Keywords
Proteasome, Storage Granules, Dynamics, Nuclear Transport, Ubiquitin System, Blm10, Importin, Karyopherin, Quiescence
Proteasome, Storage Granules, Dynamics, Nuclear Transport, Ubiquitin System, Blm10, Importin, Karyopherin, Quiescence
Proteolysis determines the half-life of proteins and thus controls protein homeostasis. If protein homeostasis is disrupted, the incidence of protein misfolding and neurodegenerative diseases such as Huntington’s, Parkinson’s and Alzheimer’s increases (Ciechanover & Brundin, 2003).
In eukaryotic cells two highly conserved degradation pathways exist: long-lived proteins are degraded within the lysosome, an organelle with membranes which protect the surrounding cytoplasm against lysosomal hydrolases (Rendueles & Wolf, 1988); short-lived proteins are degraded by proteasomes, multimeric protease complexes which move between the nucleo- and cytoplasm (Hershko & Ciechanover, 1998). Proteasomal substrates are often nuclear proteins such as proteins regulating cell cycle progression (cyclin-dependant kinases and their inhibitors), gene expression (transcriptions factors), DNA damage and stress response; although, misfolded proteins occurring during protein synthesis in the cytoplasm are also rapidly degraded by the proteasome (Kirschner, 1999; Vabulas & Hartl, 2005; von Mikecz, 2006). As a result proteasomal proteolysis serves to eliminate obsolete proteins which compete with functional proteins for binding partners and are prone to associate with irreversible and toxic protein aggregates (Goldberg, 2003).
Here, we want to address the dynamics of proteasomes, which select their substrates by specific determinants such as poly-ubiquitylation, a covalently linked chain of ubiquitin molecules (Finley, 2009). This ubiquitin-dependent proteolysis undertakes up to 90% of protein degradation in growing yeast and cultured mammalian cells and consumes considerable amounts of ATP, since the activation and conjugation of ubiquitin to the protein substrate as well as the unfolding and translocation of the protein substrate into the proteasome is ATP-dependent (Coux et al., 1996). Natively-disordered proteins also qualify as proteasome substrates and are cleaved without post-translational ubiquitin modification (Fishbain et al., 2015).
The advent of live cell imaging and GFP-labelling technologies in the 1990s (Tsien, 1998) have greatly facilitated the study of proteasome dynamics in yeast and mammalian cells. Through these non-invasive techniques, the localization of the proteasome in growing yeast and highly proliferating cancer cells has been elucidated to be primarily nuclear (Enenkel et al., 1998; Laporte et al., 2008; McDonald & Byers, 1997; Russell et al., 1999). In line with this finding, increasing evidence in the literature suggests that certain misfolded proteins are imported from the cytoplasm into the nucleus solely for proteasomal degradation (Park et al., 2013; Prasad et al., 2010). Conversely, transient nuclear proteins are exported into the cytoplasm for proteolysis, indicating a dynamic shift of proteasomal substrates between the nucleus and cytoplasm (Chen & Madura, 2014a). Under nutrient deprivation and during transition from proliferation to quiescence, yeast proteasomes gather in proteasome storage granules (PSGs) at the nuclear envelope (NE) and endoplasmic reticulum (ER) membrane (Enenkel, 2014; Knecht & Rivett, 2000; Wojcik & DeMartino, 2003). PSGs are membraneless organelles which seem to pinch off the NE/ER and are retained as stable entities in the cytoplasm. When cells resume growth, PSGs dissipate and proteasomes are rapidly imported into the nucleus to contribute their function in cell proliferation (Laporte et al., 2008). The mechanism of PSG formation and clearance is still unknown but seems to be conserved, since PSG-like structures are observed in primary cell lines of non-dividing neuronal cells and in immortalized cell lines of cancer cells, if they are chemically arrested in cell cycle progression (Kaganovich et al., 2008).
Our knowledge about proteasome dynamics in mammalian cells is poor. Thus, the focus of this review will be to critically integrate the literature about the dynamics of the proteasome, particularly based on studies in yeast. In our overview of the ubiquitin-proteasome system and common principles of nuclear transport, we cite and refer to original work and review articles written by investigators who did seminal work on these topics. In the paragraphs addressing detailed knowledge about proteasome dynamics we cite the original work.
Ubiquitylation is a post-translational modification commonly associated with proteasomal protein degradation. At least four ubiquitin molecules, conserved peptides of 76 amino acids, are required for a poly-ubiquitin chain to be recognized by the proteasome. Hershko and colleagues in the early 1980s showed that poly-ubiquitylation requires three ATP-dependent enzymes: the ubiquitin activation enzyme (E1), a family of ubiquitin conjugating enzymes (E2) and a family of ubiquitin protein ligases (E3) (Hershko & Ciechanover, 1998). First, ATP hydrolysis is required to activate the AMP linkage to the C-terminal glycine of ubiquitin which enables the transfer of the ubiquitin moiety to the active site cysteine of the E1. Second, the E1-bound ubiquitin is linked to the active site cysteine residue of an E2 by transesterification. Finally, the E3 transfers the ubiquitin onto the substrate depending on the class of the E3 enzyme (RING, HECT and U-box ligases) (Finley et al., 2012; Harper & Schulman, 2006). Upon transfer of ubiquitin onto the substrate, the C-terminal glycine forms an isopeptide bond with an ε-amino group of a lysine residue in the substrate. Elongation of the ubiquitin chain is achieved as succeeding ubiquitin form isopeptide linkages with specific lysines of the preceding ubiquitin (Hershko & Ciechanover, 1998). Prior to degradation, deubiquitinating activities within the proteasome cleave and recycle the ubiquitin molecules from the substrates (Crosas et al., 2006; Hanna et al., 2006; Lam et al., 1997; Verma et al., 2002). Deubiquitinating enzymes in the cyto- and nucleoplasm provide an additional level on the plasticity on the repertoire of proteasomal substrates (Sahtoe & Sixma, 2015). Intriguingly, GFP-labelled ubiquitin and the E1, named UBA1, is primarily nuclear in growing yeast and mammalian cells suggesting that ubiquitin-dependent proteolysis mainly occurs in the nucleus (Huh et al., 2003; Salomons et al., 2010; Sugaya et al., 2014; Sugaya et al., 2015).
Composed of over 40 subunits, the proteasome is a protein complex of 2.5 MDa which consists of two main components: the 20S core particle (CP) and the 19S regulatory particle (RP) (Coux et al., 1996).
Proteasome configurations centered on the CP can have either one or two RPs but also one or two alternative proteasome activating complexes giving rise to a variety of proteasome complex configurations. Proteasome holo-enzymes engaged in the degradation of poly-ubiquitylated proteins require the RP, thus occur either as RP-CP or RP-CP-RP, also termed the 26S and the 30S proteasome, respectively (Eytan et al., 1989).
The proteasome belongs to the family of threonine proteases and its maturation follows the concept of zymogen activation upon which proteases are activated, once they arrive at their destination. With a molecular mass of 700 kDa, the CP is composed of seven distinct α and β subunits, each of which form heptameric rings stacked into a barrel composed of two outer α rings and two inner β rings (Groll et al., 1997). The maturation of the CP involves the dimerization of two inactive precursor complexes, resembling two half-CPs. Half-CPs consist of an α ring and β ring with five of the seven β subunits synthesized with propeptides. With the dimerization of two half-CPs into the pre-holo CP, the autocatalytic processing of the propeptides is triggered and three β subunits contribute an active site threonine with different peptide cleavage specificities (Li et al., 2007; Ramos et al., 1998). CP-dedicated chaperones, namely Pac/Pba/Poc 1-4 and Ump1, assist in CP assembly. Ump1 is a natively-disordered protein, which is buried inside the pre-holo CP and later on becoming the first substrate of the nascent CP (Kusmierczyk et al., 2008; Ramos & Dohmen, 2008). The α rings are the key players in CP gating. Normally CP α rings are closed, unless they are opened by the RP to allow access of protein substrates into the proteolytic cavity (Groll et al., 2000).
As “gate keeper” of the CP, the RP is the best understood proteasome activator (Rechsteiner & Hill, 2005). The RP is divided into two parts, the base and the lid subcomplexes. The RP base is composed of six ATPases of the triple A family (ATPases Associated with diverse cellular Activities), named Rpt1-6, and five non-ATPases, Rpn1, Rpn2, Rpn10, Rpn13 and Ubp6. The base Rpn subunits are involved in the recognition of the poly-ubiquitin chain and the Rpt ATPase subunits guide the unfolding and translocation of the polypeptide substrate into the CP (Finley et al., 1998). In contrast to the RP base subunits, the subunits comprising the RP lid are only of the non-ATPase class: Rpn3, Rpn5-9, Rpn11 and Rpn12 (Glickman et al., 1998). The main known function of the RP lid is the processing of poly-ubiquitin chains. Rpn11 contributes isopeptidase activity to recycle ubiquitin moieties from the protein substrates. Ubp6 also has ubiquitin hydrolase activity and assists in trimming poly-ubiquitin chains (Crosas et al., 2006; Hanna et al., 2006; Lam et al., 1997; Verma et al., 2002). In principle, the RP ensures that only targeted substrates are degraded by the proteasome, thereby conferring the ubiquitin- and ATP-dependence towards proteasomal protein degradation.
Two competing models exist for RP assembly (Funakoshi et al., 2009; Le Tallec et al., 2009; Park et al., 2009; Roelofs et al., 2009). The first posits that RP assembly occurs in modules independent of the CP with the help of four RP-dedicated chaperones, named Hsm3, Nas2, Nas6 and Rpn14 (Funakoshi et al., 2009). In contrast, the second model proposes that the CP serves as a scaffold for the heterohexameric ATPase ring of the RP base (Park et al., 2009). The second model, however, appears less likely with regard to X-ray structure analysis showing that the RP-dedicated chaperones hinder the association between the RP base and CP α ring (Barrault et al., 2012). The CP-independent assembly model is also supported by the finding that the assembly of RP base and lid can be reconstituted from recombinant proteins with the assistance of RP-dedicated chaperones but without the CP template (Beckwith et al., 2013). However, the CP could serve as a platform for RP base assembly, if RP-dedicated chaperones are limiting.
At this point, it is important to acknowledge the importance of GFP labelling and the ease with which it has allowed localization studies to be conducted (Enenkel, 2014; Groothuis & Reits, 2005). In our species of interest, Saccharomyces cerevisiae, which is an excellent model organism for eukaryotic cells, GFP labelling of proteasomes is achieved by homologous recombination techniques into the chromosomal locus to convert an endogenous proteasomal subunit to a GFP-tagged version (Enenkel et al., 1999; Laporte et al., 2008; McDonald & Byers, 1997). Nearly all proteasomal genes are essential and could be modified by GFP fusions without interfering with their function; we prefer the CP subunits α4 and β5, the CP-dedicated chaperone Ump1, and the RP subunits Rpn1, Rpt1 and Rpn11 as GFP-labelled reporters, because their GFP fusion proteins are fully incorporated into the proteasomal subcomplexes. So far, ~30 subunits of the yeast proteasome were labelled with GFP. All of them reveal the same subcellular localization as thoroughly investigated by direct fluorescence microscopy in living yeast (Laporte et al., 2008).
Seminal studies on proteasome localization in vertebrate cells were performed by Werner Franke’s and Wolfgang Baumeister’s laboratories in the early 1990s. Proteasomes were mainly detected in the nuclei of Xenopus laevis oocytes and cultured mammalian cells (Amsterdam et al., 1993; Hügle et al., 1983; Kleinschmidt et al., 1983). Later investigations reported a shift towards cytoplasmic proteasomes dependent on the type of the cell line and the density of the cells (Wojcik & DeMartino, 2003). Proteasome localization also varies with the growth phase in yeast (Laporte et al., 2008; Weberruss et al., 2013). In growing yeast at logarithmic phase (OD~1), proteasomes are primarily nuclear. During the transition from proliferation to quiescence and the entrance into stationary phase (OD>3), proteasomes deplete from the nucleus and accumulate at the NE/ER in membraneless organelles. These enigmatic structures of proteasomes were initially observed by Isabelle Sagot and her co-workers, who coined the term proteasome storage granules (PSGs) (Laporte et al., 2008). With prolonged quiescence, one to two PSGs with a diameter of ~0.2 to 0.5 µm pinch off the NE into the cytoplasm. The PSGs are motile and stable in yeast cultures and are kept in quiescence for several weeks. If quiescent yeast cells are allowed to resume growth by replacing the glucose-depleted medium with glucose-rich medium, the PSG rapidly clears and the proteasomes are relocated into the nucleus within a few minutes.
Studies with mammalian cancer cell lines also exploited GFP-labelling techniques and fluorescence recovery after photobleaching experiments. The experiments suggested that nuclear transport of GFP-labelled CP across the NE was inefficient. Only the mitotic breakdown of the NE and its reassembly after mitosis allowed nuclear uptake of proteasomes (Reits et al., 1997). However, this nuclear uptake mechanism cannot explain the predominant nuclear localization of proteasomes in yeast cells which divide without mitotic breakdown of the NE. Proteasomes are the second most abundant protein complexes in eukaryotic cells and require continuous synthesis within the cytoplasm and nuclear import during cell division (Weberruss et al., 2013). The most common route for protein complexes to cross the NE in an organism with closed mitosis is through nuclear pore complexes (NPC). Before we address this pathway for yeast proteasomes, we will shortly summarize the concept of nuclear transport through the NPC, a pathway conserved from yeast to human.
The NE is embellished with NPCs which regulate the entry of molecules into and out of the nucleus. Their principal function is to allow free diffusion of small molecules, such as water/ions/peptides, and to block non-specific translocation of macromolecules that exceed 40kDa or a diameter larger than 5nm (Aitchison & Rout, 2012; Wente & Rout, 2010). Translocation of larger macromolecules requires specific interactions with the NPC. Protein cargoes therefore associate with soluble transport factors, called karyopherins/importins/exportins, that themselves interact with phenylalanine-glycine rich nucleoporins (FG-Nups) decorating the NPC (Wozniak et al., 1998). Importins and exportins identify their protein cargoes by nuclear localization sequences (NLSs) and nuclear export signals (NESs), which ensure their nuclear import and export, respectively. In the literature, there are variations of nuclear import and export signals, only some of which comply with the classical import/export concept. The classical concept applies for nuclear import of proteasomes. Thus, we will focus on the key components required for the classical pathway (Gorlich & Kutay, 1999).
The classical nuclear import cycle starts with the association of the importin/karyopherin αβ heretodimer, called Srp1/Kap95 in yeast, with the cargo NLS. Two types of classical NLSs exist: the monopartite NLS which contains five basic amino acid residues and the bipartite NLS in which two clusters of basic residues are spaced by 10–12 indifferent residues. Importin α has the NLS-binding grooves, and importin β mediates the interaction with FG-Nups. The directionality of nuclear transport is dictated by the Ran-GTP/GDP gradient across the NE. Ran is a small GTPase, named Gsp1 in yeast. Ran exists in its GTP-bound state in the nucleus and in its GDP-bound state in the cytoplasm due to the actions of the Ran guanine nucleotide exchange factor (RanGEF) and the RanGTPase activating protein (RanGAP) in the nucleo- and cytoplasm, respectively (Gorlich & Kutay, 1999; Moore & Blobel, 1993). In the nucleus, the cargo-importin αβ complex encounters RanGTP, which results in the release of the cargo (Rexach & Blobel, 1995). Cargo-free importin αβ is recycled into the cytoplasm for the next round of nuclear import.
Our studies in yeast strongly suggest that newly synthesized proteasomes are imported from the cytosol into the nucleus as inactive precursor complexes and that the maturation of nuclear CP proceeds to completion post-import (Lehmann et al., 2002). Although electron microscopy studies have shown that the NPC could expand to accommodate the longitudinal passage of the 30S proteasome, the permeability barriers towards macromolecules such as CP precursor complexes and RP assembly modules must be overcome by specific importins/karyopherins (Pante & Kann, 2002). Several classical NLSs exist within the N-termini of distinct α subunits which were proposed to be either accessible rendering the CP in an import-competent conformation, or to be masked rendering the CP in an import-incompatible conformation (Tanaka et al., 1990). Indeed, recent cryo-EM structure analysis revealed flexible and less structured α ring surfaces in Ump1-associated CP precursor complexes (Kock et al., 2015), in compliance with our finding that importin α recognizes CP precursor complexes but not mature CP with closed α rings (Lehmann et al., 2002). Our model upon which CP precursor complexes are imported into the nucleus was supported by the following observations (Figure 1). First, when tagged with GFP, Ump1 is predominantly nuclear in spite of the fact that CP precursor complexes are assembled from nascent subunits in the cytoplasm. Second, in importin α mutants namely srp1-49 but not in srp1-31, several groups found that the CP is mislocalized to the cytoplasm, providing another piece of evidence for the classical import pathway of proteasomes. Unprocessed and incompletely processed β5 subunits, crucial determinants of CP precursor complexes and pre-holo-CP, respectively, accumulate in srp1-49 mutants, while precursors of β5 subunits are hardly detectable in wild type cells (Lehmann et al., 2002). Third, when CP maturation is delayed by UMP1 deletion, all CP reporter proteins accumulate in the nucleus, although half of the CP is not fully matured and most likely exists as pre-holo-CP (Fehlker et al., 2003; Lehmann et al., 2008).
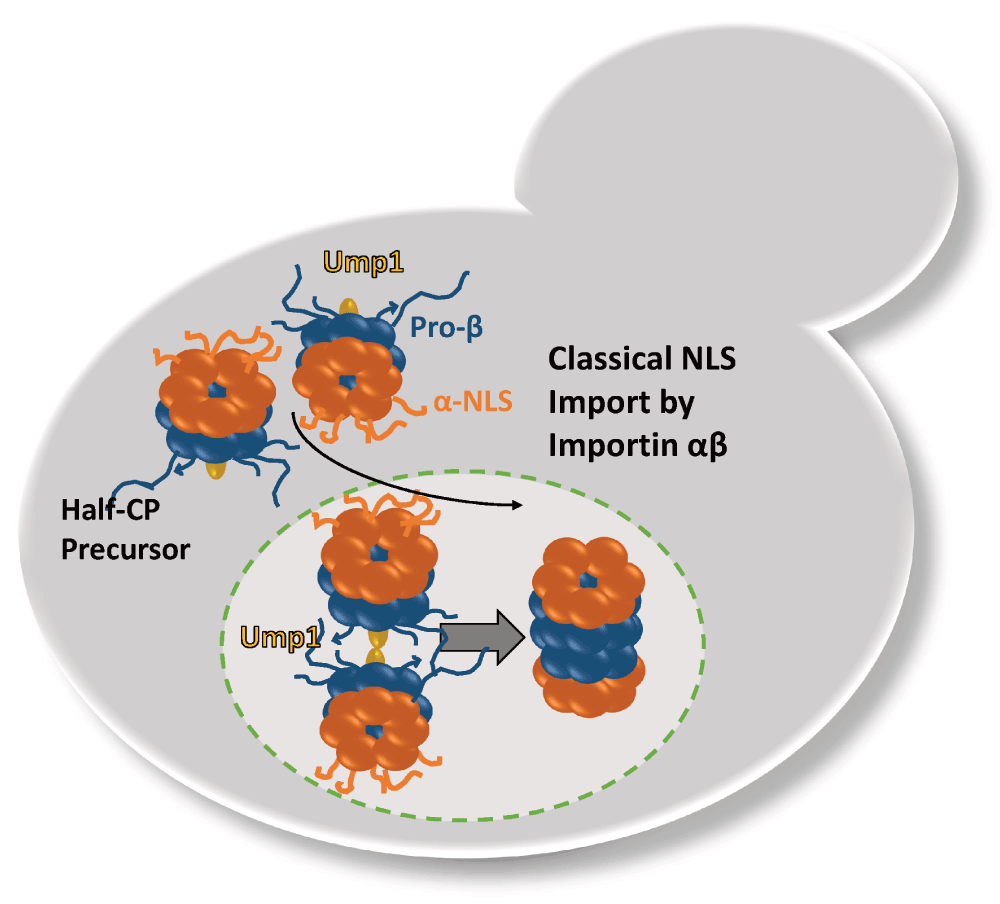
The α rings with the classical NLS are depicted in red. The β rings with propeptides are depicted in blue. The CP-dedicated chaperone and maturation factor Ump1 is depicted in yellow. The completion of CP maturation occurs in the nucleus with the degradation of Ump1.
In the case of the RP, functional NLSs were identified in RP base subunits Rpn2 and Rpt2 and are recognized by importin α (Figure 2). The deletion of the Rpn2 NLS caused a temperature sensitive phenotype and mislocalizations of the RP base into cytosolic foci, whereas the deletion of the Rpt2 NLS was compensated by the presence of the Rpn2 NLS. At permissive temperatures, neither the Rpn2 nor the Rpt2 NLS deletion had severe impact on nuclear proteasome localization suggesting a redundancy of proteasomal NLSs (Wendler et al., 2004). Isono et al. (2007) later confirmed that Rpn2 provides a crucial NLS to aid nuclear import of the RP base and that the lid is separately imported. The nuclear import of the RP lid also requires importin α, though no classical NLS has been identified within RP lid subunits; rather Sts1, a short-lived protein that itself contains a classical NLS, associates with Rpn11 to facilitate nuclear import of the RP lid by importin αβ (Chen et al., 2011). In accordance, deletion of the Sts1 NLS has downstream effects on the nuclear localization of RP lid in addition to RP base and CP, which suggests that proteasomes could also be transported as holo-enzymes (Chen & Madura, 2014b). In order to ensure comparable stoichiometry of proteasomal subcomplexes in the nucleus and similar kinetics by which they are imported into the nucleus, it is reasonable that importin αβ is used as common nuclear import receptor.
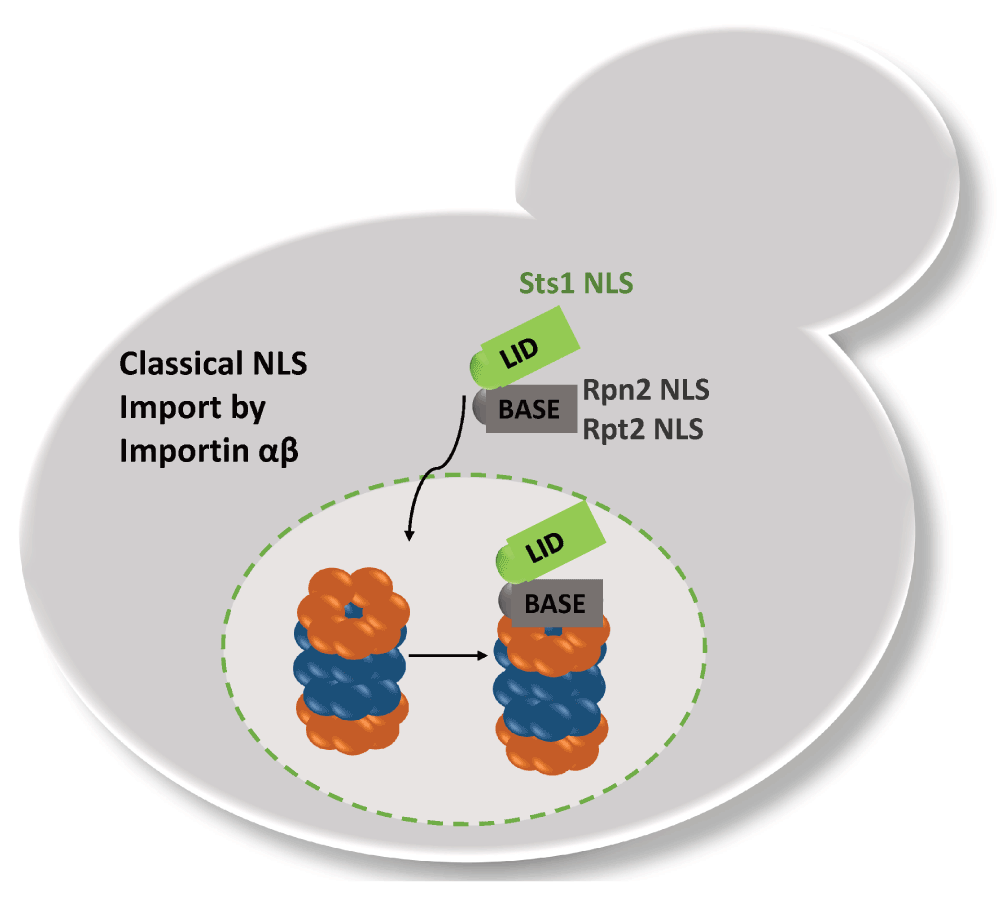
Rpn2/Rpt2 and Sts1 confer classical NLS to the RP base and lid complex, respectively. Sts1 is short-lived and most likely degraded with RP-CP assembly.
Recent fluorescence correlation spectroscopy studies also support the conclusion that proteasomes can be imported into the nucleus as holo-enzymes (Pack et al., 2014; Figure 3). However, the maturation state of the GFP-labelled proteasomes was unclear. Possibly, pre-holo-CP are the real nuclear transport intermediates which degrade Ump1 and Sts1 upon the arrival in the nucleus with the completion of proteasome maturation.
When cells experience nutrient exhaustion or enter quiescence, a drastic change in proteasome localization is observed. In prolonged quiescence, proteasomes deplete from the nucleus and reside in motile and reversible PSGs in the cytoplasm (Laporte et al., 2008). Upon addition of glucose, cells receive the signal to resume proliferation, and PSGs dissolve rapidly, where proteasomes are relocated in the nucleus. How PSGs are organized is not understood. Premature PSG formation in proliferating cells was found to depend on vacuolar ATPases and linked premature PSG formation with disregulation of the intracellular pH. In view of that, PSGs could serve as storage depots for mature proteasomes in quiescence, to protect the proteasome from cellular stress and to be eliminated by autophagocytosis (Peters et al., 2013). The storage of proteasomes during quiescence would also alleviate energy-consuming synthesis of new proteasomes with cell proliferation (Laporte et al., 2008).
The formation of PSG-like structures is also observed by chemical inhibition of proteasomes in mammalian cells or temperature sensitive proteasome mutants in yeast, conditions which result in cell cycle arrest. In spite of the differences between chemically-induced cell cycle arrest and quiescence, inhibited proteasomes are sequestered into juxta nuclear quality control compartments (JUNQs), situated at the cytoplasmic side of the NE and behaving similar to PSGs (Kaganovich et al., 2008; Weberruss et al., 2013). When the cell cycle-arrested mutants were allowed to resume growth at permissive temperatures or upon withdrawal of proteasome inhibition, JUNQs were seen to dissolve like the PSG. In the context of these studies in cell-cycle arrested yeast and mammalian cells, poly-ubiquitylated proteins were found to be accumulated in the JUNQ as well, suggesting that the JUNQ provides a major place for proteolysis (Kaganovich et al., 2008). All studies on the JUNQ and PSGs agree that these enigmatic organelles serve protective functions. Their presence protects cells against proteo- and genotoxic stress and confers cell fitness during aging. Post-translation modifications such as N-acetylation also play a role in PSG organization, but their targets are unknown (Saunier et al., 2013; van Deventer et al., 2015; Weberruss et al., 2013).
Though the CP and RP co-localize in the PSG, they seem to be loosely associated. Conflicting reports exist about the stability of RP-CP assemblies in lysates of quiescent cells (Bajorek et al., 2003; Hanna et al., 2012; Weberruss et al., 2013). The finding that RP-CP assemblies are less stable coincides with the decline in ATP during quiescence as well as the reduced proclivity of the proteasome to degrade poly-ubiquitinated substrate. Instead of an association of the CP with the RP, most CP is seen interacting with Blm10, a conserved 240 kDa HEAT repeat protein (Weberruss et al., 2013). Upon exit from quiescence, the PSGs rapidly clear and mature proteasomes are imported into the nucleus within a few minutes. The imported proteasomes must be matured and assembled, as time does not permit the new synthesis of precursor complexes (Laporte et al., 2008). Here, Blm10 plays an important role and represents the first characterized nuclear transporter which particularly facilitates nuclear import of mature CP (Figure 4). Quiescent blm10Δ mutants exhibit a significant delay in resuming cell growth due to the deficit in mature CP in the nucleus. Furthermore, Blm10 binds FG-Nups and GTP-bound Ran and dissociates from the CP upon interaction with RanGTP, suggesting that Blm10 shares functional similarities with Kap95, the classical importin β (Weberruss et al., 2013). Along this line, Blm10 belongs to the HEAT repeat family with α-solenoid fold, a structural feature shared by β karyopherins/importins (Huber & Groll, 2012). During cell proliferation, Blm10 is also expressed but to a much lesser extent (Weberruss et al., 2013). Only a minor fraction of the CP, pre-holo-CP and CP precursor complexes is associated with Blm10 in growing yeast. The Blm10-bound fraction significantly increases under geno-and proteotoxic stress suggesting a high demand for nuclear proteasomes under these growth conditions (Doherty et al., 2012; Fehlker et al., 2003; Lehmann et al., 2008). Since Blm10 associates with constitutively open or disordered CP α rings, Blm10 also plays a role in regulating α-ring gating during CP maturation (Lehmann et al., 2008). The wider α ring conformation of CP-precursor complexes seems to be preferentially bound to Blm10 and importin αβ by representing import intermediates. Thus, the Blm10-dependent import pathway complements the canonical nuclear import pathway.
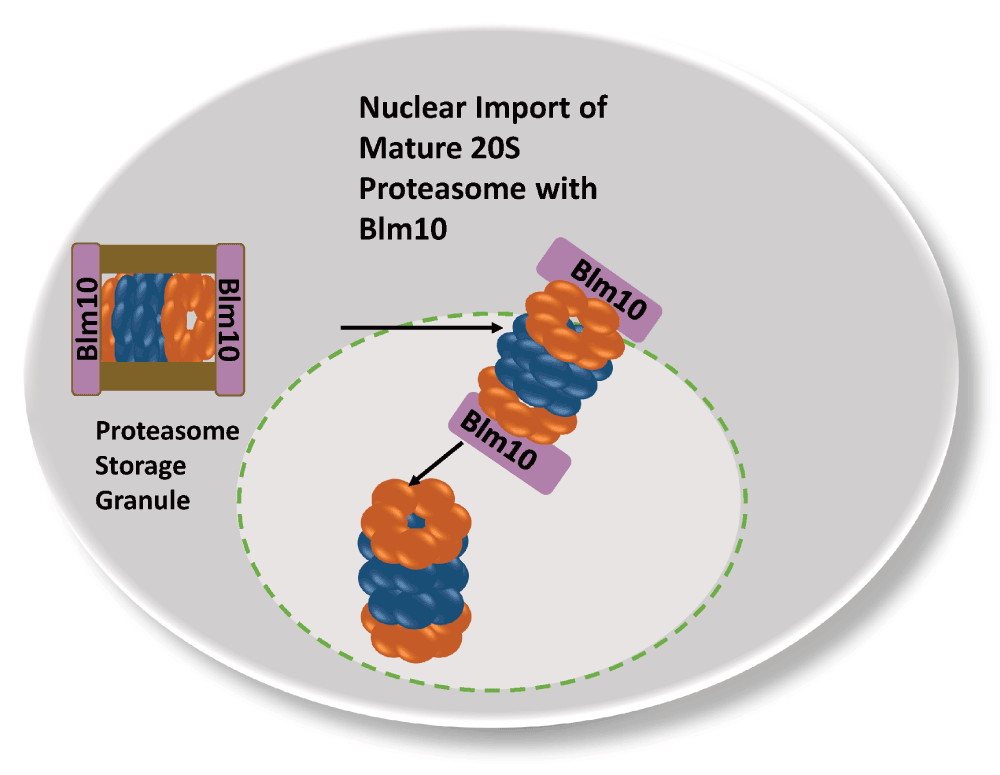
In quiescent yeast cells mature CP is stored in reversible and motile granules in the cytoplasm, which rapidly clear with the resumption of growth. Blm10 mediates the nuclear import of mature CP.
For the RP, the import pathway upon exit from quiescence is yet to be solidified. A possible candidate for a RP-dedicated nuclear import receptor is Rpn2 which exhibits a similar α-solenoid fold as Blm10 and importin β, all of which belong to the family of HEAT-repeat proteins (Huber & Groll, 2012; Kajava, 2002).
In this review, we discussed the recent literature on the dynamics of the ubiquitin-proteasome system with a major focus on the proteasome. During cell proliferation a high traffic volume of proteasomes and proteasomal substrates arises between the cyto- and nucleoplasm. In cell-cycle arrested and quiescent cells, proteasomes exit the nucleus and accumulate with poly-ubiquitylated proteins in motile and reversible PSGs in the nuclear periphery. While the basic concepts of nuclear import of proteasomes during cell proliferation and upon exit from quiescence are understood, little is known about the nuclear export of proteasomes during the transition from proliferation to quiescence. We may wonder why proteasomes exit the nucleus during quiescence. Which kind of substrates will be available in the cytoplasm, once proteasomes are sequestered into the PSG? Possibly, PSG-resident proteasomes are starving for newly synthesized proteins which arise with the resumption of cell proliferation.
The dynamics of proteasomes and their substrates are fascinating and will inspire our discussions and experiments in the future.
M.C. prepared the first draft of the manuscript and agreed to the final content.
This work was supported by grants from NSERC (4422666-2011) awarded to M.C. and C.E. and from CIHR (325477) awarded to C.E.
I confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Zhu Chao (Jerry) Gu and Julianne Burcoglu for critical reading of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
References
1. Fuertes G, Villarroya A, Knecht E: Role of proteasomes in the degradation of short-lived proteins in human fibroblasts under various growth conditions.Int J Biochem Cell Biol.2003; 35 (5): 651-664 PubMed Abstract | Publisher Full Text | Reference SourceCompeting Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 28 Sep 15 |
|||
|
Version 1 24 Jul 15 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)