Keywords
fusion, conjoined larvae, spawn slicks, inborn colonies, Platygyra daedalea
fusion, conjoined larvae, spawn slicks, inborn colonies, Platygyra daedalea
In sessile colonial marine invertebrates (e.g., sponges, cnidarians, bryozoans and ascidians), fusion among conspecifics during early ontogeny could immediately lead to a marked increase in juvenile size, thereby enhancing the performance in growth, survival and competition1,2. In addition, the allogenic fusion is expected to form chimeras which possess greater genetic variability and wider ranges of physiological resistance1. Larvae of colonial marine organisms tend to settle in a gregarious manner3–7 and their juveniles often come into physical contact through growth and then fuse8–10. These life history traits increase the opportunities for fusion, and important rates of chimerism due to allogenic fusion have been detected in wild natural populations of corals and ascidians11,12. Nevertheless, fusion of embryos or larvae during planktonic and dispersive phase (i.e. prior to settlement and metamorphosis) is rarely known to date.
Modular marine invertebrates like sponges and cnidarians usually spawn in a high synchrony and the embryos also tend to aggregate after release13–15, thus also providing the chance of contact and fusion among embryos or larvae. For instance, sticky eggs released by the oviparous sponge Cliona celata were found to adhere to each other and form flattened egg mass, within which larvae fused in twos or threes. The compound larvae metamorphosed into sponges with single oscula, indicating the cytomictical fusion among embryos or larvae13. More recently, larvae of viviparous sponge Haliclona sp. have been demonstrated to fuse and generate swimming chimeras16. Furthermore, sexually produced embryos of a non-colonial sea anemone Urticina feline were observed to fuse naturally during internal brooding, generating pre-metamorphic cytomictical and sectorial chimeras17,18. These findings suggested that the direct contact between embryos and larvae would facilitate fusion either during internal brooding or pelagic phase.
For broadcast spawning corals, synchronous spawning events usually result in billions of naked embryos floating at the sea surface in the form of spawn slicks19,20. The direct contact between naked embryos highlights the possibility of fusion of coral embryos while sticking together in slicks. Moreover, previous studies have demonstrated there is a window in ontogeny, before allorecognition system matures, when newly settled polyps can fuse21. Time for allorecognition maturation in reef corals varied from 4 months following settlement in brooding species22, to 1–3 years in spawning species9,10. This further supports the possibility of fusion at embryonic stage when allorecognition may be weak in corals. As yet, the possible occurrence of fused embryos and conjoined larvae in broadcast spawning corals has not been investigated.
Here, we happened to test this unexplored probability of fusion of embryos in broadcast spawning reef corals. We experimentally mimicked spawn slicks using gametes collected from 4 mature colonies of Platygyra daedalea, and followed the fate and development of embryos within lab-generated slicks.
Ten gravid colonies of P. daedalea (20–30 cm in diameter) were collected at depth between 2–4 m from Luhuitou fringing reef in Sanya, China (18°12′N, 109°28′E). Corals were maintained in an outdoor tank with flowing sand-filtered seawater in Tropical Marine Biological Research Station in Hainan, Sanya. Four colonies spawned around 22:00 on May 18, 2014 (5 nights after full moon). Egg-sperm bundles were collected using pipettes, then mixed and gently agitated to facilitate bundle disintegration and cross-fertilization. Fertilization was allowed to take place for about 2 hours, after which eggs (ca. 300, 000) were washed two times with fresh seawater and suspended in a 15 cm-diameter jar. Because of the logistical constraints, eggs were left undisturbed and they formed dense slicks on the seawater surface. The next morning around 08:30, embryos were inspected under a dissecting microscope and we accidentally discovered that some embryos fused. Embryos were washed and seawater was changed twice daily thereafter. Two days after fertilization, 500 larvae were randomly sampled to count the proportion of each type of fusion. Seven days after fertilization, chips of crustose coralline algae Hydrolithon onkodes were used to induce the settlement of larvae and the recruits were reared in the lab at 28°C until June 26.
Embryos became bowl shaped (cushion stage) 8 h after fertilization. Notably, some embryos fused (Figure 1A) and a substantial proportion even stuck together into dense aggregates (Figure 1B). It could be deduced that fusion of embryos took place some time during blastulation. Mortality of embryos within the first 2 days was extremely high (>50%) and the dense aggregates all died and decomposed. Unitary larvae became pear-shaped and began to rotate actively 20 h after fertilization, while conjoined larvae were highly variable in shape. Bi-fused larvae were dominantly peanut-shaped, and multi-fused larvae were arranged in chains or triangles, or in the form of the letter “T” or “L” (Figure 1C, D).
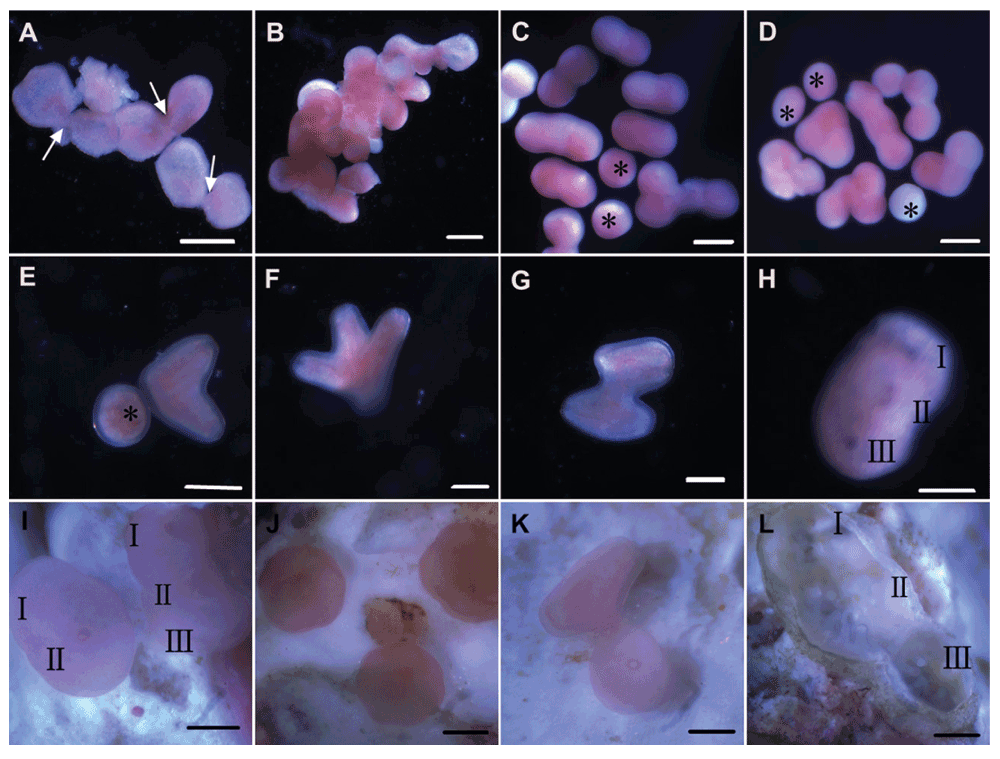
(A) Fused embryos (arrows point to the fusing areas). (B) A dense aggregate comprising more than 20 embryos. (C–G) Unitary (asterisks) and conjoined larvae. (H, I) Inborn colonies. (J) Single settlers. (K) Incomplete settlement of perpendicularly bi-fused larvae, with the left partner being parallel to the substrate. (L) An inborn colony 26 days post-settlement. Roman numbers indicate visible individuals within inborn colonies. Scale bars 250 μm.
Of the 500 randomly sampled larvae, 174 (34.8%) were conjoined with 2–4 partners. Conjoined larvae clearly showed their spatial arrangement after elongation and fusion was apparently without polarity. Larvae either joined at the aboral end (Figure 1E, F), or united side by side (Figure 1G), or even fused perpendicularly (Figure 1K). Furthermore, 56 out of the 174 conjoined larvae (32.2%) united at the aboral extremity and only these larvae were potentially competent to metamorphose normally into inborn colonies (Figure 1H, I), which were prominently larger in size than the single settlers (Figure 1J). In contrast, perpendicularly bi-fused larvae settled incompletely, with one partner metamorphosing and firmly attaching while the other still being parallel to the substrate and not able to settle (Figure 1K), ultimately leading to the death of the whole entity 3 days later. Since the coralline algae provided here was not suitable for the settlement of P. daedalea larvae, only 12 inborn colonies were obtained in total and they persisted for 26 days post-settlement when the study ended (Figure 1L).
The present study documented for the first time the fusion of embryos, the subsequent formation of conjoined larvae and inborn colonies in a broadcast spawning coral. Fusion of P. daedalea embryos was spontaneous, resulting simply from the aggregation and contact of embryos in mimicked slicks, which was analogous with that in sponge C. celata13. While unlike the cytomictical compound larvae in sponge C. celata, partners within the conjoined P. daedalea larvae still retained a degree of individuality, suggesting sectorial fusion of coral embryos and supporting the assumption that corals typically exhibit sectorial fusion1.
Corals often spawn their gametes during seasonally calm periods and low-amplitude tides19,20,23 and spawn slicks extending up to few km in length were often observed in the field20,24. Given that slicks remained aggregated 1–2 d after spawning19,20 and embryos can fuse during embryogenesis within 8 h post-fertilization, fusion of coral embryos is highly favored in situ. On the other hand, although mass coral spawning events usually involved several species, significant temporal differences in spawning to ensure fertilization and reproductive isolation have been demonstrated for many sympatric species25–27, which considerably increase the encounters between embryos of the same species in slicks. Taken together, fusion of coral embryos might be a natural phenomenon. However, the density of embryos here was 1700 cm-2 and likely to be much higher than that in the field. Therefore, the extent to which fusion of embryos occurs in natural slicks and whether there is a density effect on the formation of conjoined larvae remain to be fully evaluated.
At last, approximately 12 percent of all the larvae had the potential to form inborn colonies, which persisted for one month and exhibited no sign of rejection. The inborn colonies here originated from fusion of embryos and settlement of conjoined larvae, contrasting the traditional concept that the asexual budding of the primary polyp leads to the formation of a young coral colony28, and thus fusion of embryos could be an unexpected shortcut to colony formation in reef corals. These facts raise questions as to the ecological implications of inborn colonies formed as a consequence of fusion of embryos in corals. Firstly, larger coral colonies composed of multiple fused partners are known to yield remarkable gained benefits, such as enhanced survival and growth5,8. Hence, the larger initial size and the status of multi-polyp at settlement may confer these inborn colonies better capacities to compete for space and survive partial mortality.
Fusion of coral embryos also shed new light on the chimerism in scleractinian corals, which was often attributed to fusion of gregariously settling larvae5,7, or of juveniles that come into contact through growth7,9,10. However, our study documented fusion between individuals in P. daedalea occurred at the embryonic stage, earlier than any other corals studied to date. It should be pointed out that though embryos here were produced sexually from 4 parent colonies, it did not denote they were genetically distinct. Therefore, the possibility of isogenic fusion can not be totally ruled out7. However, it is reasonable to assume the presence of allogenic fusion, suggesting fusion of embryos might add a novel mechanism to form chimeric larvae and colonies in scleractinian corals. In that case, the increased genetic diversity within these inborn colonies may translate into versatile physiological qualities, thus enabling them to better cope with environmental changes unless negative interactions occur1,29,30.
Overall, this is the first report of fused embryos and pre-metamorphic conjoined larvae in reef corals. Fusion of coral embryos could be an adaptive strategy to form larger and chimeric recruits, thereby promoting growth and survival during the vulnerable early stages5,8. Clearly, future studies are required to explore whether fusion of embryos is common in spawning corals and fully evaluate its biological and ecological implications.
Coral sampling was permitted by the Administration of Sanya Coral Reef National Nature Reserve, the Department of Ocean and Fisheries of Hainan Province.
LJ conceived and performed the study. All authors wrote the manuscript and gave final consent for publication.
This work was supported by Public Science and Technology Research Funds Projects of Ocean (201305030-3) and the National Natural Science Foundation of China (41306144 and U1301232).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 05 Mar 15 |
read | |
|
Version 1 13 Feb 15 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)