial chemotherapy.
Cell.
2014; 158(4): 722–33. PubMed Abstract
| Publisher Full Text
- 351.
Helaine S, Cheverton AM, Watson KG, et al.:
Internalization of Salmonella by macrophages induces formation of nonreplicating persisters.
Science.
2004; 343(6167): 204–8. PubMed Abstract
| Publisher Full Text
- 352.
Holden DW:
Microbiology. Persisters unmasked.
Science.
2015; 347(6217): 30–2. PubMed Abstract
| Publisher Full Text
- 353.
Thwaites GE, Gant V:
Are bloodstream leukocytes Trojan Horses for the metastasis of Staphylococcus aureus?
Nat Rev Microbiol.
2011; 9(3): 215–22. PubMed Abstract
| Publisher Full Text
- 354.
Prajsnar TK, Hamilton R, Garcia-Lara J, et al.:
A privileged intraphagocyte niche is responsible for disseminated infection of Staphylococcus aureus in a zebrafish model.
Cell Microbiol.
2012; 14(10): 1600–19. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 355.
Proctor RA, Kriegeskorte A, Kahl BC, et al.:
Staphylococcus aureus Small Colony Variants (SCVs): a road map for the metabolic pathways involved in persistent infections.
Front Cell Infect Microbiol.
2014; 4: 99. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 356.
Kahl BC:
Small colony variants (SCVs) of Staphylococcus aureus--a bacterial survival strategy.
Infect Genet Evol.
2014; 21: 515–22. PubMed Abstract
| Publisher Full Text
- 357.
Fredricks DN, Relman DA:
Improved amplification of microbial DNA from blood cultures by removal of the PCR inhibitor sodium polyanetholesulfonate.
J Clin Microbiol.
1998; 36(10): 2810–6. PubMed Abstract
| Free Full Text
- 358.
Tanner MA, Goebel BM, Dojka MA, et al.:
Specific ribosomal DNA sequences from diverse environmental settings correlate with experimental contaminants.
Appl Environ Microbiol.
1998; 64(8): 3110–3. PubMed Abstract
| Free Full Text
- 359.
Millar BC, Xu J, Moore JE:
Risk assessment models and contamination management: implications for broad-range ribosomal DNA PCR as a diagnostic tool in medical bacteriology.
J Clin Microbiol.
2002; 40(5): 1575–80. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 360.
Schroeter J, Wilkemeyer I, Schiller RA, et al.:
Validation of the Microbiological Testing of Tissue Preparations Using the BACTECTM Blood Culture System.
Transfus Med Hemother.
2012; 39(6): 387–90. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 361.
Salter SJ, Cox MJ, Turek EM, et al.:
Reagent and laboratory contamination can critically impact sequence-based microbiome analyses.
BMC Biol.
2014; 12(1): 87. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 362.
Mylotte JM, Tayara A:
Blood cultures: clinical aspects and controversies.
Eur J Clin Microbiol Infect Dis.
2000; 19(3): 157–63. PubMed Abstract
- 363.
Ribault S, Faucon A, Grave L, et al.:
Detection of bacteria in red blood cell concentrates by the Scansystem method.
J Clin Microbiol.
2005; 43(5): 2251–5. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 364.
Amar J, Serino M, Lange C:
Involvement of tissue bacteria in the onset of diabetes in humans: evidence for a concept.
Diabetologia.
2011; 54(12): 3055–61. PubMed Abstract
| Publisher Full Text
- 365.
Dinakaran V, Rathinavel A, Pushpanathan M, et al.:
Elevated levels of circulating DNA in cardiovascular disease patients: metagenomic profiling of microbiome in the circulation.
PLoS One.
2014; 9(8): e105221. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 366.
Didelot X, Bowden R, Wilson DJ, et al.:
Transforming clinical microbiology with bacterial genome sequencing.
Nat Rev Genet.
2012; 13(9): 601–12. PubMed Abstract
| Publisher Full Text
- 367.
Loman NJ, Constantinidou C, Chan JZ, et al.:
High-throughput bacterial genome sequencing: an embarrassment of choice, a world of opportunity.
Nat Rev Microbiol.
2012; 10(9): 599–606. PubMed Abstract
| Publisher Full Text
- 368.
Shendure J, Lieberman Aiden E:
The expanding scope of DNA sequencing.
Nat Biotechnol.
2012; 30(11): 1084–94. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 369.
Fichot EB, Norman RS:
Microbial phylogenetic profiling with the Pacific Biosciences sequencing platform.
Microbiome.
2013; 1(1): 10. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 370.
Padmanabhan R, Mishra AK, Raoult D, et al.:
Genomics and metagenomics in medical microbiology.
J Microbiol Methods.
2013; 95(3): 415–24. PubMed Abstract
| Publisher Full Text
- 371.
Fricke WF, Rasko DA:
Bacterial genome sequencing in the clinic: bioinformatic challenges and solutions.
Nat Rev Genet.
2014; 15(1): 49–55. PubMed Abstract
| Publisher Full Text
- 372.
Ryu H, Henson M, Elk M, et al.:
Development of quantitative PCR assays targeting the 16S rRNA genes of Enterococcus spp. and their application to the identification of Enterococcus species in environmental samples.
Appl Environ Microbiol.
2013; 79(1): 196–204. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 373.
Clarridge JE 3rd:
Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases.
Clin Microbiol Rev.
2004; 17(4): 840–62. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 374.
Petti CA, Polage CR, Schreckenberger P:
The role of 16S rRNA gene sequencing in identification of microorganisms misidentified by conventional methods.
J Clin Microbiol.
2005; 43(12): 6123–5. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 375.
Dreier J, Störmer M, Kleesiek K:
Real-time polymerase chain reaction in transfusion medicine: applications for detection of bacterial contamination in blood products.
Transfus Med Rev.
2007; 21(3): 237–54. PubMed Abstract
| Publisher Full Text
- 376.
Jiang W, Lederman MM, Hunt P, et al.:
Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection.
J Infect Dis.
2009; 199(8): 1177–85. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 377.
Varani S, Stanzani M, Paolucci M, et al.:
Diagnosis of bloodstream infections in immunocompromised patients by real-time PCR.
J Infect.
2009; 58(5): 346–51. PubMed Abstract
| Publisher Full Text
- 378.
Grif K, Heller I, Prodinger WM, et al.:
Improvement of detection of bacterial pathogens in normally sterile body sites with a focus on orthopedic samples by use of a commercial 16S rRNA broad-range PCR and sequence analysis.
J Clin Microbiol.
2012; 50(7): 2250–4. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 379.
Grif K, Fille M, Würzner R, et al.:
Rapid detection of bloodstream pathogens by real-time PCR in patients with sepsis.
Wien Klin Wochenschr.
2012; 124(7–8): 266–70. PubMed Abstract
| Publisher Full Text
- 380.
Pence MA, McElvania TeKippe E, Burnham CA:
Diagnostic assays for identification of microorganisms and antimicrobial resistance determinants directly from positive blood culture broth.
Clin Lab Med.
2013; 33(3): 651–84. PubMed Abstract
| Publisher Full Text
- 381.
Riedel S, Carroll KC:
Laboratory detection of sepsis: biomarkers and molecular approaches.
Clin Lab Med.
2013; 33(3): 413–37. PubMed Abstract
| Publisher Full Text
- 382.
Salipante SJ, Sengupta DJ, Rosenthal C, et al.:
Rapid 16S rRNA next-generation sequencing of polymicrobial clinical samples for diagnosis of complex bacterial infections.
PLoS One.
2013; 8(5): e65226. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 383.
Buchan BW, Ledeboer NA:
Emerging technologies for the clinical microbiology laboratory.
Clin Microbiol Rev.
2014; 27(4): 783–822. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 384.
Kothari A, Morgan M, Haake DA:
Emerging technologies for rapid identification of bloodstream pathogens.
Clin Infect Dis.
2014; 59(2): 272–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 385.
Loonen AJ, Wolffs PF, Bruggeman CA, et al.:
Developments for improved diagnosis of bacterial bloodstream infections.
Eur J Clin Microbiol Infect Dis.
2014; 33(10): 1687–702. PubMed Abstract
| Publisher Full Text
- 386.
Harris KA, Hartley JC:
Development of broad-range 16S rDNA PCR for use in the routine diagnostic clinical microbiology service.
J Med Microbiol.
2003; 52(Pt 8): 685–91. PubMed Abstract
| Publisher Full Text
- 387.
Woo PC, Lau SK, Teng JL, et al.:
Then and now: use of 16S rDNA gene sequencing for bacterial identification and discovery of novel bacteria in clinical microbiology laboratories.
Clin Microbiol Infect.
2008; 14(10): 908–34. PubMed Abstract
| Publisher Full Text
- 388.
Dark P, Blackwood B, Gates S, et al.:
Accuracy of LightCycler(®) SeptiFast for the detection and identification of pathogens in the blood of patients with suspected sepsis: a systematic review and meta-analysis.
Intensive Care Med.
2015; 41(1): 21–33. PubMed Abstract
| Publisher Full Text
- 389.
Warhurst G, Maddi S, Dunn G, et al.:
Diagnostic accuracy of SeptiFast multi-pathogen real-time PCR in the setting of suspected healthcare-associated bloodstream infection.
Intensive Care Med.
2015; 41(1): 86–93. PubMed Abstract
| Publisher Full Text
- 390.
Gauduchon V, Chalabreysse L, Etienne J, et al.:
Molecular diagnosis of infective endocarditis by PCR amplification and direct sequencing of DNA from valve tissue.
J Clin Microbiol.
2003; 41(2): 763–6. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 391.
Schabereiter-Gurtner C, Nehr M, Apfalter P, et al.:
Evaluation of a protocol for molecular broad-range diagnosis of culture-negative bacterial infections in clinical routine diagnosis.
J Appl Microbiol.
2008; 104(4): 1228–37. PubMed Abstract
| Publisher Full Text
- 392.
Sontakke S, Cadenas MB, Maggi RG, et al.:
Use of broad range16S rDNA PCR in clinical microbiology.
J Microbiol Methods.
2009; 76(3): 217–25. PubMed Abstract
| Publisher Full Text
- 393.
Domingue GJ, Ghoniem GM, Bost KL, et al.:
Dormant microbes in interstitial cystitis.
J Urol.
1995; 153(4): 1321–6. PubMed Abstract
| Publisher Full Text
- 394.
Fournier PE, Thuny F, Richet H, et al.:
Comprehensive diagnostic strategy for blood culture-negative endocarditis: a prospective study of 819 new cases.
Clin Infect Dis.
2010; 51(2): 131–40. PubMed Abstract
| Publisher Full Text
- 395.
Tattevin P, Watt G, Revest M, et al.:
Update on blood culture-negative endocarditis.
Med Mal Infect.
2015; 45(1–2): 1–8. PubMed Abstract
| Publisher Full Text
- 396.
Aarthi P, Harini R, Sowmiya M, et al.:
Identification of bacteria in culture negative and polymerase chain reaction (PCR) positive intraocular specimen from patients with infectious endopthalmitis.
J Microbiol Methods.
2011; 85(1): 47–52. PubMed Abstract
| Publisher Full Text
- 397.
Rampini SK, Bloemberg GV, Keller PM, et al.:
Broad-range 16S rRNA gene polymerase chain reaction for diagnosis of culture-negative bacterial infections.
Clin Infect Dis.
2011; 53(12): 1245–51. PubMed Abstract
| Publisher Full Text
- 398.
Sleigh J, Cursons R, La Pine M:
Detection of bacteraemia in critically ill patients using 16S rDNA polymerase chain reaction and DNA sequencing.
Intensive Care Med.
2001; 27(8): 1269–73. PubMed Abstract
| Publisher Full Text
- 399.
Bloos F, Sachse S, Kortgen A, et al.:
Evaluation of a polymerase chain reaction assay for pathogen detection in septic patients under routine condition: an observational study.
PLoS One.
2012; 7(9): e46003. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 400.
Lodes U, Bohmeier B, Lippert H, et al.:
PCR-based rapid sepsis diagnosis effectively guides clinical treatment in patients with new onset of SIRS.
Langenbecks Arch Surg.
2012; 397(3): 447–55. PubMed Abstract
| Publisher Full Text
- 401.
Bloos F, Hinder F, Becker K, et al.:
A multicenter trial to compare blood culture with polymerase chain reaction in severe human sepsis.
Intensive Care Med.
2010; 36(2): 241–7. PubMed Abstract
| Publisher Full Text
- 402.
Lucignano B, Ranno S, Liesenfeld O, et al.:
Multiplex PCR allows rapid and accurate diagnosis of bloodstream infections in newborns and children with suspected sepsis.
J clin microbiol.
2011; 49(6): 2252–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 403.
Liu CL, Ai HW, Wang WP, et al.:
Comparison of 16S rRNA gene PCR and blood culture for diagnosis of neonatal sepsis.
Arch Pediatr.
2014; 21(2): 162–9. PubMed Abstract
| Publisher Full Text
- 404.
Levy PY, Fournier PE, Fenollar F, et al.:
Systematic PCR detection in culture-negative osteoarticular infections.
Am J Med.
2013; 126(12): 1143.e25–33. PubMed Abstract
| Publisher Full Text
- 405.
Renvoisé A, Brossier F, Sougakoff W, et al.:
Broad-range PCR: past, present, or future of bacteriology?
Med Mal Infect.
2013; 43(8): 322–30. PubMed Abstract
| Publisher Full Text
- 406.
Lleo MM, Ghidini V, Tafi MC, et al.:
Detecting the presence of bacterial DNA by PCR can be useful in diagnosing culture-negative cases of infection, especially in patients with suspected infection and antibiotic therapy.
FEMS Microbiol Lett.
2014; 354(2): 153–60. PubMed Abstract
| Publisher Full Text
- 407.
Welinder-Olsson C, Dotevall L, Hogevik H, et al.:
Comparison of broad-range bacterial PCR and culture of cerebrospinal fluid for diagnosis of community-acquired bacterial meningitis.
Clin Microbiol Infect.
2007; 13(9): 879–86. PubMed Abstract
| Publisher Full Text
- 408.
Pandit L, Kumar S, Karunasagar I, et al.:
Diagnosis of partially treated culture-negative bacterial meningitis using 16S rRNA universal primers and restriction endonuclease digestion.
J Med Microbiol.
2005; 54(Pt 6): 539–42. PubMed Abstract
| Publisher Full Text
- 409.
Saglani S, Harris KA, Wallis C, et al.:
Empyema: the use of broad range 16S rDNA PCR for pathogen detection.
Arch Dis Child.
2005; 90(1): 70–3. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 410.
Tran NK, Wisner DH, Albertson TE, et al.:
Multiplex polymerase chain reaction pathogen detection in patients with suspected septicemia after trauma, emergency, and burn surgery.
Surgery.
2012; 151(3): 456–63. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 411.
Billings F:
Focal infection. New York: Appleton, 1915.
- 412.
Price WA:
Dental infections oral and systemic, being a contribution to the pathology of dental infections, focal infections and the degenerative diseases, Parts I and II. Cleveland: Penton Press, 1923.
Reference Source
- 413.
Domingue GJ:
Electron dense cytoplasmic particles and chronic infection: a bacterial pleomorphy hypothesis.
Endocytobiosis & Cell Res.
1995; 11: 19–40. Reference Source
- 414.
Domingue GJ Sr, Woody HB:
Bacterial persistence and expression of disease.
Clin Microbiol Rev.
1997; 10(2): 320–44. PubMed Abstract
| Free Full Text
- 415.
Domingue GJ:
Demystifying pleomorphic forms in persistence and expression of disease: Are they bacteria, and is peptidoglycan the solution?
Discov Med.
2010; 10(52): 234–46. PubMed Abstract
- 416.
Mattman L:
Cell Wall Deficient Forms, Third Edition: Stealth Pathogens. Boca Raton: CRC Press.
2001.
Reference Source
- 417.
Ewald PW:
Plague time: the new germ theory of disease.
New York: Anchor Books.
2002.
Reference Source
- 418.
Onwuamaegbu ME, Belcher RA, Soare C:
Cell wall-deficient bacteria as a cause of infections: a review of the clinical significance.
J Int Med Res.
2005; 33(1): 1–20. PubMed Abstract
| Publisher Full Text
- 419.
Domingue GJ, Schlegel JU:
Novel bacterial structures in human blood: cultural isolation.
Infect Immun.
1977; 15(2): 621–7. PubMed Abstract
| Free Full Text
- 420.
Clasener H:
Pathogenicity of the L-phase of bacteria.
Annu Rev Microbiol.
1972; 26: 55–84. PubMed Abstract
| Publisher Full Text
- 421.
Lipinski B, Pretorius E:
The role of iron-induced fibrin in the pathogenesis of Alzheimer's disease and the protective role of magnesium.
Front Hum Neurosci.
2013; 7: 735. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 422.
Pretorius E, Swanepoel AC, Buys AV, et al.:
Eryptosis as a marker of Parkinson’s disease.
Aging (Albany NY).
2014; 6(10): 788–819. PubMed Abstract
| Free Full Text
- 423.
Bester J, Buys AV, Lipinski B, et al.:
High ferritin levels have major effects on the morphology of erythrocytes in Alzheimer's disease.
Front Aging Neurosci.
2013; 5: 88. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 424.
Pretorius E, Vermeulen N, Bester J, et al.:
Novel use of scanning electron microscopy for detection of iron-induced morphological changes in human blood.
Microsc Res Tech.
2013; 76(3): 268–71. PubMed Abstract
| Publisher Full Text
- 425.
Pretorius E, Lipinski B:
Thromboembolic ischemic stroke changes red blood cell morphology.
Cardiovasc Pathol.
2013; 22(3): 241–2. PubMed Abstract
| Publisher Full Text
- 426.
Pretorius E, Lipinski B:
Iron alters red blood cell morphology.
Blood.
2013; 121(1): 9. PubMed Abstract
| Publisher Full Text
- 427.
Pretorius E, Bester J, Vermeulen N, et al.:
Profound morphological changes in the erythrocytes and fibrin networks of patients with hemochromatosis or with hyperferritinemia, and their normalization by iron chelators and other agents.
PLoS One.
2014; 9(1): e85271. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 428.
Pretorius E, du Plooy J, Soma P, et al.:
An ultrastructural analysis of platelets, erythrocytes, white blood cells, and fibrin network in systemic lupus erythematosus.
Rheumatol Int.
2014; 34(7): 1005–9. PubMed Abstract
| Publisher Full Text
- 429.
Pretorius E, Kell DB:
Diagnostic morphology: biophysical indicators for iron-driven inflammatory diseases.
Integr Biol (Camb).
2014; 6(5): 486–510. PubMed Abstract
| Publisher Full Text
- 430.
Pretorius E, Bester J, Vermeulen N, et al.:
Extreme morphological changes in the erythrocytes and fibrin networks of patients with Hepatitis C. 2015.
- 431.
Pretorius E, Bester J, Vermeulen N, et al.:
Poorly controlled type 2 diabetes is accompanied by significant morphological and ultrastructural changes in both erythrocytes and in thrombin-generated fibrin: implications for diagnostics.
Cardiovasc Diabetol.
2015; 14: 30. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 432.
Kell DB, Pretorius E:
Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells.
Metallomics.
2014; 6(4): 748–73. PubMed Abstract
| Publisher Full Text
- 433.
McLaughlin RW, Vali H, Lau PC, et al.:
Are there naturally occurring pleomorphic bacteria in the blood of healthy humans?
J Clin Microbiol.
2002; 40(12): 4771–5. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 434.
Pohlod DJ, Mattman LH, Tunstall L:
Structures suggesting cell-wall-deficient forms detected in circulating erythrocytes by fluorochrome staining.
Appl Microbiol.
1972; 23(2): 262–7. PubMed Abstract
| Free Full Text
- 435.
Tedeschi GG, Bondi A, Paparelli M, et al.:
Electron microscopical evidence of the evolution of corynebacteria-like microorganisms within human erythrocytes.
Experientia.
1978; 34(4): 458–60. PubMed Abstract
| Publisher Full Text
- 436.
Tedeschi GG, Sprovieri G, Prete DP:
Cocci and diphtheroids in blood cultures from patients in various pathological situations.
Experientia.
1978; 34(5): 596–8. PubMed Abstract
| Publisher Full Text
- 437.
Tedeschi GG, Di Iorio EE:
Penetration and interaction with haemoglobin of corynebacteria-like microorganisms into erythrocytes in vitro.
Experientia.
1979; 35(3): 330–2. PubMed Abstract
| Publisher Full Text
- 438.
Damgaard C, Magnussen K, Enevold C, et al.:
Viable bacteria associated with red blood cells and plasma in freshly drawn blood donations.
PLoS One.
2015; 10(3): e0120826. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 439.
Kunishima S, Inoue C, Kamiya T, et al.:
Presence of Propionibacterium acnes in blood components.
Transfusion.
2001; 41(9): 1126–9. PubMed Abstract
| Publisher Full Text
- 440.
Walther-Wenke G:
Incidence of bacterial transmission and transfusion reactions by blood components.
Clin Chem Lab Med.
2008; 46(7): 919–25. PubMed Abstract
| Publisher Full Text
- 441.
Montag T:
Strategies of bacteria screening in cellular blood components.
Clin Chem Lab Med.
2008; 46(7): 926–32. PubMed Abstract
| Publisher Full Text
- 442.
Rohde JM, Dimcheff DE, Blumberg N, et al.:
Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis.
JAMA.
2014; 311(13): 1317–26. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 443.
Carson JL:
Blood transfusion and risk of infection: new convincing evidence.
JAMA.
2014; 311(13): 1293–4; discussion 716-7. PubMed Abstract
| Publisher Full Text
- 444.
Offner PJ, Moore EE, Biffl WL, et al.:
Increased rate of infection associated with transfusion of old blood after severe injury.
Arch Surg.
2002; 137(6): 711–6; discussion 716–7. PubMed Abstract
| Publisher Full Text
- 445.
Perez P, Salmi LR, Follea G, et al.:
Determinants of transfusion-associated bacterial contamination: results of the French BACTHEM Case-Control Study.
Transfusion.
2001; 41(7): 862–72. PubMed Abstract
| Publisher Full Text
- 446.
Vasconcelos E, Seghatchian J:
Bacterial contamination in blood components and preventative strategies: an overview.
Transfus Apher Sci.
2004; 31(2): 155–63. PubMed Abstract
| Publisher Full Text
- 447.
Klausen SS, Hervig T, Seghatchian J, et al.:
Bacterial contamination of blood components: Norwegian strategies in identifying donors with higher risk of inducing septic transfusion reactions in recipients.
Transfus Apher Sci.
2014; 51(2): 97–102. PubMed Abstract
| Publisher Full Text
- 448.
Nelson RA Jr:
The immune-adherence phenomenon; an immunologically specific reaction between microorganisms and erythrocytes leading to enhanced phagocytosis.
Science.
1953; 118(3077): 733–7. PubMed Abstract
| Publisher Full Text
- 449.
Belstrøm D, Holmstrup P, Damgaard C, et al.:
The atherogenic bacterium Porphyromonas gingivalis evades circulating phagocytes by adhering to erythrocytes.
Infect Immun.
2011; 79(4): 1559–65. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 450.
Ebringer A, Rashid T, Wilson C:
Rheumatoid arthritis, Proteus, anti-CCP antibodies and Karl Popper.
Autoimmun Rev.
2010; 9(4): 216–23. PubMed Abstract
| Publisher Full Text
- 451.
Cani PD, Amar J, Iglesias MA, et al.:
Metabolic endotoxemia initiates obesity and insulin resistance.
Diabetes.
2007; 56(7): 1761–72. PubMed Abstract
| Publisher Full Text
- 452.
Manco M, Putignani L, Bottazzo GF:
Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk.
Endocr Rev.
2010; 31(6): 817–44. PubMed Abstract
| Publisher Full Text
- 453.
Lawrence CB, Brough D, Knight EM:
Obese mice exhibit an altered behavioural and inflammatory response to lipopolysaccharide.
Dis Model Mech.
2012; 5(5): 649–59. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 454.
Jin C, Flavell RA:
Innate sensors of pathogen and stress: linking inflammation to obesity.
J Allergy Clin Immunol.
2013; 132(2): 287–94. PubMed Abstract
| Publisher Full Text
- 455.
Jin C, Henao-Mejia J, Flavell RA:
Innate immune receptors: key regulators of metabolic disease progression.
Cell Metab.
2013; 17(6): 873–82. PubMed Abstract
| Publisher Full Text
- 456.
Zhao L:
The gut microbiota and obesity: from correlation to causality.
Nat Rev Microbiol.
2013; 11(9): 639–47. PubMed Abstract
| Publisher Full Text
- 457.
Cunningham C, Wilcockson DC, Campion S, et al.:
Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration.
J Neurosci.
2005; 25(40): 9275–84. PubMed Abstract
| Publisher Full Text
- 458.
Heneka MT, Kummer MP, Latz E:
Innate immune activation in neurodegenerative disease.
Nat Rev Immunol.
2014; 14(7): 463–77. PubMed Abstract
| Publisher Full Text
- 459.
Heneka MT, Carson MJ, Khoury JE, et al.:
Neuroinflammation in Alzheimer's disease.
Lancet Neurol.
2015; 14(4): 388–405. PubMed Abstract
| Publisher Full Text
- 460.
Tufekci KU, Genc S, Genc K:
The endotoxin-induced neuroinflammation model of Parkinson's disease.
Parkinsons Dis.
2011; 2011: 487450. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 461.
Naser SA, Ghobrial G, Romero C, et al.:
Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn's disease.
Lancet.
2004; 364(9439): 1039–44. PubMed Abstract
| Publisher Full Text
- 462.
Feller M, Huwiler K, Stephan R, et al.:
Mycobacterium avium subspecies paratuberculosis and Crohn's disease: a systematic review and meta-analysis.
Lancet Infect Dis.
2007; 7(9): 607–13. PubMed Abstract
| Publisher Full Text
- 463.
Hermon-Taylor J:
Mycobacterium avium subspecies paratuberculosis, Crohn's disease and the Doomsday scenario.
Gut Pathog.
2009; 1(1): 15. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 464.
Parkin DM:
The global health burden of infection-associated cancers in the year 2002.
Int J Cancer.
2006; 118(12): 3030–44. PubMed Abstract
| Publisher Full Text
- 465.
De Spiegeleer B, Verbeke F, D'Hondt M, et al.:
The quorum sensing peptides PhrG, CSP and EDF promote angiogenesis and invasion of breast cancer cells in vitro.
PLoS One.
2015; 10(3): e0119471. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 466.
Louis P, Hold GL, Flint HJ:
The gut microbiota, bacterial metabolites and colorectal cancer.
Nat Rev Microbiol.
2014; 12(10): 661–72. PubMed Abstract
| Publisher Full Text
- 467.
Sheflin AM, Whitney AK, Weir TL:
Cancer-promoting effects of microbial dysbiosis.
Curr Oncol Rep.
2014; 16(10): 406. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 468.
Urbaniak C, Cummins J, Brackstone M, et al.:
Microbiota of human breast tissue.
Appl Environ Microbiol.
2014; 80(10): 3007–14. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 469.
Xuan C, Shamonki JM, Chung A, et al.:
Microbial dysbiosis is associated with human breast cancer.
PLoS One.
2014; 9(1): e83744. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 470.
Ebringer R, Cooke D, Cawdell DR, et al.:
Ankylosing spondylitis: klebsiella and HL-A B27.
Rheumatol Rehabil.
1977; 16(3): 190–6. PubMed Abstract
| Publisher Full Text
- 471.
Ahmadi K, Wilson C, Tiwana H, et al.:
Antibodies to Klebsiella pneumoniae lipopolysaccharide in patients with ankylosing spondylitis.
Br J Rheumatol.
1998; 37(12): 1330–3. PubMed Abstract
| Publisher Full Text
- 472.
Rashid T, Ebringer A:
Ankylosing spondylitis is linked to Klebsiella - the evidence.
Clin Rheumatol.
2007; 26(6): 858–64. PubMed Abstract
| Publisher Full Text
- 473.
Rashid T, Wilson C, Ebringer A:
The link between ankylosing spondylitis, Crohn's disease, Klebsiella, and starch consumption.
Clin Dev Immunol.
2013; 2013: 872632. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 474.
Rumah KR, Linden J, Fischetti VA, et al.:
Isolation of Clostridium perfringens type B in an individual at first clinical presentation of multiple sclerosis provides clues for environmental triggers of the disease.
PLoS One.
2013; 8(10): e76359. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 475.
Sriram S, Stratton CW, Yao S, et al.:
Chlamydia pneumoniae infection of the central nervous system in multiple sclerosis.
Ann Neurol.
1999; 46(1): 6–14. PubMed Abstract
- 476.
Layh-Schmitt G, Bendl C, Hildt U, et al.:
Evidence for infection with Chlamydia pneumoniae in a subgroup of patients with multiple sclerosis.
Ann Neurol.
2000; 47(5): 652–5. PubMed Abstract
- 477.
Hao Q, Miyashita N, Matsui M, et al.:
Chlamydia pneumoniae infection associated with enhanced MRI spinal lesions in multiple sclerosis.
Mult Scler.
2002; 8(5): 436–40. PubMed Abstract
| Publisher Full Text
- 478.
Grimaldi LM, Pincherle A, Martinelli-Boneschi F, et al.:
An MRI study of Chlamydia pneumoniae infection in Italian multiple sclerosis patients.
Mult Scler.
2003; 9(5): 467–71. PubMed Abstract
| Publisher Full Text
- 479.
Giovannoni G, Cutter GR, Lunemann J, et al.:
Infectious causes of multiple sclerosis.
Lancet Neurol.
2006; 5(10): 887–94. PubMed Abstract
| Publisher Full Text
- 480.
Stratton CW, Wheldon DB:
Multiple sclerosis: an infectious syndrome involving Chlamydophila pneumoniae.
Trends Microbiol.
2006; 14(11): 474–9. PubMed Abstract
| Publisher Full Text
- 481.
Tang YW, Sriram S, Li H, et al.:
Qualitative and quantitative detection of Chlamydophila pneumoniae DNA in cerebrospinal fluid from multiple sclerosis patients and controls.
PLoS One.
2009; 4(4): e5200. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 482.
Martinez-Martinez RE, Abud-Mendoza C, Patiño-Marin N, et al.:
Detection of periodontal bacterial DNA in serum and synovial fluid in refractory rheumatoid arthritis patients.
J Clin Periodontol.
2009; 36(12): 1004–10. PubMed Abstract
| Publisher Full Text
- 483.
Mikuls TR, Payne JB, Reinhardt RA, et al.:
Antibody responses to Porphyromonas gingivalis (P. gingivalis) in subjects with rheumatoid arthritis and periodontitis.
Int Immunopharmacol.
2009; 9(1): 38–42. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 484.
Hitchon CA, Chandad F, Ferucci ED, et al.:
Antibodies to Porphyromonas gingivalis are associated with anticitrullinated protein antibodies in patients with rheumatoid arthritis and their relatives.
J Rheumatol.
2010; 37(6): 1105–12. PubMed Abstract
| Publisher Full Text
- 485.
Mikuls TR, Thiele GM, Deane KD, et al.:
Porphyromonas gingivalis and disease-related autoantibodies in individuals at increased risk of rheumatoid arthritis.
Arthritis Rheum.
2012; 64(11): 3522–30. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 486.
de Smit M, Westra J, Vissink A, et al.:
Periodontitis in established rheumatoid arthritis patients: a cross-sectional clinical, microbiological and serological study.
Arthritis Res Ther.
2012; 14(5): R222. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 487.
Ebringer A, Khalafpour S, Wilson C:
Rheumatoid arthritis and Proteus: a possible aetiological association.
Rheumatol Int.
1989; 9(3–5): 223–8. PubMed Abstract
- 488.
Kjeldsen-Kragh J, Rashid T, Dybwad A, et al.:
Decrease in anti-Proteus mirabilis but not anti-Escherichia coli antibody levels in rheumatoid arthritis patients treated with fasting and a one year vegetarian diet.
Ann Rheum Dis.
1995; 54(3): 221–4. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 489.
Rashid T, Tiwana H, Wilson C, et al.:
Rheumatoid arthritis as an autoimmune disease caused by Proteus urinary tract infections: a proposal for a therapeutic protocol.
Isr Med Assoc J.
2001; 3(9): 675–80. PubMed Abstract
- 490.
Newkirk MM, Goldbach-Mansky R, Senior BW, et al.:
Elevated levels of IgM and IgA antibodies to Proteus mirabilis and IgM antibodies to Escherichia coli are associated with early rheumatoid factor (RF)-positive rheumatoid arthritis.
Rheumatology (Oxford).
2005; 44(11): 1433–41. PubMed Abstract
| Publisher Full Text
- 491.
Rashid T, Jayakumar KS, Binder A, et al.:
Rheumatoid arthritis patients have elevated antibodies to cross-reactive and non cross-reactive antigens from Proteus microbes.
Clin Exp Rheumatol.
2007; 25(2): 259–67. PubMed Abstract
- 492.
Rashid T, Ebringer A:
Rheumatoid arthritis is linked to Proteus - the evidence.
Clin Rheumatol.
2007; 26(7): 1036–43. PubMed Abstract
| Publisher Full Text
- 493.
Ebringer A, Rashid T:
Rheumatoid arthritis is caused by Proteus: the molecular mimicry theory and Karl Popper.
Front Biosci (Elite Ed).
2009; 1: 577–86. PubMed Abstract
- 494.
Arabski M, Fudala R, Koza A, et al.:
The presence of anti-LPS antibodies and human serum activity against Proteus mirabilis S/R forms in correlation with TLR4 (Thr399Ile) gene polymorphism in rheumatoid arthritis.
Clin Biochem.
2012; 45(16–17): 1374–82. PubMed Abstract
| Publisher Full Text
- 495.
Ebringer A, Rashid T:
Rheumatoid arthritis is caused by a Proteus urinary tract infection.
APMIS.
2014; 122(5): 363–8. PubMed Abstract
| Publisher Full Text
- 496.
Newkirk MM, Duffy WKN, Leclerc J, et al.:
Detection of cytomegalovirus, Epstein-Barr virus and herpes virus-6 in patients with rheumatoid arthritis with or without Sjögren's syndrome.
Br J Rheumatol.
1994; 33(4): 317–22. PubMed Abstract
| Publisher Full Text
- 497.
Takeda T, Mizugaki Y, Matsubara L, et al.:
Lytic Epstein-Barr virus infection in the synovial tissue of patients with rheumatoid arthritis.
Arthritis Rheum.
2000; 43(6): 1218–25. PubMed Abstract
- 498.
Balandraud N, Meynard JB, Auger I, et al.:
Epstein-Barr virus load in the peripheral blood of patients with rheumatoid arthritis: accurate quantification using real-time polymerase chain reaction.
Arthritis Rheum.
2003; 48(5): 1223–8. PubMed Abstract
| Publisher Full Text
- 499.
Croia C, Serafini B, Bombardieri M, et al.:
Epstein-Barr virus persistence and infection of autoreactive plasma cells in synovial lymphoid structures in rheumatoid arthritis.
Ann Rheum Dis.
2013; 72(9): 1559–68. PubMed Abstract
| Publisher Full Text
- 500.
Schaeverbeke T, Renaudin H, Clerc M, et al.:
Systematic detection of mycoplasmas by culture and polymerase chain reaction (PCR) procedures in 209 synovial fluid samples.
Br J Rheumatol.
1997; 36(3): 310–4. PubMed Abstract
| Publisher Full Text
- 501.
Sawitzke A, Joyner D, Knudtson K, et al.:
Anti-MAM antibodies in rheumatic disease: evidence for a MAM-like superantigen in rheumatoid arthritis?
J Rheumatol.
2000; 27(2): 358–64. PubMed Abstract
- 502.
da Rocha Sobrinho HM, Jarach R, da Silva NA, et al.:
Mycoplasmal lipid-associated membrane proteins and Mycoplasma arthritidis mitogen recognition by serum antibodies from patients with rheumatoid arthritis.
Rheumatol Int.
2011; 31(7): 951–7. PubMed Abstract
| Publisher Full Text
- 503.
Leirisalo-Repo M:
Early arthritis and infection.
Curr Opin Rheumatol.
2005; 17(4): 433–9. PubMed Abstract
| Publisher Full Text
- 504.
Schrama JC, Lutro O, Langvatn H, et al.:
Bacterial findings in infected hip joint replacements in patients with rheumatoid arthritis and osteoarthritis: a study of 318 revisions for infection reported to the Norwegian arthroplasty register.
ISRN Orthop.
2012; 2012: 437675. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 505.
Hill Gaston JS, Lillicrap MS:
Arthritis associated with enteric infection.
Best Pract Res Clin Rheumatol.
2003; 17(2): 219–39. PubMed Abstract
| Publisher Full Text
- 506.
Levy O, Iyer S, Atoun E, et al.:
Propionibacterium acnes: an underestimated etiology in the pathogenesis of osteoarthritis?
J Shoulder Elbow Surg.
2013; 22(4): 505–11. PubMed Abstract
| Publisher Full Text
- 507.
Jolly M, Curran JJ:
Chlamydial infection preceding the development of rheumatoid arthritis: a brief report.
Clin Rheumatol.
2004; 23(5): 453–5. PubMed Abstract
| Publisher Full Text
- 508.
Carter JD, Gerard HC, Whittum-Hudson JA, et al.:
The molecular basis for disease phenotype in chronic Chlamydia-induced arthritis.
Int J Clin Rheumtol.
2012; 7(6): 627–40. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 509.
Cantwell AR Jr, Kelso DW, Jones JE:
Histologic observations of coccoid forms suggestive of cell wall deficient bacteria in cutaneous and systemic lupus erythematosus.
Int J Dermatol.
1982; 21(9): 526–37. PubMed Abstract
| Publisher Full Text
- 510.
Zonana-Nacach A, Camargo-Coronel A, Yañez P, et al.:
Infections in outpatients with systemic lupus erythematosus: a prospective study.
Lupus.
2001; 10(7): 505–10. PubMed Abstract
| Publisher Full Text
- 511.
Yang CD, Wang XD, Ye S, et al.:
Clinical features, prognostic and risk factors of central nervous system infections in patients with systemic lupus erythematosus.
Clin Rheumatol.
2007; 26(6): 895–901. PubMed Abstract
| Publisher Full Text
- 512.
Charuvanij S, Houghton KM:
Acute epiglottitis as the initial presentation of pediatric Systemic Lupus Erythematosus.
Pediatr Rheumatol Online J.
2009; 7: 19. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 513.
Shaughnessy MK, Williams DN, Segal B:
Severe infection with encapsulated bacteria as the initial presentation of systemic lupus erythematosus: two case reports and a review of the literature.
JMM Case Rep.
2014; 1(2): e001362. Publisher Full Text
- 514.
Samad I, Wang MC, Chong VH:
Intracerebral coinfection with Burkholderia pseudomallei and Cryptococcus neoformans in a patient with systemic lupus erythematosus.
Southeast Asian J Trop Med Public Health.
2014; 45(2): 352–6. PubMed Abstract
- 515.
Rodríguez-Pla A, Stone JH:
Vasculitis and systemic infections.
Curr Opin Rheumatol.
2006; 18(1): 39–47. PubMed Abstract
| Publisher Full Text
- 516.
Belizna CC, Hamidou MA, Levesque H, et al.:
Infection and vasculitis.
Rheumatology (Oxford).
2009; 48(5): 475–82. PubMed Abstract
| Publisher Full Text
- 517.
Soto ME, Del Carmen Ávila-Casado M, Huesca-Gómez C, et al.:
Detection of IS6110 and HupB gene sequences of Mycobacterium tuberculosis and bovis in the aortic tissue of patients with Takayasu's arteritis.
BMC Infect Dis.
2012; 12: 194. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 518.
Guillevin L:
Infections in vasculitis.
Best Pract Res Clin Rheumatol.
2013; 27(1): 19–31. PubMed Abstract
| Publisher Full Text
- 519.
Kallenberg CG, Tadema H:
Vasculitis and infections: contribution to the issue of autoimmunity reviews devoted to "autoimmunity and infection".
Autoimmun Rev.
2008; 8(1): 29–32. PubMed Abstract
| Publisher Full Text
- 520.
Lidar M, Lipschitz N, Langevitz P, et al.:
The infectious etiology of vasculitis.
Autoimmunity.
2009; 42(5): 432–8. PubMed Abstract
| Publisher Full Text
- 521.
van Timmeren MM, Heeringa P, Kallenberg CGM:
Infectious triggers for vasculitis.
Curr Opin Rheumatol.
2014; 26(4): 416–23. PubMed Abstract
| Publisher Full Text
- 522.
Kiechl S, Egger G, Mayr M, et al.:
Chronic infections and the risk of carotid atherosclerosis: prospective results from a large population study.
Circulation.
2001; 103(8): 1064–70. PubMed Abstract
| Publisher Full Text
- 523.
Reyes L, Herrera D, Kozarov E, et al.:
Periodontal bacterial invasion and infection: contribution to atherosclerotic pathology.
J Periodontol.
2013; 84(4 Suppl): S30–50. PubMed Abstract
| Publisher Full Text
- 524.
Zhang T, Kurita-Ochiai T, Hashizume T, et al.:
Aggregatibacter actinomycetemcomitans accelerates atherosclerosis with an increase in atherogenic factors in spontaneously hyperlipidemic mice.
FEMS Immunol Med Microbiol.
2010; 59(2): 143–51. PubMed Abstract
| Publisher Full Text
- 525.
Grayston JT:
Antibiotic treatment of Chlamydia pneumoniae for secondary prevention of cardiovascular events.
Circulation.
1998; 97(17): 1669–70. PubMed Abstract
| Publisher Full Text
- 526.
Ewald PW, Cochran GM:
Chlamydia pneumoniae and cardiovascular disease: an evolutionary perspective on infectious causation and antibiotic treatment.
J Infect Dis.
2000; 181(Suppl 3): S394–401. PubMed Abstract
| Publisher Full Text
- 527.
Kaplan M, Yavuz SS, Cinar B, et al.:
Detection of Chlamydia pneumoniae and Helicobacter pylori in atherosclerotic plaques of carotid artery by polymerase chain reaction.
Int J Infect Dis.
2006; 10(2): 116–23. PubMed Abstract
| Publisher Full Text
- 528.
Choroszy-Król I, Frej-Mądrzak M, Hober M, et al.:
Infections caused by Chlamydophila pneumoniae.
Adv Clin Exp Med.
2014; 23(1): 123–6. PubMed Abstract
| Publisher Full Text
- 529.
Campbell LA, Rosenfeld ME:
Persistent C. pneumoniae infection in atherosclerotic lesions: rethinking the clinical trials.
Front Cell Infect Microbiol.
2014; 4: 34. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 530.
Khan S, Rahman HN, Okamoto T, et al.:
Promotion of atherosclerosis by Helicobacter cinaedi infection that involves macrophage-driven proinflammatory responses.
Sci Rep.
2014; 4: 4680. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 531.
Li L, Messas E, Batista EL Jr, et al.:
Porphyromonas gingivalis infection accelerates the progression of atherosclerosis in a heterozygous apolipoprotein E-deficient murine model.
Circulation.
2002; 105(7): 861–7. PubMed Abstract
| Publisher Full Text
- 532.
Toyofuku T, Inoue Y, Kurihara N, et al.:
Differential detection rate of periodontopathic bacteria in atherosclerosis.
Surg Today.
2011; 41(10): 1395–400. PubMed Abstract
| Publisher Full Text
- 533.
Yang J, Wu J, Liu Y, et al.:
Porphyromonas gingivalis infection reduces regulatory T cells in infected atherosclerosis patients.
PLoS One.
2014; 9(1): e86599. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 534.
Hajishengallis G:
Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response.
Trends Immunol.
2014; 35(1): 3–11. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 535.
Velsko IM, Chukkapalli SS, Rivera MF, et al.:
Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis.
PLoS One.
2014; 9(5): e97811. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 536.
Hajishengallis G:
Periodontitis: from microbial immune subversion to systemic inflammation.
Nat Rev Immunol.
2015; 15(1): 30–44. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 537.
Dénes Á, Pradillo JM, Drake C, et al.:
Streptococcus pneumoniae worsens cerebral ischemia via interleukin 1 and platelet glycoprotein Ibα.
Ann Neurol.
2014; 75(5): 670–83. PubMed Abstract
| Publisher Full Text
- 538.
Portugal LR, Fernandes LR, Cesar GC, et al.:
Infection with Toxoplasma gondii increases atherosclerotic lesion in ApoE-deficient mice.
Infect Immun.
2004; 72(6): 3571–6. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 539.
Mattman L:
Cell wall deficient bacteria: Their surprising role in health and illness. World out of Balance: The Microbial-Pollution Connection. Wake up Call. 1995; 141–5.
- 540.
Kotze MJ:
Antibiotic prophylaxis for preventing endocarditis and infection in joint prosthesis after dental treatment: a review of new trends and recommendations in the literature.
SADJ.
2008; 63(8): 440–4. PubMed Abstract
- 541.
Koren O, Spor A, Felin J, et al.:
Human oral, gut and plaque microbiota in patients with atherosclerosis.
Proc Natl Acad Sci U S A.
2011; 108(Suppl 1): 4592–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 542.
Mosquera JD, Zabalza M, Lantero M, et al.:
Endocarditis due to Gemella haemolysans in a patient with hemochromatosis.
Clin Microbiol Infect.
2000; 6(10): 566–8. PubMed Abstract
| Publisher Full Text
- 543.
Sinkovics JG, Cormia F, Plager C:
Hemochromatosis and Listeria infection.
Arch Intern Med.
1980; 140(2): 284. PubMed Abstract
| Publisher Full Text
- 544.
van Asbeck BS, Verbrugh HA, van Oost BA, et al.:
Listeria monocytogenes meningitis and decreased phagocytosis associated with iron overload.
Br Med J (Clin Res Ed).
1982; 284(6315): 542–4. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 545.
Delforge ML, Devriendt J, Glupczynski Y, et al.:
Plesiomonas shigelloides septicemia in a patient with primary hemochromatosis.
Clin Infect Dis.
1995; 21(3): 692–3. PubMed Abstract
| Publisher Full Text
- 546.
Barton JC, Acton RT:
Hemochromatosis and Vibrio vulnificus wound infections.
J Clin Gastroenterol.
2009; 43(9): 890–3. PubMed Abstract
| Publisher Full Text
- 547.
Arezes J, Jung G, Gabayan V, et al.:
Hepcidin-Induced hypoferremia is a critical host defense mechanism against the siderophilic bacterium Vibrio vulnificus.
Cell Host Microbe.
2015; 17(1): 47–57. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 548.
Fernández JM, Serrano M, De Arriba JJ, et al.:
Bacteremic cellulitis caused by Non-01, Non-0139 Vibrio cholerae: report of a case in a patient with hemochromatosis.
Diagn Microbiol Infect Dis.
2000; 37(1): 77–80. PubMed Abstract
| Publisher Full Text
- 549.
Capron JP, Capron-Chivrac D, Tossou H, et al.:
Spontaneous Yersinia enterocolitica peritonitis in idiopathic hemochromatosis.
Gastroenterology.
1984; 87(6): 1372–5. PubMed Abstract
- 550.
de Cuenca-Moron B, Solis-Herruzo JA, Moreno D, et al.:
Spontaneous bacterial peritonitis due to Yersinia enterocolitica in secondary alcoholic hemochromatosis.
J Clin Gastroenterol.
1989; 11(6): 675–8. PubMed Abstract
| Publisher Full Text
- 551.
Vadillo M, Corbella X, Pac V, et al.:
Multiple liver abscesses due to Yersinia enterocolitica discloses primary hemochromatosis: three cases reports and review.
Clin Infect Dis.
1994; 18(6): 938–41. PubMed Abstract
| Publisher Full Text
- 552.
Höpfner M, Nitsche R, Rohr A, et al.:
Yersinia enterocolitica infection with multiple liver abscesses uncovering a primary hemochromatosis.
Scand J Gastroenterol.
2001; 36(2): 220–4. PubMed Abstract
| Publisher Full Text
- 553.
Conway SP, Dudley N, Sheridan P, et al.:
Haemochromatosis and aldosterone deficiency presenting with Yersinia pseudotuberculosis septicaemia.
Postgrad Med J.
1989; 65(761): 174–6. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 554.
Mennecier D, Lapprand M, Hernandez E, et al.:
[Liver abscesses due to Yersinia pseudotuberculosis discloses a genetic hemochromatosis].
Gastroenterol Clin Biol.
2001; 25(12): 1113–5. PubMed Abstract
- 555.
Desvarieux M, Demmer RT, Jacobs DR, et al.:
Periodontal bacteria and hypertension: the oral infections and vascular disease epidemiology study (INVEST).
J Hypertens.
2010; 28(7): 1413–21. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 556.
Mangin M:
Hypertension and inflammation: the infection connection.
J Amer Soc Hypertens.
2014; 8: e7. Publisher Full Text
- 557.
Mattila KJ, Nieminen MS, Valtonen VV, et al.:
Association between dental health and acute myocardial infarction.
BMJ.
1989; 298(6676): 779–81. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 558.
Kaisare S, Rao J, Dubashi N:
Periodontal disease as a risk factor for acute myocardial infarction. A case-control study in Goans highlighting a review of the literature.
Br Dent J.
2007; 203(3): E5; discussion 144–5. PubMed Abstract
| Publisher Full Text
- 559.
Willershausen B, Kasaj A, Willershausen I, et al.:
Association between chronic dental infection and acute myocardial infarction.
J Endod.
2009; 35(5): 626–30. PubMed Abstract
| Publisher Full Text
- 560.
Meier CR, Derby LE, Jick SS, et al.:
Antibiotics and risk of subsequent first-time acute myocardial infarction.
JAMA.
1999; 281(5): 427–31. PubMed Abstract
| Publisher Full Text
- 561.
Smeeth L, Thomas SL, Hall AJ, et al.:
Risk of myocardial infarction and stroke after acute infection or vaccination.
N Engl J Med.
2004; 351(25): 2611–8. PubMed Abstract
| Publisher Full Text
- 562.
Mattila KJ:
Viral and bacterial infections in patients with acute myocardial infarction.
J Intern Med.
1989; 225(5): 293–6. PubMed Abstract
| Publisher Full Text
- 563.
Warren-Gash C, Bhaskaran K, Hayward A, et al.:
Circulating influenza virus, climatic factors, and acute myocardial infarction: a time series study in England and Wales and Hong Kong.
J Infect Dis.
2011; 203(12): 1710–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 564.
Brown AO, Mann B, Gao G, et al.:
Streptococcus pneumoniae translocates into the myocardium and forms unique microlesions that disrupt cardiac function.
PLoS Pathog.
2014; 10(9): e1004383. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 565.
Emsley HCA, Tyrrell PJ:
Inflammation and infection in clinical stroke.
J Cereb Blood Flow Metab.
2002; 22(12): 1399–419. PubMed Abstract
- 566.
Emsley HCA, Smith CJ, Gavin CM, et al.:
An early and sustained peripheral inflammatory response in acute ischaemic stroke: relationships with infection and atherosclerosis.
J Neuroimmunol.
2003; 139(1–2): 93–101. PubMed Abstract
| Publisher Full Text
- 567.
Lindsberg PJ, Grau AJ:
Inflammation and infections as risk factors for ischemic stroke.
Stroke.
2003; 34(10): 2518–32. PubMed Abstract
| Publisher Full Text
- 568.
Smith CJ, Emsley HC, Vail A, et al.:
Variability of the systemic acute phase response after ischemic stroke.
J Neurol Sci.
2006; 251(1–2): 77–81. PubMed Abstract
| Publisher Full Text
- 569.
Emsley HC, Hopkins SJ:
Acute ischaemic stroke and infection: recent and emerging concepts.
Lancet Neurol.
2008; 7(4): 341–53. PubMed Abstract
| Publisher Full Text
- 570.
Piñol-Ripoll G, de la Puerta I, Santos S, et al.:
Chronic bronchitis and acute infections as new risk factors for ischemic stroke and the lack of protection offered by the influenza vaccination.
Cerebrovasc Dis.
2008; 26(4): 339–47. PubMed Abstract
| Publisher Full Text
- 571.
Emsley HCA, Chamorro A:
Stroke bugs: current and emerging concepts relevant to infection in cerebrovascular disease.
Infect Disord Drug Targets.
2010; 10(2): 65–6. PubMed Abstract
| Publisher Full Text
- 572.
Worthmann H, Tryc AB, Deb M, et al.:
Linking infection and inflammation in acute ischemic stroke.
Ann N Y Acad Sci.
2010; 1207: 116–22. PubMed Abstract
| Publisher Full Text
- 573.
Lee JT, Chung WT, Lin JD, et al.:
Increased risk of stroke after septicaemia: a population-based longitudinal study in Taiwan.
PLoS One.
2014; 9(2): e89386. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 574.
Levine DA, Langa KM, Rogers MA:
Acute infection contributes to racial disparities in stroke mortality.
Neurology.
2014; 82(11): 914–21. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 575.
Manousakis G, Jensen MB, Chacon MR, et al.:
The interface between stroke and infectious disease: infectious diseases leading to stroke and infections complicating stroke.
Curr Neurol Neurosci Rep.
2009; 9(1): 28–34. PubMed Abstract
| Publisher Full Text
- 576.
Armingohar Z, Jørgensen JJ, Kristoffersen AK, et al.:
Bacteria and bacterial DNA in atherosclerotic plaque and aneurysmal wall biopsies from patients with and without periodontitis.
J Oral Microbiol.
2014; 6. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 577.
Dalager-Pedersen M, Sogaard M, Schonheyder HC, et al.:
Risk for myocardial infarction and stroke after community-acquired bacteremia: a 20-year population-based cohort study.
Circulation.
2014; 129(13): 1387–96. PubMed Abstract
| Publisher Full Text
- 578.
Schut ES, Lucas MJ, Brouwer MC, et al.:
Cerebral infarction in adults with bacterial meningitis.
Neurocrit Care.
2012; 16(3): 421–7. PubMed Abstract
| Publisher Full Text
- 579.
May EF, Jabbari B:
Stroke in neuroborreliosis.
Stroke.
1990; 21(8): 1232–5. PubMed Abstract
| Publisher Full Text
- 580.
Bingöl A, Togay-Işıkay C:
Neurobrucellosis as an exceptional cause of transient ischemic attacks.
Eur J Neurol.
2006; 13(5): 544–8. PubMed Abstract
| Publisher Full Text
- 581.
Elkind MSV, Lin IF, Grayston JT, et al.:
Chlamydia pneumoniae and the risk of first ischemic stroke : The Northern Manhattan Stroke Study.
Stroke.
2000; 31(7): 1521–5. PubMed Abstract
| Publisher Full Text
- 582.
Njamnshi AK, Blackett KN, Mbuagbaw JN, et al.:
Chronic Chlamydia pneumoniae infection and stroke in Cameroon: a case-control study.
Stroke.
2006; 37(3): 796–9. PubMed Abstract
| Publisher Full Text
- 583.
Eini P, Keramat F, Farajpoor N:
The Association Between Chlamydia pneumoniae Infection and Ischemic Stroke.
Avicenna J Clin Microb Infec.
2014; 1(3): e22165. Reference Source
- 584.
Salih MA, Abdel-Gader AG, Al-Jarallah AA, et al.:
Infectious and inflammatory disorders of the circulatory system as risk factors for stroke in Saudi children.
Saudi Med J.
2006; 27(Suppl 1): S41–52. PubMed Abstract
- 585.
Sheu JJ, Chiou HY, Kang JH, et al.:
Tuberculosis and the risk of ischemic stroke: a 3–year follow-up study.
Stroke.
2010; 41(2): 244–9. PubMed Abstract
| Publisher Full Text
- 586.
Chiang CH, Huang CC, Chan WL, et al.:
Association between Mycoplasma pneumonia and increased risk of ischemic stroke: a nationwide study.
Stroke.
2011; 42(10): 2940–3. PubMed Abstract
| Publisher Full Text
- 587.
Garcia AV, Fingeret AL, Thirumoorthi AS, et al.:
Severe Mycoplasma pneumoniae infection requiring extracorporeal membrane oxygenation with concomitant ischemic stroke in a child.
Pediatr Pulmonol.
2013; 48(1): 98–101. PubMed Abstract
| Publisher Full Text
- 588.
Kim GH, Seo WH, Je BK, et al.:
Mycoplasma pneumoniae associated stroke in a 3–year-old girl.
Korean J Pediatr.
2013; 56(9): 411–5. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 589.
de Souza AL, de Oliveira AC, Romano CC, et al.:
Interleukin-6 activation in ischemic stroke caused by Neisseria meningitidis serogroup C.
Int J Cardiol.
2008; 127(3): e160–3. PubMed Abstract
| Publisher Full Text
- 590.
Hart RG, Foster JW, Luther MF, et al.:
Stroke in infective endocarditis.
Stroke.
1990; 21(5): 695–700. PubMed Abstract
| Publisher Full Text
- 591.
Fowler VGJr, Miro JM, Hoen B, et al.:
Staphylococcus aureus endocarditis: a consequence of medical progress.
JAMA.
2005; 293(24): 3012–21. PubMed Abstract
| Publisher Full Text
- 592.
Stöllberger C, Finsterer J, Pratter A, et al.:
Ischemic stroke and splenic rupture in a case of Streptococcus bovis endocarditis.
J Clin Microbiol.
2003; 41(6): 2654–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 593.
Nakano K, Hokamura K, Taniguchi N, et al.:
The collagen-binding protein of Streptococcus mutans is involved in haemorrhagic stroke.
Nat Commun.
2011; 2: 485. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 594.
Chen LF, Chen HP, Huang YS, et al.:
Pneumococcal pneumonia and the risk of stroke: a population-based follow-up study.
PLoS One.
2012; 7(12): e51452. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 595.
López J, San Román JA, Revilla A, et al.:
Clinical, echocardiographic and prognostic profile of Streptococcus viridans left-sided endocarditis.
Rev Esp Cardiol.
2005; 58(2): 153–8. PubMed Abstract
| Publisher Full Text
- 596.
Ahamed S, Varghese M, El Agib el N, et al.:
Case of neurosyphilis presented as recurrent stroke.
Oman Med J.
2009; 24(2): 134–6. PubMed Abstract
| Free Full Text
- 597.
Dharmasaroja PA, Dharmasaroja P:
Serum and cerebrospinal fluid profiles for syphilis in Thai patients with acute ischaemic stroke.
Int J STD AIDS.
2012; 23(5): 340–5. PubMed Abstract
| Publisher Full Text
- 598.
Rafferty B, Jönsson D, Kalachikov S, et al.:
Impact of monocytic cells on recovery of uncultivable bacteria from atherosclerotic lesions.
J Intern Med.
2011; 270(3): 273–80. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 599.
Bartenjev I, Rogl Butina M, Potocnik M:
Subclinical microbial infection in patients with chronic plaque psoriasis.
Acta Derm Venereol Suppl (Stockh).
2000; (211): 17–8. PubMed Abstract
| Publisher Full Text
- 600.
Ramírez-Boscá A, Navarro-López V, Martínez-Andrés A, et al.:
Identification of bacterial DNA in the peripheral blood of patients with active psoriasis.
JAMA Dermatol.
2015; 151(6): 670–1. PubMed Abstract
| Publisher Full Text
- 601.
Fry L, Baker BS:
Triggering psoriasis: the role of infections and medications.
Clin Dermatol.
2007; 25(6): 606–15. PubMed Abstract
| Publisher Full Text
- 602.
Fry L, Baker BS, Powles AV:
Psoriasis--a possible candidate for vaccination.
Autoimmun Rev.
2007; 6(5): 286–9. PubMed Abstract
| Publisher Full Text
- 603.
Munz OH, Sela S, Baker BS, et al.:
Evidence for the presence of bacteria in the blood of psoriasis patients.
Arch Dermatol Res.
2010; 302(7): 495–8. PubMed Abstract
| Publisher Full Text
- 604.
Joshi N, Caputo GM, Weitekamp MR, et al.:
Infections in patients with diabetes mellitus.
N Engl J Med.
1999; 341(25): 1906–12. PubMed Abstract
| Publisher Full Text
- 605.
Casqueiro J, Casqueiro J, Alves C:
Infections in patients with diabetes mellitus: A review of pathogenesis.
Indian J Endocrinol Metab.
2012; 16(Suppl 1): S27–36. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 606.
Peräneva L, Fogarty CL, Pussinen PJ, et al.:
Systemic exposure to Pseudomonal bacteria: a potential link between type 1 diabetes and chronic inflammation.
Acta Diabetol.
2013; 50(3): 351–61. PubMed Abstract
| Publisher Full Text
- 607.
Oldstone MB, Nerenberg M, Southern P, et al.:
Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response.
Cell.
1991; 65(2): 319–31. PubMed Abstract
| Publisher Full Text
- 608.
Kumar A, Turney JH, Brownjohn AM, et al.:
Unusual bacterial infections of the urinary tract in diabetic patients--rare but frequently lethal.
Nephrol Dial Transplant.
2001; 16(5): 1062–5. PubMed Abstract
| Publisher Full Text
- 609.
Yeung WC, Rawlinson WD, Craig ME:
Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies.
BMJ.
2011; 342: d35. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 610.
Serino M, Blasco-Baque V, Burcelin R:
Microbes on-air: gut and tissue microbiota as targets in type 2 diabetes.
J Clin Gastroenterol.
2012; 46(Suppl): S27–8. PubMed Abstract
| Publisher Full Text
- 611.
Peterson LW, Artis D:
Intestinal epithelial cells: regulators of barrier function and immune homeostasis.
Nat Rev Immunol.
2014; 14(3): 141–53. PubMed Abstract
| Publisher Full Text
- 612.
Li X, Kolltveit KM, Tronstad L, et al.:
Systemic diseases caused by oral infection.
Clin Microbiol Rev.
2000; 13(4): 547–58. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 613.
Sato J, Kanazawa A, Ikeda F, et al.:
Gut dysbiosis and detection of "live gut bacteria" in blood of Japanese patients with type 2 diabetes.
Diabetes Care.
2014; 37(8): 2343–50. PubMed Abstract
| Publisher Full Text
- 614.
Nicolson GL, Haier J:
Role of chronic bacterial and viral infections in neurodegenerative, neurobehavioural, psychiatric, autoimmune and fatiguing illnesses: part 1.
Br J Med Pract.
2009; 2(4): 20–8. Reference Source
- 615.
Nicolson GL, Haier J:
Role of chronic bacterial and viral infections in neurodegenerative, neurobehavioural, psychiatric, autoimmune and fatiguing illnesses: part 2.
Br J Med Pract.
2010; 3(1): 301–10. Reference Source
- 616.
De Chiara G, Marcocci ME, Sgarbanti R, et al.:
Infectious agents and neurodegeneration.
Mol Neurobiol.
2012; 46(3): 614–38. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 617.
Bibi F, Yasir M, Sohrab SS, et al.:
Link between chronic bacterial inflammation and Alzheimer disease.
CNS Neurol Disord Drug Targets.
2014; 13(7): 1140–7. PubMed Abstract
| Publisher Full Text
- 618.
Bu XL, Yao XQ, Jiao SS, et al.:
A study on the association between infectious burden and Alzheimer's disease.
Eur J Neurol.
2014. PubMed Abstract
| Publisher Full Text
- 619.
Poole S, Singhrao SK, Kesavalu L, et al.:
Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer's disease brain tissue.
J Alzheimers Dis.
2013; 36(4): 665–77. PubMed Abstract
| Publisher Full Text
- 620.
Miklossy J:
Alzheimer's disease--a spirochetosis?
Neuroreport.
1993; 4(7): 841–8. PubMed Abstract
| Publisher Full Text
- 621.
Balin BJ, Gérard HC, Arking EJ, et al.:
Identification and localization of Chlamydia pneumoniae in the Alzheimer's brain.
Med Microbiol Immunol.
1998; 187(1): 23–42. PubMed Abstract
| Publisher Full Text
- 622.
Arking EJ, Appelt DM, Abrams JT, et al.:
Ultrastructural Analysis of Chlamydia pneumoniae in the Alzheimer's Brain.
Pathogenesis (Amst).
1999; 1(3): 201–11. PubMed Abstract
| Free Full Text
- 623.
Balin BJ, Appelt DM:
Role of infection in Alzheimer's disease.
J Am Osteopath Assoc.
2001; 101(12 Suppl Pt 1): S1–6. PubMed Abstract
- 624.
Little CS, Hammond CJ, MacIntyre A, et al.:
Chlamydia pneumoniae induces Alzheimer-like amyloid plaques in brains of BALB/c mice.
Neurobiol Aging.
2004; 25(4): 419–29. PubMed Abstract
| Publisher Full Text
- 625.
Gérard HC, Dreses-Werringloer U, Wildt KS, et al.:
Chlamydophila (Chlamydia) pneumoniae in the Alzheimer's brain.
FEMS Immunol Med Microbiol.
2006; 48(3): 355–66. PubMed Abstract
| Publisher Full Text
- 626.
Balin BJ, Little CS, Hammond CJ, et al.:
Chlamydophila pneumoniae and the etiology of late-onset Alzheimer's disease.
J Alzheimers Dis.
2008; 13(4): 371–80. PubMed Abstract
- 627.
MacDonald AB:
Plaques of Alzheimer's disease originate from cysts of Borrelia burgdorferi, the Lyme disease spirochete.
Med Hypotheses.
2006; 67(3): 592–600. PubMed Abstract
| Publisher Full Text
- 628.
Hammond CJ, Hallock LR, Howanski RJ, et al.:
Immunohistological detection of Chlamydia pneumoniae in the Alzheimer's disease brain.
BMC Neurosci.
2010; 11: 121. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 629.
Miklossy J:
Alzheimer's disease - a neurospirochetosis. Analysis of the evidence following Koch's and Hill's criteria.
J Neuroinflammation.
2011; 8: 90. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 630.
Hill JM, Clement C, Pogue AI, et al.:
Pathogenic microbes, the microbiome, and Alzheimer’s disease (AD).
Front Aging Neurosci.
2014; 6: 127. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 631.
Little CS, Joyce TA, Hammond CJ, et al.:
Detection of bacterial antigens and Alzheimer's disease-like pathology in the central nervous system of BALB/c mice following intranasal infection with a laboratory isolate of Chlamydia pneumoniae.
Front Aging Neurosci.
2014; 6: 304. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 632.
Maheshwari P, Eslick GD:
Bacterial infection and Alzheimer's disease: a meta-analysis.
J Alzheimers Dis.
2015; 43(3): 957–66. PubMed Abstract
| Publisher Full Text
- 633.
Miklossy J:
Historic evidence to support a causal relationship between spirochetal infections and Alzheimer’s disease.
Front Aging Neurosci.
2015; 7: 46. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 634.
Kountouras J, Tsolaki M, Gavalas E, et al.:
Relationship between Helicobacter pylori infection and Alzheimer disease.
Neurology.
2006; 66(6): 938–40. PubMed Abstract
| Publisher Full Text
- 635.
Chang YP, Chiu GF, Kuo FC, et al.:
Eradication of Helicobacter pylori Is Associated with the Progression of Dementia: A Population-Based Study.
Gastroenterol Res Pract.
2013; 2013: 175729. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 636.
Wang XL, Zeng J, Feng J, et al.:
Helicobacter pylori filtrate impairs spatial learning and memory in rats and increases β-amyloid by enhancing expression of presenilin-2.
Front Aging Neurosci.
2014; 6: 66. Publisher Full Text
- 637.
Noble JM, Scarmeas N, Celenti RS, et al.:
Serum IgG antibody levels to periodontal microbiota are associated with incident Alzheimer disease.
PLoS One.
2014; 9(12): e114959. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 638.
Halperin JJ, Kaplan GP, Brazinsky S, et al.:
Immunologic reactivity against Borrelia burgdorferi in patients with motor neuron disease.
Arch Neurol.
1990; 47(5): 586–94. PubMed Abstract
| Publisher Full Text
- 639.
Nicolson GL, Nasralla MY, Haier J, et al.:
High frequency of systemic mycoplasmal infections in Gulf War veterans and civilians with Amyotrophic Lateral Sclerosis (ALS).
J Clin Neurosci.
2002; 9(5): 525–9. PubMed Abstract
| Publisher Full Text
- 640.
Gil C, González AAS, León IL, et al.:
Detection of Mycoplasmas in Patients with Amyotrophic Lateral Sclerosis.
Adv Microbiol.
2014; 4: 712–9. Publisher Full Text
- 641.
Nicolson GL, Berns P, Gan R, et al.:
Chronic Mycoplasmal Infections in Gulf War Veterans’ Children and Autism Patients.
Med Ver.
2005; 2: 383–7. Reference Source
- 642.
Koch AL:
Cell wall-deficient (CWD) bacterial pathogens: could amylotrophic lateral sclerosis (ALS) be due to one?
Crit Rev Microbiol.
2003; 29(3): 215–21. PubMed Abstract
- 643.
Nicolson GL, Gan R, Nicolson NL, et al.:
Evidence for Mycoplasma ssp., Chlamydia pneunomiae, and human herpes virus-6 coinfections in the blood of patients with autistic spectrum disorders.
J Neurosci Res.
2007; 85(5): 1143–8. PubMed Abstract
| Publisher Full Text
- 644.
Atladóttir HÓ, Thorsen P, Østergaard L, et al.:
Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders.
J Autism Dev Disord.
2010; 40(12): 1423–30. Publisher Full Text
- 645.
Maes M, Kubera M, Leunis JC, et al.:
Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut.
J Affect Disord.
2012; 141(1): 55–62. PubMed Abstract
| Publisher Full Text
- 646.
Nafisah WY, Hamdi Najman A, Hamizah R, et al.:
High prevalence of Helicobacter pylori infection in Malaysian Parkinson’s disease patients.
J Parkinsonism Restless Legs Syndrome.
2013; 3: 63–7. Reference Source
- 647.
Nielsen HH, Qiu J, Friis S, et al.:
Treatment for Helicobacter pylori infection and risk of Parkinson's disease in Denmark.
Eur J Neurol.
2012; 19(6): 864–9. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 648.
Dobbs SM, Dobbs RJ, Weller C, et al.:
Differential effect of Helicobacter pylori eradication on time-trends in brady/hypokinesia and rigidity in idiopathic parkinsonism.
Helicobacter.
2010; 15(4): 279–94. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 649.
Tan AH, Mahadeva S, Marras C, et al.:
Helicobacter pylori infection is associated with worse severity of Parkinson's disease.
Parkinsonism Relat Disord.
2015; 21(3): 221–5. PubMed Abstract
| Publisher Full Text
- 650.
Miman O, Kusbeci OY, Aktepe OC, et al.:
The probable relation between Toxoplasma gondii and Parkinson's disease.
Neurosci Lett.
2010; 475(3): 129–31. PubMed Abstract
| Publisher Full Text
- 651.
Blaecher C, Smet A, Flahou B, et al.:
Significantly higher frequency of Helicobacter suis in patients with idiopathic parkinsonism than in control patients.
Aliment Pharmacol Ther.
2013; 38(11–12): 1347–53. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 652.
Torrey EF, Yolken RH:
Could schizophrenia be a viral zoonosis transmitted from house cats?
Schizophr Bull.
1995; 21(2): 167–71. PubMed Abstract
| Publisher Full Text
- 653.
Torrey EF, Rawlings R, Yolken RH:
The antecedents of psychoses: a case-control study of selected risk factors.
Schizophr Res.
2000; 46(1): 17–23. PubMed Abstract
| Publisher Full Text
- 654.
Knobler SL, O'Connor S, Lemon SM:
The Infectious Etiology of Chronic Diseases: Defining the Relationship, Enhancing the Research, and Mitigating the Effects - Workshop Summary. Washington: National Academies Press; 2004. Reference Source
- 655.
Torrey EF, Bartko JJ, Yolken RH:
Toxoplasma gondii and other risk factors for schizophrenia: an update.
Schizophr Bull.
2012; 38(3): 642–7. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 656.
Torrey EF, Yolken RH:
The urban risk and migration risk factors for schizophrenia: are cats the answer?
Schizophr Res.
2014; 159(2–3): 299–302. PubMed Abstract
| Publisher Full Text
- 657.
Sørensen HJ, Mortensen EL, Reinisch JM, et al.:
Association between prenatal exposure to bacterial infection and risk of schizophrenia.
Schizophr Bull.
2009; 35(3): 631–7. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 658.
Krause DL, Weidinger E, Matz J, et al.:
Infectious Agents are Associated with Psychiatric Diseases.
Ment Illn.
2012; 4(1): e10. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 659.
Krause DL, Muller N:
The Relationship between Tourette's Syndrome and Infections.
Open Neurol J.
2012; 6: 124–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 660.
Earl CS, An SQ, Ryan RP:
The changing face of asthma and its relation with microbes.
Trends Microbiol.
2015. PubMed Abstract
| Publisher Full Text
- 661.
Friedman R, Ackerman M, Wald E, et al.:
Asthma and bacterial sinusitis in children.
J Allergy Clin Immunol.
1984; 74(2): 185–9. PubMed Abstract
| Publisher Full Text
- 662.
Martin RJ, Kraft M, Chu HW, et al.:
A link between chronic asthma and chronic infection.
J Allergy Clin Immunol.
2001; 107(4): 595–601. PubMed Abstract
| Publisher Full Text
- 663.
Bisgaard H, Hermansen MN, Buchvald F, et al.:
Childhood asthma after bacterial colonization of the airway in neonates.
N Engl J Med.
2007; 357(15): 1487–95. PubMed Abstract
| Publisher Full Text
- 664.
Bisgaard H, Hermansen MN, Bonnelykke K, et al.:
Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study.
BMJ.
2010; 341: c4978. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 665.
Monsó E, Ruiz J, Rosell A, et al.:
Bacterial infection in chronic obstructive pulmonary disease. A study of stable and exacerbated outpatients using the protected specimen brush.
Am J Respir Crit Care Med.
1995; 152(4 Pt 1): 1316–20. PubMed Abstract
| Publisher Full Text
- 666.
Sethi S, Murphy TF:
Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review.
Clin Microbiol Rev.
2001; 14(2): 336–63. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 667.
Papi A, Bellettato CM, Braccioni F, et al.:
Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations.
Am J Respir Crit Care Med.
2006; 173(10): 1114–21. PubMed Abstract
| Publisher Full Text
- 668.
Wark PA, Tooze M, Powell H, et al.:
Viral and bacterial infection in acute asthma and chronic obstructive pulmonary disease increases the risk of readmission.
Respirology.
2013; 18(6): 996–1002. PubMed Abstract
| Publisher Full Text
- 669.
Barak S, Oettinger-Barak O, Machtei EE, et al.:
Evidence of periopathogenic microorganisms in placentas of women with preeclampsia.
J Periodontol.
2007; 78(4): 670–6. PubMed Abstract
| Publisher Full Text
- 670.
Herrera JA, Chaudhuri G, López-Jaramillo P:
Is infection a major risk factor for preeclampsia?
Med Hypotheses.
2001; 57(3): 393–7. PubMed Abstract
| Publisher Full Text
- 671.
von Dadelszen P, Magee LA:
Could an infectious trigger explain the differential maternal response to the shared placental pathology of preeclampsia and normotensive intrauterine growth restriction?
Acta Obstet Gynecol Scand.
2002; 81(7): 642–8. PubMed Abstract
| Publisher Full Text
- 672.
Conde-Agudelo A, Villar J, Lindheimer M:
Maternal infection and risk of preeclampsia: systematic review and metaanalysis.
Am J Obstet Gynecol.
2008; 198(1): 7–22. PubMed Abstract
| Publisher Full Text
- 673.
Karmon A, Sheiner E:
The relationship between urinary tract infection during pregnancy and preeclampsia: causal, confounded or spurious?
Arch Gynecol Obstet.
2008; 277(6): 479–81. PubMed Abstract
| Publisher Full Text
- 674.
Rustveld LO, Kelsey SF, Sharma R:
Association between maternal infections and preeclampsia: a systematic review of epidemiologic studies.
Matern Child Health J.
2008; 12(2): 223–42. PubMed Abstract
| Publisher Full Text
- 675.
Xie F, Hu Y, Magee LA, et al.:
Chlamydia pneumoniae infection in preeclampsia.
Hypertens Pregnancy.
2010; 29(4): 468–77. PubMed Abstract
| Publisher Full Text
- 676.
Chrisoulidou A, Goulis DG, Iliadou PK, et al.:
Acute and chronic Chlamydia pneumoniae infection in pregnancy complicated with preeclampsia.
Hypertens Pregnancy.
2011; 30(2): 164–8. PubMed Abstract
| Publisher Full Text
- 677.
Haggerty CL, Klebanoff MA, Panum I, et al.:
Prenatal Chlamydia trachomatis infection increases the risk of preeclampsia.
Pregnancy Hypertens.
2013; 3(3): 151–4. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 678.
Üstün Y, Engin-Üstün Y, Ozkaplan E, et al.:
Association of Helicobacter pylori infection with systemic inflammation in preeclampsia.
J Matern Fetal Neonatal Med.
2010; 23(4): 311–4. PubMed Abstract
| Publisher Full Text
- 679.
Tersigni C, Franceschi F, Todros T, et al.:
Insights into the Role of Helicobacter pylori Infection in Preeclampsia: From the Bench to the Bedside.
Front Immunol.
2014; 5: 484. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 680.
Maes M, Mihaylova I, Leunis JC:
Increased serum IgA and IgM against LPS of enterobacteria in chronic fatigue syndrome (CFS): indication for the involvement of gram-negative enterobacteria in the etiology of CFS and for the presence of an increased gut-intestinal permeability.
J Affect Disord.
2007; 99(1–3): 237–40. PubMed Abstract
| Publisher Full Text
- 681.
Maes M:
Leaky gut in chronic fatigue syndrome: A review.
Activitas Nervosa Superior Rediviva.
2009; 51(1–2): 21–8. Reference Source
- 682.
Maes M, Twisk FN:
Chronic fatigue syndrome: Harvey and Wessely's (bio)psychosocial model versus a bio(psychosocial) model based on inflammatory and oxidative and nitrosative stress pathways.
BMC Med.
2010; 8: 35. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 683.
Maes M, Twisk FN, Kubera M, et al.:
Increased IgA responses to the LPS of commensal bacteria is associated with inflammation and activation of cell-mediated immunity in chronic fatigue syndrome.
J Affect Disord.
2012; 136(3): 909–17. PubMed Abstract
| Publisher Full Text
- 684.
Nicolson GL, Nasralla MY, De Meirleir K, et al.:
Bacterial and Viral Co-Infections in Chronic Fatigue Syndrome (CFS/ME) Patients.
Proc Clin Sci Conference on Myalgic Encephalopathy/Chronic Fatigue Syndrome.
2002: 1–12. Reference Source
- 685.
Proal AD, Albert PJ, Marshall TG, et al.:
Immunostimulation in the treatment for chronic fatigue syndrome/myalgic encephalomyelitis.
Immunol Res.
2013; 56(2–3): 398–412. PubMed Abstract
| Publisher Full Text
- 686.
Mangin M, Sinha R, Fincher K:
Inflammation and vitamin D: the infection connection.
Inflamm Res.
2014; 63(10): 803–19. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 687.
Martin E, Winn R, Nugent K:
Catastrophic antiphospholipid syndrome in a community-acquired methicillin-resistant Staphylococcus aureus infection: a review of pathogenesis with a case for molecular mimicry.
Autoimmun Rev.
2011; 10(4): 181–8. PubMed Abstract
| Publisher Full Text
- 688.
Sène D, Piette JC, Cacoub P:
Antiphospholipid antibodies, antiphospholipid syndrome and infections.
Autoimmun Rev.
2008; 7(4): 272–7. PubMed Abstract
| Publisher Full Text
- 689.
García-Carrasco M, Galarza-Maldonado C, Mendoza-Pinto C, et al.:
Infections and the antiphospholipid syndrome.
Clin Rev Allergy Immunol.
2009; 36(2–3): 104–8. PubMed Abstract
| Publisher Full Text
- 690.
Cruz-Tapias P, Blank M, Anaya JM, et al.:
Infections and vaccines in the etiology of antiphospholipid syndrome.
Curr Opin Rheumatol.
2012; 24(4): 389–93. PubMed Abstract
| Publisher Full Text
- 691.
Zinger H, Sherer Y, Goddard G, et al.:
Common infectious agents prevalence in antiphospholipid syndrome.
Lupus.
2009; 18(13): 1149–53. PubMed Abstract
| Publisher Full Text
- 692.
Weber MA, Klein NJ, Hartley JC, et al.:
Infection and sudden unexpected death in infancy: a systematic retrospective case review.
Lancet.
2008; 371(9627): 1848–53. PubMed Abstract
| Publisher Full Text
- 693.
Goldwater PN:
Sterile site infection at autopsy in sudden unexpected deaths in infancy.
Arch Dis Child.
2009; 94(4): 303–7. PubMed Abstract
| Publisher Full Text
- 694.
Alfelali M, Khandaker G:
Infectious causes of sudden infant death syndrome.
Paediatr Respir Rev.
2014; 15(4): 307–11. PubMed Abstract
| Publisher Full Text
- 695.
Blood-Siegfried J:
The role of infection and inflammation in sudden infant death syndrome.
Immunopharmacol Immunotoxicol.
2009; 31(4): 516–23. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 696.
Blood-Siegfried J, Bowers MT, Lorimer M:
Is shock a key element in the pathology of sudden infant death syndrome (SIDS)?
Biol Res Nurs.
2009; 11(2): 187–94. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 697.
Sayers NM, Drucker DB, Hutchinson IV, et al.:
Preliminary investigation of lethally toxic sera of sudden infant death syndrome victims and neutralisation by commercially available immunoglobulins and adult sera.
FEMS Immunol Med Microbiol.
1999; 25(1–2): 193–8. PubMed Abstract
| Publisher Full Text
- 698.
Highet AR:
An infectious aetiology of sudden infant death syndrome.
J Appl Microbiol.
2008; 105(3): 625–35. PubMed Abstract
| Publisher Full Text
- 699.
Sartor RB:
Microbial influences in inflammatory bowel diseases.
Gastroenterology.
2008; 134(2): 577–94. PubMed Abstract
| Publisher Full Text
- 700.
Manichanh C, Borruel N, Casellas F, et al.:
The gut microbiota in IBD.
Nat Rev Gastroenterol Hepatol.
2012; 9(10): 599–608. PubMed Abstract
| Publisher Full Text
- 701.
Wu GD, Bushmanc FD, Lewis JD:
Diet, the human gut microbiota, and IBD.
Anaerobe.
2013; 24: 117–20. PubMed Abstract
| Publisher Full Text
- 702.
Petersen C, Round JL:
Defining dysbiosis and its influence on host immunity and disease.
Cell Microbiol.
2014; 16(7): 1024–33. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 703.
Hold GL, Smith M, Grange C, et al.:
Role of the gut microbiota in inflammatory bowel disease pathogenesis: what have we learnt in the past 10 years?
World J Gastroenterol.
2014; 20(5): 1192–210. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 704.
Huttenhower C, Kostic AD, Xavier RJ:
Inflammatory bowel disease as a model for translating the microbiome.
Immunity.
2014; 40(6): 843–54. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 705.
Kerman DH, Deshpande AR:
Gut microbiota and inflammatory bowel disease: the role of antibiotics in disease management.
Postgrad Med.
2014; 126(4): 7–19. PubMed Abstract
| Publisher Full Text
- 706.
Sartor RB:
The intestinal microbiota in inflammatory bowel diseases.
Nestle Nutr Inst Workshop Ser.
2014; 79: 29–39. PubMed Abstract
| Publisher Full Text
- 707.
Cammarota G, Ianiro G, Cianci R, et al.:
The involvement of gut microbiota in inflammatory bowel disease pathogenesis: potential for therapy.
Pharmacol Ther.
2015. PubMed Abstract
| Publisher Full Text
- 708.
Eishi Y:
Etiologic aspect of sarcoidosis as an allergic endogenous infection caused by Propionibacterium acnes.
Biomed Res Int.
2013; 2013: 935289. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 709.
Eishi Y:
Etiologic link between sarcoidosis and Propionibacterium acnes.
Respir Investig.
2013; 51(2): 56–68. PubMed Abstract
| Publisher Full Text
- 710.
Omori M, Bito T, Yamada M, et al.:
Systemic sarcoidosis with bone marrow involvement showing Propionibacterium acnes in the lymph nodes.
J Eur Acad Dermatol Venereol.
2014. PubMed Abstract
| Publisher Full Text
- 711.
Faraji F, Zarinfar N, Zanjani AT, et al.:
The effect of Helicobacter pylori eradication on migraine: a randomized, double blind, controlled trial.
Pain physician.
2012; 15(6): 495–8. PubMed Abstract
- 712.
Su J, Zhou XY, Zhang GX:
Association between Helicobacter pylori infection and migraine: a meta-analysis.
World J Gastroenterol.
2014; 20(40): 14965–72. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 713.
Kell DB, Pretorius E:
The simultaneous occurrence of both hypercoagulability and hypofibrinolysis in blood and serum during systemic inflammation, and the roles of iron and fibrin(ogen).
Integr Biol (Camb).
2015; 7(1): 24–52. PubMed Abstract
| Publisher Full Text
- 714.
Weinberg ED:
Iron withholding: a defense against infection and neoplasia.
Physiol Rev.
1984; 64(1): 65–102. PubMed Abstract
- 715.
Galley HF, Webster NR:
Elevated serum bleomycin-detectable iron concentrations in patients with sepsis syndrome.
Intensive Care Med.
1996; 22(3): 226–9. PubMed Abstract
| Publisher Full Text
- 716.
Galley HF, Davies MJ, Webster NR:
Ascorbyl radical formation in patients with sepsis: effect of ascorbate loading.
Free Radic Biol Med.
1996; 20(1): 139–43. PubMed Abstract
| Publisher Full Text
- 717.
Galley HF, Howdle PD, Walker BE, et al.:
The effects of intravenous antioxidants in patients with septic shock.
Free Radic Biol Med.
1997; 23(5): 768–74. PubMed Abstract
| Publisher Full Text
- 718.
Ghio AJ, Carter JD, Richards JH, et al.:
Iron and iron-related proteins in the lower respiratory tract of patients with acute respiratory distress syndrome.
Crit Care Med.
2003; 31(2): 395–400. PubMed Abstract
| Publisher Full Text
- 719.
Duvigneau JC, Piskernik C, Haindl S, et al.:
A novel endotoxin-induced pathway: upregulation of heme oxygenase 1, accumulation of free iron, and free iron-mediated mitochondrial dysfunction.
Lab Invest.
2008; 88(1): 70–7. PubMed Abstract
| Publisher Full Text
- 720.
Lagan AL, Melley DD, Evans TW, et al.:
Pathogenesis of the systemic inflammatory syndrome and acute lung injury: role of iron mobilization and decompartmentalization.
Am J Physiol Lung Cell Mol Physiol.
2008; 294(2): L161–74. PubMed Abstract
| Publisher Full Text
- 721.
Lagan AL, Quinlan GJ, Mumby S, et al.:
Variation in iron homeostasis genes between patients with ARDS and healthy control subjects.
Chest.
2008; 133(6): 1302–11. PubMed Abstract
| Publisher Full Text
- 722.
Weinberg ED:
Iron availability and infection.
Biochim Biophys Acta.
2009; 1790(7): 600–5. PubMed Abstract
| Publisher Full Text
- 723.
Goldenberg RL, Tamura T, DuBard M, et al.:
Plasma ferritin and pregnancy outcome.
Am J Obstet Gynecol.
1996; 175(5): 1356–9. PubMed Abstract
| Publisher Full Text
- 724.
Goldenberg RL, Mercer BM, Miodovnik M, et al.:
Plasma ferritin, premature rupture of membranes, and pregnancy outcome.
Am J Obstet Gynecol.
1998; 179(6 Pt 1): 1599–604. PubMed Abstract
| Publisher Full Text
- 725.
Garcia PC, Longhi F, Branco RG, et al.:
Ferritin levels in children with severe sepsis and septic shock.
Acta paediatr.
2007; 96(12): 1829–31. PubMed Abstract
| Publisher Full Text
- 726.
Bennett TD, Hayward KN, Farris RW, et al.:
Very high serum ferritin levels are associated with increased mortality and critical care in pediatric patients.
Pediatr Crit Care Med.
2011; 12(6): e233–6. PubMed Abstract
| Publisher Full Text
- 727.
Suárez-Santamaría M, Santolaria F, Pérez-Ramírez A, et al.:
Prognostic value of inflammatory markers (notably cytokines and procalcitonin), nutritional assessment, and organ function in patients with sepsis.
Eur Cytokine Netw.
2010; 21(1): 19–26. PubMed Abstract
| Publisher Full Text
- 728.
Muench KH:
Hemochromatosis and infection: alcohol and iron, oysters and sepsis.
Am J Med.
1989; 87(3N): 40N–43N. PubMed Abstract
- 729.
Oppenheimer SJ:
Iron and infection: the clinical evidence.
Acta Paediatr Scand Suppl.
1989; 361: 53–62. PubMed Abstract
- 730.
Khan FA, Fisher MA, Khakoo RA:
Association of hemochromatosis with infectious diseases: expanding spectrum.
Int J Infect Dis.
2007; 11(6): 482–7. PubMed Abstract
| Publisher Full Text
- 731.
Larson JA, Higashi DL, Stojiljkovic I, et al.:
Replication of Neisseria meningitidis within epithelial cells requires TonB-dependent acquisition of host cell iron.
Infect Immun.
2002; 70(3): 1461–7. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 732.
Braun V:
Bacterial iron transport related to virulence.
Contrib Microbiol.
2005; 12: 210–33. PubMed Abstract
| Publisher Full Text
- 733.
Gao Q, Wang X, Xu H, et al.:
Roles of iron acquisition systems in virulence of extraintestinal pathogenic Escherichia coli: salmochelin and aerobactin contribute more to virulence than heme in a chicken infection model.
BMC Microbiol.
2012; 12: 143. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 734.
Mittal R, Sharma S, Chhibber S, et al.:
Iron dictates the virulence of Pseudomonas aeruginosa in urinary tract infections.
J Biomed Sci.
2008; 15(6): 731–41. PubMed Abstract
| Publisher Full Text
- 735.
Nevitt T:
War-Fe-re: iron at the core of fungal virulence and host immunity.
Biometals.
2011; 24(3): 547–58. PubMed Abstract
| Publisher Full Text
- 736.
Rakin A, Schneider L, Podladchikova O:
Hunger for iron: the alternative siderophore iron scavenging systems in highly virulent Yersinia.
Front Cell Infect Microbiol.
2012; 2: 151. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 737.
Rodriguez GM, Smith I:
Mechanisms of iron regulation in mycobacteria: role in physiology and virulence.
Mol Microbiol.
2003; 47(6): 1485–94. PubMed Abstract
| Publisher Full Text
- 738.
Russo TA, Olson R, Macdonald U, et al.:
Aerobactin mediates virulence and accounts for increased siderophore production under iron-limiting conditions by hypervirulent (hypermucoviscous) Klebsiella pneumoniae.
Infect Immun.
2014; 82(6): 2356–67. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 739.
Sritharan M:
Iron and bacterial virulence.
Indian J Med Microbiol.
2006; 24(3): 163–4. PubMed Abstract
- 740.
Sutak R, Lesuisse E, Tachezy J, et al.:
Crusade for iron: iron uptake in unicellular eukaryotes and its significance for virulence.
Trends Microbiol.
2008; 16(6): 261–8. PubMed Abstract
| Publisher Full Text
- 741.
Vasil ML, Ochsner UA:
The response of Pseudomonas aeruginosa to iron: genetics, biochemistry and virulence.
Mol Microbiol.
1999; 34(3): 399–413. PubMed Abstract
| Publisher Full Text
- 742.
Williams PH, Carbonetti NH:
Iron, siderophores, and the pursuit of virulence: independence of the aerobactin and enterochelin iron uptake systems in Escherichia coli.
Infect Immun.
1986; 51(3): 942–7. PubMed Abstract
| Free Full Text
- 743.
Yep A, McQuade T, Kirchhoff P, et al.:
Inhibitors of TonB function identified by a high-throughput screen for inhibitors of iron acquisition in uropathogenic Escherichia coli CFT073.
MBio.
2014; 5(2): e01089–13. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 744.
Gaitonde S, Pathan E, Sule A, et al.:
Efficacy of isoniazid prophylaxis in patients with systemic lupus erythematosus receiving long term steroid treatment.
Ann Rheum Dis.
2002; 61(3): 251–3. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 745.
Gilliland WR, Tsokos GC:
Prophylactic use of antibiotics and immunisations in patients with SLE.
Ann Rheum Dis.
2002; 61(3): 191–2. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 746.
Filgueiras LR, Brandt SL, Wang S, et al.:
Leukotriene B4–mediated sterile inflammation promotes susceptibility to sepsis in a mouse model of type 1 diabetes.
Sci Signal.
2015; 8(361): ra10. PubMed Abstract
| Publisher Full Text
- 747.
Syrjänen J, Valtonen VV, Iivanainen M, et al.:
Preceding infection as an important risk factor for ischaemic brain infarction in young and middle aged patients.
Br Med J (Clin Res Ed).
1988; 296(6630): 1156–60. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 748.
Grau AJ, Buggle F, Steichen-Wiehn C, et al.:
Clinical and biochemical analysis in infection-associated stroke.
Stroke.
1995; 26(9): 1520–6. PubMed Abstract
| Publisher Full Text
- 749.
Grau AJ, Buggle F, Heindl S, et al.:
Recent infection as a risk factor for cerebrovascular ischemia.
Stroke.
1995; 26(3): 373–9. PubMed Abstract
| Publisher Full Text
- 750.
Palasik W, Fiszer U, Lechowicz W, et al.:
Assessment of relations between clinical outcome of ischemic stroke and activity of inflammatory processes in the acute phase based on examination of selected parameters.
Eur Neurol.
2005; 53(4): 188–93. PubMed Abstract
| Publisher Full Text
- 751.
Zeller JA, Lenz A, Eschenfelder CC, et al.:
Platelet-leukocyte interaction and platelet activation in acute stroke with and without preceding infection.
Arterioscler Thromb Vasc Biol.
2005; 25(7): 1519–23. PubMed Abstract
| Publisher Full Text
- 752.
McColl BW, Allan SM, Rothwell NJ:
Systemic infection, inflammation and acute ischemic stroke.
Neuroscience.
2009; 158(3): 1049–61. PubMed Abstract
| Publisher Full Text
- 753.
Grau AJ, Urbanek C, Palm F:
Common infections and the risk of stroke.
Nat Rev Neurol.
2010; 6(12): 681–94. PubMed Abstract
| Publisher Full Text
- 754.
Ionita CC, Siddiqui AH, Levy EI, et al.:
Acute ischemic stroke and infections.
J Stroke Cerebrovasc Dis.
2011; 20(1): 1–9. PubMed Abstract
| Publisher Full Text
- 755.
Mayr FB, Yende S, Angus DC:
Epidemiology of severe sepsis.
Virulence.
2014; 5(1): 4–11. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 756.
Srinivasan G, Aitken JD, Zhang B, et al.:
Lipocalin 2 deficiency dysregulates iron homeostasis and exacerbates endotoxin-induced sepsis.
J Immunol.
2012; 189(4): 1911–9. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 757.
Lehmann C, Sharawi N, Al-Banna N, et al.:
Novel approaches to the development of anti-sepsis drugs.
Expert Opin Drug Discov.
2014; 9(5): 523–31. PubMed Abstract
| Publisher Full Text
- 758.
Luo G, Spellberg B, Gebremariam T, et al.:
Combination therapy with iron chelation and vancomycin in treating murine staphylococcemia.
Eur J Clin Microbiol Infect Dis.
2014; 33(5): 845–51. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 759.
Zeng C, Chen Q, Zhang K, et al.:
Hepatic Hepcidin Protects against Polymicrobial Sepsis in Mice by Regulating Host Iron Status.
Anesthesiology.
2015; 122(2): 374–86. PubMed Abstract
| Publisher Full Text
- 760.
Dellinger RP, Levy MM, Carlet JM, et al.:
Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008.
Crit Care Med.
2008; 36(1): 296–327. PubMed Abstract
- 761.
Currin A, Swainston N, Day PJ, et al.:
Synthetic biology for the directed evolution of protein biocatalysts: navigating sequence space intelligently.
Chem Soc Rev.
2015; 44(5): 1172–239. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 762.
Xie L, Xie L, Bourne PE:
Structure-based systems biology for analyzing off-target binding.
Curr Opin Struct Biol.
2011; 21(2): 189–99. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 763.
Mestres J, Gregori-Puigjané E, Valverde S, et al.:
The topology of drug-target interaction networks: implicit dependence on drug properties and target families.
Mol Biosyst.
2009; 5(9): 1051–7. PubMed Abstract
| Publisher Full Text
- 764.
Marshall TG, Marshall FE:
Sarcoidosis succumbs to antibiotics--implications for autoimmune disease.
Autoimmun Rev.
2004; 3(4): 295–300. PubMed Abstract
| Publisher Full Text
- 765.
O'Dell JR, Paulsen G, Haire CE, et al.:
Treatment of early seropositive rheumatoid arthritis with minocycline: four-year followup of a double-blind, placebo-controlled trial.
Arthritis Rheum.
1999; 42(8): 1691–5. PubMed Abstract
| Publisher Full Text
- 766.
Astrauskiene D, Bernotiene E:
New insights into bacterial persistence in reactive arthritis.
Clin Exp Rheumatol.
2007; 25(3): 470–9. PubMed Abstract
- 767.
Ogrendik M, Karagoz N:
Treatment of rheumatoid arthritis with roxithromycin: a randomized trial.
Postgrad Med.
2011; 123(5): 220–7. PubMed Abstract
| Publisher Full Text
- 768.
Kwiatkowska B, Maślińska M:
Macrolide therapy in chronic inflammatory diseases.
Mediators Inflamm.
2012; 2012: 636157. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 769.
Garrido-Mesa N, Zarzuelo A, Gálvez J:
Minocycline: far beyond an antibiotic.
Br J Pharmacol.
2013; 169(2): 337–52. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 770.
Ogrendik M:
Rheumatoid arthritis is an autoimmune disease caused by periodontal pathogens.
Int J Gen Med.
2013; 6: 383–6. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 771.
Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, et al.:
Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis.
J Immunol.
2009; 183(10): 6041–50. PubMed Abstract
| Publisher Full Text
- 772.
Yokote H, Miyake S, Croxford JL, et al.:
NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora.
Am J Pathol.
2008; 173(6): 1714–23. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 773.
Ochoa-Repáraz J, Mielcarz DW, Begum-Haque S, et al.:
Gut, bugs, and brain: role of commensal bacteria in the control of central nervous system disease.
Ann Neurol.
2011; 69(2): 240–7. PubMed Abstract
| Publisher Full Text
- 774.
Berer K, Mues M, Koutrolos M, et al.:
Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination.
Nature.
2011; 479(7374): 538–41. PubMed Abstract
| Publisher Full Text
- 775.
Berer K, Krishnamoorthy G:
Commensal gut flora and brain autoimmunity: a love or hate affair?
Acta Neuropathol.
2012; 123(5): 639–51. PubMed Abstract
| Publisher Full Text
- 776.
Wang Y, Kasper LH:
The role of microbiome in central nervous system disorders.
Brain Behav Immun.
2014; 38: 1–12. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 777.
Ochoa-Repáraz J, Kasper LH:
Gut microbiome and the risk factors in central nervous system autoimmunity.
FEBS Lett.
2014; 588(22): 4214–22. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 778.
Saxena VN, Dogra J:
Long-term use of penicillin for the treatment of chronic plaque psoriasis.
Eur J Dermatol.
2005; 15(5): 359–62. PubMed Abstract
- 779.
Saxena VN, Dogra J:
Long-term oral azithromycin in chronic plaque psoriasis: a controlled trial.
Eur J Dermatol.
2010; 20(3): 329–33. PubMed Abstract
| Publisher Full Text
- 780.
Alzolibani AA, Zedan K:
Macrolides in Chronic Inflammatory Skin Disorders.
Mediators Inflamm.
2012; 2012: 159354. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 781.
Vila-Corcoles A, Ochoa-Gondar O, Rodriguez-Blanco T, et al.:
Clinical effectiveness of pneumococcal vaccination against acute myocardial infarction and stroke in people over 60 years: the CAPAMIS study, one-year follow-up.
BMC Public Health.
2012; 12: 222. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 782.
Vila-Corcoles A, Ochoa-Gondar O, Rodriguez-Blanco T, et al.:
Evaluating clinical effectiveness of pneumococcal vaccination in preventing stroke: the CAPAMIS Study, 3-year follow-up.
J Stroke Cerebrovasc Dis.
2014; 23(6): 1577–84. PubMed Abstract
| Publisher Full Text
- 783.
Goodfellow M, Fiedler HP:
A guide to successful bioprospecting: informed by actinobacterial systematics.
Antonie Van Leeuwenhoek.
2010; 98(2): 119–42. PubMed Abstract
| Publisher Full Text
- 784.
Yarwood JM, Leung DYM, Schlievert PM:
Evidence for the involvement of bacterial superantigens in psoriasis, atopic dermatitis, and Kawasaki syndrome.
FEMS Microbiol Lett.
2000; 192(1): 1–7. PubMed Abstract
| Publisher Full Text
- 785.
Proal AD, Albert PJ, Marshall TG:
Inflammatory disease and the human microbiome.
Discov Med.
2014; 17(95): 257–65. PubMed Abstract
- 786.
Reddick LE, Alto NM:
Bacteria fighting back: how pathogens target and subvert the host innate immune system.
Mol Cell.
2014; 54(2): 321–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 787.
Liu D: editor. Molecular Detection of Human Bacterial Pathogens. Boca Raton: CRC Press; 2011. Reference Source
- 788.
Swearingen MC, Porwollik S, Desai PT, et al.:
Virulence of 32 Salmonella strains in mice.
PLoS One.
2012; 7(4): e36043. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 789.
Bleibtreu A, Gros PA, Laouenan C, et al.:
Fitness, stress resistance, and extraintestinal virulence in Escherichia coli.
Infect Immun.
2013; 81(8): 2733–42. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 790.
Hacker J, Bender L, Ott M, et al.:
Deletions of chromosomal regions coding for fimbriae and hemolysins occur in vitro and in vivo in various extraintestinal Escherichia coli isolates.
Microb Pathog.
1990; 8(3): 213–25. PubMed Abstract
| Publisher Full Text
- 791.
Hacker J, Kaper JB:
Pathogenicity islands and the evolution of microbes.
Annu Rev Microbiol.
2000; 54: 641–79. PubMed Abstract
| Publisher Full Text
- 792.
Falkow S:
Molecular Koch's postulates applied to bacterial pathogenicity--a personal recollection 15 years later.
Nat Rev Microbiol.
2004; 2(1): 67–72. PubMed Abstract
| Publisher Full Text
- 793.
Asad S, Opal SM:
Bench-to-bedside review: Quorum sensing and the role of cell-to-cell communication during invasive bacterial infection.
Crit Care.
2008; 12(6): 236. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 794.
Gal-Mor O, Finlay BB:
Pathogenicity islands: a molecular toolbox for bacterial virulence.
Cell Microbiol.
2006; 8(11): 1707–19. PubMed Abstract
| Publisher Full Text
- 795.
Che D, Hasan MS, Chen B:
Identifying pathogenicity islands in bacterial pathogenomics using computational approaches.
Pathogens.
2014; 3(1): 36–56. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 796.
Unsworth KE, Holden DW:
Identification and analysis of bacterial virulence genes in vivo.
Philos Trans R Soc Lond B Biol Sci.
2000; 355(1397): 613–22. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 797.
Penadés JR, Chen J, Quiles-Puchalt N, et al.:
Bacteriophage-mediated spread of bacterial virulence genes.
Curr Opin Microbiol.
2015; 23: 171–8. PubMed Abstract
| Publisher Full Text
- 798.
Novick RP:
Autoinduction and signal transduction in the regulation of staphylococcal virulence.
Mol Microbiol.
2003; 48(6): 1429–49. PubMed Abstract
| Publisher Full Text
- 799.
Ewald PW:
Evolution of infectious disease. New York: Oxford University Press; 1994. Publisher Full Text
- 800.
Landraud L, Jauréguy F, Frapy E, et al.:
Severity of Escherichia coli bacteraemia is independent of the intrinsic virulence of the strains assessed in a mouse model.
Clin Microbiol Infect.
2013; 19(1): 85–90. PubMed Abstract
| Publisher Full Text
- 801.
Wester AL, Melby KK, Wuyller TB, et al.:
E. coli bacteremia strains - high diversity and associations with age-related clinical phenomena.
Clin Microbiol.
2014; 3(2): 140. Publisher Full Text
- 802.
Rook GA, Brunet LR:
Give us this day our daily germs.
Biologist (London).
2002; 49(4): 145–9. PubMed Abstract
- 803.
Rook GA:
Hygiene hypothesis and autoimmune diseases.
Clin Rev Allergy Immunol.
2012; 42(1): 5–15. PubMed Abstract
| Publisher Full Text
- 804.
Rook GA:
Regulation of the immune system by biodiversity from the natural environment: an ecosystem service essential to health.
Proc Natl Acad Sci U S A.
2013; 110(46): 18360–7. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 805.
Rook GA, Raison CL, Lowry CA:
Microbiota, immunoregulatory old friends and psychiatric disorders.
Adv Exp Med Biol.
2014; 817: 319–56. PubMed Abstract
| Publisher Full Text
- 806.
Mändle T, Einsele H, Schaller M, et al.:
Infection of human CD34+ progenitor cells with Bartonella henselae results in intraerythrocytic presence of B. henselae.
Blood.
2005; 106(4): 1215–22. PubMed Abstract
| Publisher Full Text
- 807.
Pitassi LH, Magalhães RF, Barjas-Castro ML, et al.:
Bartonella henselae infects human erythrocytes.
Ultrastruct Pathol.
2007; 31(6): 369–72. PubMed Abstract
| Publisher Full Text
- 808.
Pitassi LHU, Cintra ML, Ferreira MR, et al.:
Blood cell findings resembling Bartonella spp.
Ultrastruct Pathol.
2010; 34(1): 2–6. PubMed Abstract
| Publisher Full Text
- 809.
Groebel K, Hoelzle K, Wittenbrink MM, et al.:
Mycoplasma suis invades porcine erythrocytes.
Infect Immun.
2009; 77(2): 576–84. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 810.
Horzempa J, O'Dee DM, Stolz DB, et al.:
Invasion of erythrocytes by Francisella tularensis.
J Infect Dis.
2011; 204(1): 51–9. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 811.
Sagan L:
On the origin of mitosing cells.
J Theor Biol.
1967; 14(3): 255–74. PubMed Abstract
| Publisher Full Text
- 812.
Margulis L, Chapman MJ:
Endosymbioses: cyclical and permanent in evolution.
Trends Microbiol.
1998; 6(9): 342–5; discussion 345–6. PubMed Abstract
| Publisher Full Text
- 813.
Kell DB, Oliver SG:
Here is the evidence, now what is the hypothesis? The complementary roles of inductive and hypothesis-driven science in the post-genomic era.
Bioessays.
2004; 26(1): 99–105. PubMed Abstract
| Publisher Full Text
- 814.
Kell DB:
What would be the observable consequences if phospholipid bilayer diffusion of drugs into cells is negligible?
Trends Pharmacol Sci.
2015; 36(1): 15–21. PubMed Abstract
| Publisher Full Text
- 815.
Evans AS:
Causation and disease: the Henle-Koch postulates revisited.
Yale J Biol Med.
1976; 49(2): 175–95. PubMed Abstract
| Free Full Text
- 816.
Harden VA:
Koch's postulates and the etiology of AIDS: an historical perspective.
Hist Philos Life Sci.
1992; 14(2): 249–69. PubMed Abstract
- 817.
Thagard P:
How scientists explain disease. Princeton, NJ Princeton University Press; 1999. Reference Source
- 818.
Gradmann C:
A spirit of scientific rigour: Koch's postulates in twentieth-century medicine.
Microbes Infect.
2014; 16(11): 885–92. PubMed Abstract
| Publisher Full Text
- 819.
Fredricks DN, Relman DA:
Sequence-based identification of microbial pathogens: a reconsideration of Koch's postulates.
Clin Micr Rev.
1996; 9(1): 18–33. PubMed Abstract
| Free Full Text
- 820.
Lowe AM, Yansouni CP, Behr MA:
Causality and gastrointestinal infections: Koch, Hill, and Crohn's.
Lancet Infect Dis.
2008; 8(11): 720–6. PubMed Abstract
| Publisher Full Text
- 821.
Segre JA:
What does it take to satisfy Koch's postulates two centuries later? Microbial genomics and Propionibacteria acnes.
J Invest Dermatol.
2013; 133(9): 2141–2. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 822.
Silvers RB:
Hidden histories of science. New York: New York Review; 1995. Reference Source
- 823.
Hook EB:
Prematurity in scientific discovery: on resistance and neglect. Berkeley, CA: University of California Press; 2002. Reference Source
- 824.
Kell D:
Dormant microbes: time to revive some old ideas.
Nature.
2009; 458(7240): 831. PubMed Abstract
| Publisher Full Text
- 825.
Finkel SE:
Long-term survival during stationary phase: evolution and the GASP phenotype.
Nat Rev Microbiol.
2006; 4(2): 113–20. PubMed Abstract
| Publisher Full Text
- 826.
Buzan T:
How to mind map. London: Thorsons; 2002. Reference Source
- 827.
Withell ER:
The significance of the variation in shape of time-survivor curves.
J Hyg (Lond).
1942; 42(2): 124–83. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 828.
Chu BC, Garcia-Herrero A, Johanson TH, et al.:
Siderophore uptake in bacteria and the battle for iron with the host; a bird's eye view.
Biometals.
2010; 23(4): 601–11. PubMed Abstract
| Publisher Full Text
- 829.
Armitage AE, Drakesmith H:
Genetics. The battle for iron.
Science.
2014; 346(6215): 1299–300. PubMed Abstract
| Publisher Full Text
- 830.
Haley KP, Skaar EP:
A battle for iron: host sequestration and Staphylococcus aureus acquisition.
Microbes Infect.
2012; 14(3): 217–27. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 831.
Subashchandrabose S, Mobley HLT:
Back to the metal age: battle for metals at the host-pathogen interface during urinary tract infection.
Metallomics.
2015; 7(6): 935–42. PubMed Abstract
| Publisher Full Text
- 832.
Zhang H, Niesel DW, Peterson JW, et al.:
Lipoprotein release by bacteria: potential factor in bacterial pathogenesis.
Infect Immun.
1998; 66(11): 5196–201. PubMed Abstract
| Free Full Text
- 833.
Kotsaki A, Giamarellos-Bourboulis EJ:
Emerging drugs for the treatment of sepsis.
Expert Opin Emerg Drugs.
2012; 17(3): 379–91. PubMed Abstract
| Publisher Full Text
- 834.
Balakrishnan A, Marathe SA, Joglekar M, et al.:
Bactericidal/permeability increasing protein: a multifaceted protein with functions beyond LPS neutralization.
Innate Immun.
2013; 19(4): 339–47. PubMed Abstract
| Publisher Full Text
- 835.
Noble F, Rubira E, Boulanouar M, et al.:
Acute systemic inflammation induces central mitochondrial damage and mnesic deficit in adult Swiss mice.
Neurosci Lett.
2007; 424(2): 106–10. PubMed Abstract
| Publisher Full Text
- 836.
Lee DC, Rizer J, Selenica ML, et al.:
LPS- induced inflammation exacerbates phospho-tau pathology in rTg4510 mice.
J Neuroinflammation.
2010; 7: 56. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 837.
Small BG, McColl BW, Allmendinger R, et al.:
Efficient discovery of anti-inflammatory small-molecule combinations using evolutionary computing.
Nature Chem Biol.
2011; 7(12): 902–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 838.
Bode JG, Ehlting C, Häussinger D:
The macrophage response towards LPS and its control through the p38MAPK-STAT3 axis.
Cell Signal.
2012; 24(6): 1185–94. PubMed Abstract
| Publisher Full Text
- 839.
Murray KN, Buggey HF, Denes A, et al.:
Systemic immune activation shapes stroke outcome.
Mol Cell Neurosci.
2013; 53: 14–25. PubMed Abstract
| Publisher Full Text
- 840.
Belkaid Y, Hand TW:
Role of the microbiota in immunity and inflammation.
Cell.
2014; 157(1): 121–41. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 841.
Płóciennikowska A, Hromada-Judycka A, Borzęcka K, et al.:
Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling.
Cell Mol Life Sci.
2015; 72(3): 557–81. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 842.
Ji S, Choi YS, Choi Y:
Bacterial invasion and persistence: critical events in the pathogenesis of periodontitis?
J Periodontal Res.
2014. PubMed Abstract
| Publisher Full Text
- 843.
Akiyama H, Barger S, Barnum S, et al.:
Inflammation and Alzheimer's disease.
Neurobiol Aging.
2000; 21(3): 383–421. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 844.
Hotamisligil GS:
Inflammation and metabolic disorders.
Nature.
2006; 444(7121): 860–7. PubMed Abstract
| Publisher Full Text
- 845.
Hotamisligil GS, Erbay E:
Nutrient sensing and inflammation in metabolic diseases.
Nat Rev Immunol.
2008; 8(12): 923–34. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 846.
Tan Y, Kagan JC:
A cross-disciplinary perspective on the innate immune responses to bacterial lipopolysaccharide.
Mol Cell.
2014; 54(2): 212–23. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 847.
Ong WY, Farooqui AA:
Iron, neuroinflammation, and Alzheimer's disease.
J Alzheimers Dis.
2005; 8(2): 183–200; discussion 209–15. PubMed Abstract
- 848.
Marques F, Falcao AM, Sousa JC, et al.:
Altered iron metabolism is part of the choroid plexus response to peripheral inflammation.
Endocrinology.
2009; 150(6): 2822–8. PubMed Abstract
| Publisher Full Text
- 849.
Levi M, Schouten M, van der Poll T:
Sepsis, coagulation, and antithrombin: old lessons and new insights.
Semin Thromb Hemost.
2008; 34(8): 742–6. PubMed Abstract
| Publisher Full Text
- 850.
Schouten M, Wiersinga WJ, Levi M, et al.:
Inflammation, endothelium, and coagulation in sepsis.
J Leukoc Biol.
2008; 83(3): 536–45. PubMed Abstract
| Publisher Full Text
- 851.
Levi M, van der Poll T:
Inflammation and coagulation.
Crit Care Med.
2010; 38(2 Suppl): S26–34. PubMed Abstract
| Publisher Full Text
- 852.
Levi M:
The coagulant response in sepsis and inflammation.
Hamostaseologie.
2010; 30(1): 10–2, 14–6. PubMed Abstract
- 853.
van der Poll T, de Boer JD, Levi M:
The effect of inflammation on coagulation and vice versa.
Curr Opin Infect Dis.
2011; 24(3): 273–8. PubMed Abstract
| Publisher Full Text
- 854.
Levi M, Schultz M, van der Poll T:
Sepsis and thrombosis.
Semin Thromb Hemost.
2013; 39(5): 559–66. PubMed Abstract
| Publisher Full Text
- 855.
Levi M, Poll TV:
Coagulation in patients with severe sepsis.
Semin Thromb Hemost.
2015; 41(1): 9–15. PubMed Abstract
| Publisher Full Text
- 856.
Guadarrama-López AL, Valdés-Ramos R, Martínez-Carrillo BE:
Type 2 diabetes, PUFAs, and vitamin D: their relation to inflammation.
J Immunol Res.
2014; 2014: 860703. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 857.
Arner P:
Insulin resistance in type 2 diabetes -- role of the adipokines.
Curr Mol Med.
2005; 5(3): 333–9. PubMed Abstract
| Publisher Full Text
- 858.
Kadowaki T, Yamauchi T, Kubota N, et al.:
Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome.
J Clin Invest.
2006; 116(7): 1784–92. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 859.
Anderson SG, Dunn WB, Banerjee M, et al.:
Evidence that multiple defects in lipid regulation occur before hyperglycemia during the prodrome of type-2 diabetes.
PLoS One.
2014; 9(9): e103217. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 860.
Aregbesola AO, Voutilainen S, Virtanen JK, et al.:
Body iron stores and the risk of type 2 diabetes in middle-aged men.
Eur J Endocrinol.
2013; 169(2): 247–53. PubMed Abstract
| Publisher Full Text
- 861.
Simcox JA, McClain DA:
Iron and diabetes risk.
Cell Metab.
2013; 17(3): 329–41. PubMed Abstract
| Publisher Full Text
| Free Full Text









Comments on this article Comments (0)