Keywords
membrane fusion, liposome, fusion mechanism, freeze-fracture, fusion intermediate
membrane fusion, liposome, fusion mechanism, freeze-fracture, fusion intermediate
While tempered with the limitation of observing one lipid vesicle composition and one fusogenic agent providing a limited number of fusion events, the reviewers recognized the noteworthy attribute of the lipid vesicle fusion intermediate in this study.
The scarcity of visual information on lipid vesicle interactions captured at different time-points leads us to present these images as possible additional information. Literature published in the past two decades indicates that capturing a fusion event at the most revealing time point is indeed a challenge.
It is recognized that alternative interpretations for fusion intermediate structure and differences between different lipid systems could also be contemplated. However, due to the rarity of such image, it is important to present this to the scientific community. As we indicate the serendipity of this finding, it is recognized that this paper can only be a small piece of information that stimulates further studies.
We have revised the paper to clarify that the morphological observation and the schematic interpretation are from a single examination and limited to one lipid system and fusogenic agent combination that will require other studies and confirmation in the future.
See the author's detailed response to the review by Jesse C. Hay
See the author's detailed response to the review by M Joseph Costello
See the author's detailed response to the review by James McNew
Phospholipid vesicles (liposomes) are suitable models for studying biological membrane behaviour. Liposomes can be made from a single lipid or lipid mixtures that can provide opportunities to investigate membrane-related events under different conditions and in the presence of additives. The fusion process has been investigated in various artificial membrane systems using electron microscopic, small-angle X-ray scattering (SAXS), nuclear magnetic resonance (NMR) and fluorescence-based kinetic techniques1–5. Despite the efforts, complete understanding of the molecular structural and kinetic details of the fusion event is still lacking.
The first fusion mechanism from early studies proposed ‘lipidic particles’ (intermediates between lamellar and HII hexagonal phases of the phospholipids or inverted micelles) as the possible intermediate in the fusion process of model lipid vesicles6–8, since these particles seemed to be present at attachment sites of lipid vesicles and could be visualized by freeze-fracture EM. These intermediates could also be detected in fusion processes of biological membranes (exocytosis, myoblast fusion, protoplast fusion). For example, Satir et al.9 observed small particles arranged in rosettes at the site where fusion of the mucocysts in Tetrahymena pyriformis was initiated. Other authors questioned the existence of lipidic particles as dynamic fusion intermediates10,11 because these particles could not be observed every time at fusion interfaces, and suggested that ‘lipidic particles’ develop subsequent to the fusion process. However, it was suspected that some type of a non-bilayer structure formed in the fusion event. Bearer et al.10 specifically suggested the existence of an “’elusive’ intermediate” that has not been visualized in published morphological studies and suggest that the absence of lipidic particles or other intermediate structures may not mean that some dynamic process is taking place and that fusion intermediates could be unstable and convert to different polymorphic forms.
Another mechanism proposed was the ‘stalk mechanism’12–14, which involved the formation of a trilaminar structure between the closely apposed bilayers such that the outer monolayers bend to the side to allow joining of the inner monolayers (trans-monolayer contact (TMC)15), which form a stalk at the attachment site of the two membranes12,15–17.
The theoretical sequence of events in model lipid membrane fusion can be summarized as follows: 1) close apposition of the two bilayers (<1 nm); 2) local dehydration of phosphorus head groups; 3) destabilization of bilayers; and either 4a) formation of inverted micelle intermediates (IMI) at the attachment site; or 4b) formation of stalk and TMC); and 5) completion of fusion, i.e. the leakless mixing of contents of two vesicles.
Most of these stages of membrane fusion were described and indirectly measured or modeled, but direct visual evidence is still lacking. Siegel18 and Cullis et al.19, and more recently Lentz et al.20,21 speculated that the reason why only some of the actual intermediate structures were detected or visualized is the short lifetime (1 msec or less18) of any given fusion intermediate, making the capture very challenging even with rapid freezing, 31P-NMR or SAXS techniques.
In this study, we have observed some fusion intermediate structures in a liposome system in the presence of glycerol by freeze-fracture EM. One of these intermediates, the ‘fusion rim intermediate’ may provide new structural/morphological information on membrane fusion events.
Liposomes (small unilamellar vesicles, SUVs) were prepared with soybean lipids (Centrolex P; Central Soya, Fort Wayne, IN). The liposomes were prepared by high shear dispersion using Microfluidizer M110 (Microfluidics Inc. Newton, MA). The liposomes were freeze-fractured without glycerol or after preincubation in 30% v/v glycerol for 30 minutes at room temperature. A drop of the liposome suspension was placed on a gold specimen stub and rapidly frozen in liquid nitrogen cooled Freon 22 (-158°C). All samples were fractured at -105°C in a Balzers 360 freeze-fracture unit. The fracture surfaces were shadowed at 45° angle with a thin layer of platinum-carbon followed by vertical deposition of a carbon layer for replica support. The replicas were floated onto the surface of distilled water and subsequently cleaned with sodium hypochlorite (5% chlorine) and 60% sulfuric acid. After the final washing in distilled water the replicas were picked up on 200 mesh copper grids and examined in a Phillips 200 EM and photographed on Kodak fine grain positive film.
The fusion of liposomes was induced by glycerol and several fusion intermediates were captured in the replicas (Figure 1). Without glycerol, there was no liposome fusion (Figure 1A). The relatively low concentration of glycerol provided slow dehydration at the phospholipid head group regions which made it possible to observe vesicles still in the fusion process. The micrographs captured vesicles (assumed to be) at various stages of the membrane fusion event (Figure 1A–C). Figure 1B (large arrow) depicts the initial contact between vesicles – their external monolayers fused but no communication between the two aqueous compartments started. Figure 1D shows a previously unseen moment of liposome-liposome fusion. During the freeze-fracturing procedure one of the liposomes was fractured on the outside surface (it shows the E face), while the other shows the cytoplasmic (P) face (the interior surface of liposome). The leakless intermixing of the aqueous contents of the liposomes had started. At the perimeter of the fusion interface small, 10–12 nm diameter, particles can be distinguished, which probably correspond to the inverted micellar intermediates, so called, lipidic particles. This fusion rim intermediate structure is depicted in the insert of Figure 2. The presence of these lipidic particles could be suspected from another micrograph (Figure 1C, large arrow), which may represent a preceding intermediate state of fusion.
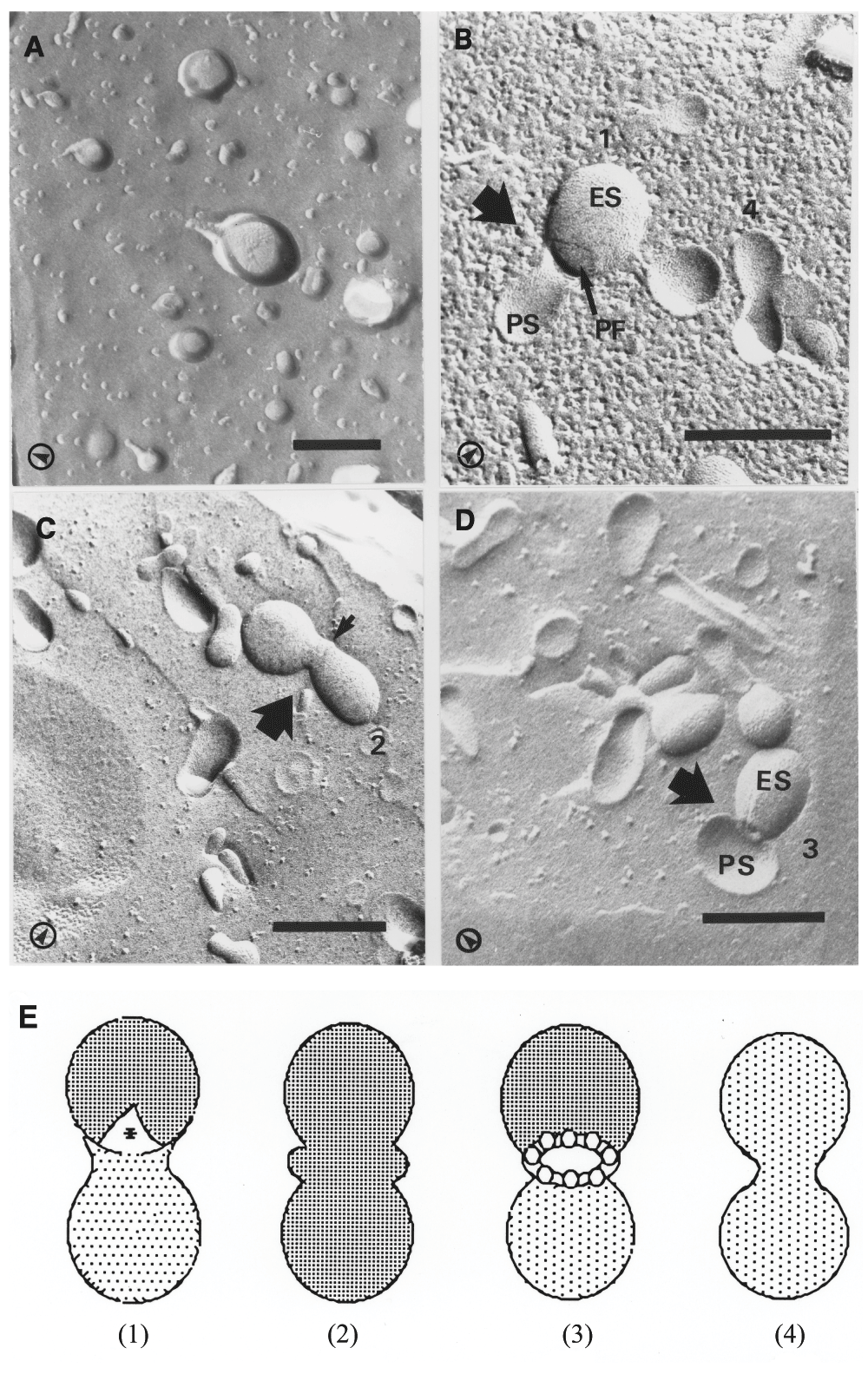
Various stages of liposome-liposome fusion event were captured by rapid freezing. A) Liposomes were freeze-fractured without the addition of glycerol. B–D) Liposomes were preincubated with 30% v/v for 30 minutes before freezing. B) The first stage of liposome-liposome fusion: the joining of the outer monolayers of the liposomes (large arrow) is apparent without communication between their internal spaces. C) Early liposome-liposome fusion intermediate (large arrow) fractured at the exterior surface of the membrane. The rim between the two vesicles (small arrow) is indicative of non-bilayer intermediate structures. D) Late liposome-liposome fusion intermediate (large arrow). One of the liposomes was fractured on the external surface, the other on the internal surface with its internal space is being visible and exposing the fusion interface. The presence of small (10–12 nm) particles can be distinguished at the fusion rim. E) Schematic representation of the identified fusion intermediates. Numbers on micrographs correspond to the numbers of the diagrams. Freeze-fracture nomenclature27: ES - external surface; PS - cytoplasmic surface (in this case interior surface of liposome); PF and * - fractured face of the lipid monolayer adjacent to the interior space of the vesicle; arrows in left corners of micrographs indicate the shadowing direction. Bars 250 nm.
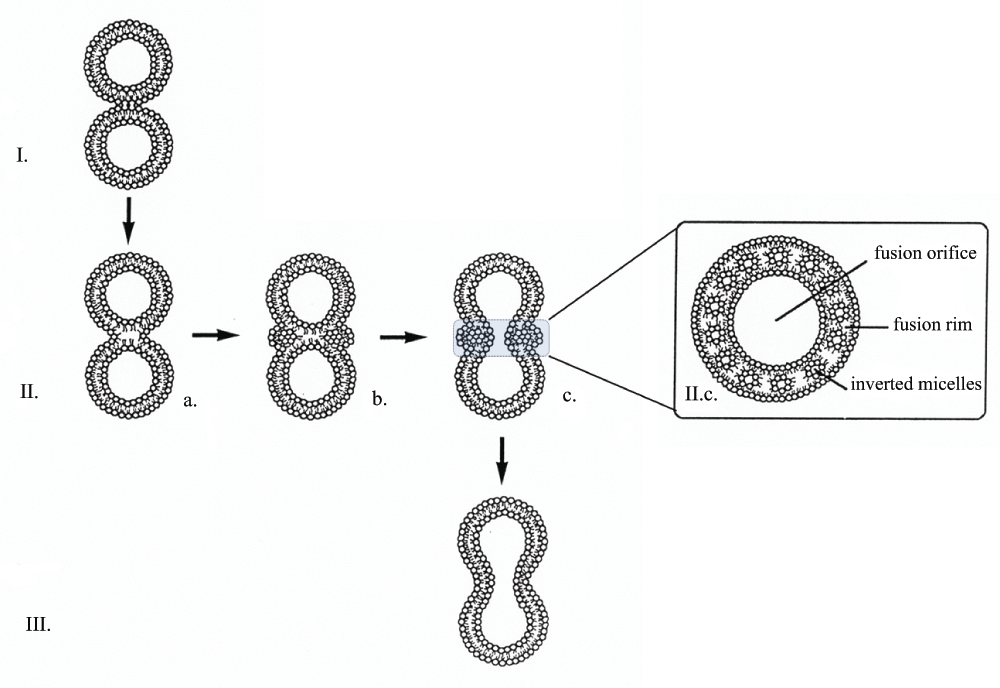
I. Close apposition of two bilayers and formation of an aggregation site when bilayers touch each other. II. Merging of two bilayers. a) formation of the initial fusion product: the outer leaflets of the bilayers of both vesicles join (fuse), while their inner bilayer leaflets form one common bilayer at the attachment site. b) phospholipid molecules from the outer leaflet of the vesicle bilayers, ie. From the attachment site are pushed sideways and form inverted micelles; at the attachment site transient bilayer form composed of the inner bilayer leaflets of the vesicles. c) formation of the fusion orifice: the phospholipid molecules from the attachment site are used for the formation of the outer monolayer of the inverted micelles. Inverted micelles are situated all around the fusion orifice. Mixing of the contents of the vesicles has started. Boxed insert shows a horizontal section of the fusion area viewed from above. III. Expansion of the bilayer to form a single larger liposome.
On the basis of this morphological evidence we constructed a schematic set of drawings to represent a modified model for phospholipid vesicle fusion (Figure 2). When the bilayers of two separate vesicles are in close apposition (Figure 2 I), an initial fusion product will form. The outer leaflets of the bilayers of the two vesicles fuse, while their inner leaflets form one common bilayer at the attachment site (Figure 2 IIa). This stage is followed by the formation of inverted micelles (lipidic particles) around the attachment site (Figure 2 IIb). The organization of the next fusion intermediate (Figure 2 IIc and Figure 1D) involves the formation of a fusion orifice. At the perimeter of this orifice, the fusion rim can be seen (Figure 2, insert), which contains inverted micelles all around. The development of an intermediate like this appears feasible from both energetic and morphological viewpoints, if we take into consideration that the excess phospholipid molecules cleared from the attachment site at this particular stage should be accommodated somewhere, until incorporated into the expanded bilayer of the single larger liposome.
Glycerol-induced fusion seen in this study may bear similarities to polyethylene glycol (PEG)-induced fusion20,22 with dehydration at the liposome attachment site contributing to close contact between the bilayers. It is recognized that fusion is a very dynamic event and certain stages of the fusion are easier to demonstrate than others. Due to the low frequency of vesicle collisions and short lifetime of the actual fusion event and the fact that fusion of vesicles in an aqueous medium is not a synchronous event, visualization of intermediary fusion features on all liposomes in a sample is difficult.
Most of the morphological freeze-fracture studies in the literature show liposomes just before fusion or at the stage already well undergoing fusion. It would be important to clarify events at the stage, where bilayers of the two vesicles are merging and communication between their aqueous spaces begins. The freeze-fracture results in this work supplement those previously reported in the literature and potentially add a new visual image of an intermediate structure to the model of membrane fusion. The initial fusion product (Figure 2 I) must be very similar to the one proposed by Kozlov and Markin12 on the basis of theoretical considerations. The molecular arrangement of lipids in the ‘fusion rim intermediate’ (Figure 1D and Figure 2 IIc) could provide an alternative interpretation of the IMI8,23 or could be considered the next step after the previously described stalk and TMC intermediate15,17 (this latter may correspond to the image on Figure 1B), and may also be similar to membrane hemifusion events involving proteins21,24–26. However, this study has limitation in that the morphological observation and the schematic interpretation are from a single examination and limited to one lipid system and fusogenic agent combination that will require other studies and confirmation in the future.
This paper is dedicated to the memory of Dr Michael Mezei, my mentor and friend. I specifically thank Dr Michael Mezei for providing the liposome dispersion and inspiring the study. I am also grateful to Dr Gary T. Faulkner for guidance with the freeze-fracturing and useful discussions of the micrographs.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 18 Mar 15 |
read | read | read |
|
Version 1 08 Jan 15 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)