Keywords
Pheochromocytoma, Heart transplantation, catecholamine cardiomyopathy
Pheochromocytoma, Heart transplantation, catecholamine cardiomyopathy
Pheochromocytomas are rare catecholamine-producing neuroendocrine tumors. The common signs and symptoms of these tumors (headaches, sweating, palpitations and hypertension) can be attributed to the direct effects of catecholamines at various receptor sites throughout the body1–4. Catecholamine-induced cardiomyopathy is a potentially deadly outcome in patients with pheochromocytomas; it is caused by direct injury to the cardiac myocardium by catecholamines2,3,5,6. Histological changes found in catecholamine-induced cardiomyopathy are characterized by progression from diffuse edema and mild changes in the nuclei of myocytes to fibrotic changes with inflammatory infiltrates, granular cytoplasm, and contraction band necrosis7.
Decreased ejection fraction (EF; measurement of how much blood is being pumped out of the left ventricle of the heart with each contraction) in those with catecholamine-induced cardiomyopathy is attributed to both myofibrillar damage and down-regulation of beta 1 and 2 adrenergic receptor, which leads to dilated cardiomyopathy in most cases but rarely to hypertrophic cardiomyopathy7,8. Damage to the myocardium is a result of enhanced lipid mobility leading to increased atherosclerosis, hypoxia during coronary vasospasm, increased calcium influx due to changes in the permeability of the sarcolemmal membrane, and free radical insult by the oxidized products of catecholamines7,8. Additionally, catecholamines are thought to stimulate protein synthesis that may contribute to left ventricular hypertrophy independently of pressure overload8. Changes seen in catecholamine-induced cardiomyopathy are usually reversible by surgical excision of the tumor or medical adrenergic blockade8. In the case presented here, removal of the pheochromocytoma did not result in reversal of the cardiomyopathy. Thus, the patient underwent orthotopic heart transplantation, which gave a successful outcome and stable left ventricular systolic function at three years.
A 28-year-old Caucasian man presented in July 2008 with dyspnea, cough and chest pain. He had been treated for pneumonia a month before, and computed tomography (CT) had revealed a right adrenal mass. Elevated catecholamines confirmed pheochromocytoma (Table 1). Upon further questioning the patient admitted to right shoulder pain for the past year, paroxysmal headaches for 10 years, and episodes thought to be “panic attacks” with tachycardia, palpitations, diaphoresis and red-purple discoloration of extremities. He had had a myocardial infarction (MI) three years previously, for which he had received a bare metal stent placed to the left anterior descending artery; his EF was 30%. While myocardial infarctions can cause reduction in EF, this was unusually low for the extent of his MI and for his age. Other conditions included hypertension, with blood pressure occasionally exceeding 130/90 mmHg, dyslipidemia and a pyloric stenosis repair in childhood. He used no tobacco, was a vegetarian and had a family history of hypertension and obesity.
Physical examination revealed a lean appearing, young male who was tachycardic with scattered rhonchi in bilateral lung fields, tenderness to palpation in the right upper quadrant of the abdomen and right lower chest, and bilateral minimal edema of ankles. He had no neurofibromata or café-au-lait spots. His extremities were observed to turn bluish and pale during spells of anxiety accompanied by modest hypertension of no more than 160/85 mmHg. There were no Cushingoid features. The thyroid gland was not enlarged and there were no nodules. Vital signs were as follows: temperature 37°C, blood pressure 128/83 mmHg, heart rate 120 beats/min, respirations 20/min, and oxygen saturation of 99% on room air. Brain natriuretic peptide (BNP) levels were 3810 pg/ml (normal range 34–42) pg/ml. Lipid analysis showed total cholesterol levels of 165 mg/dl (normal range 110–200 mg/dl), HDL 30 mg/dl (normal range 40–59 mg/dl), LDL 116 mg/dl (normal range 50–99 mg/dl), and triglycerides 97 mg/dl (normal range 40–149 mg/dl). Catecholamines remained significantly elevated (Table 1). Electrocardiogram showed sinus tachycardia, biatrial enlargement, left ventricular hypertrophy with repolarization abnormalities and old anteroseptal myocardial infarction. Chest X-ray showed bilateral pulmonary parenchymal densities and cardiomegaly. Initial ejection fraction was 10% (Table 2) and cardiac catheterization revealed decreased cardiac index and elevated pulmonary capillary wedge pressure (Table 3).
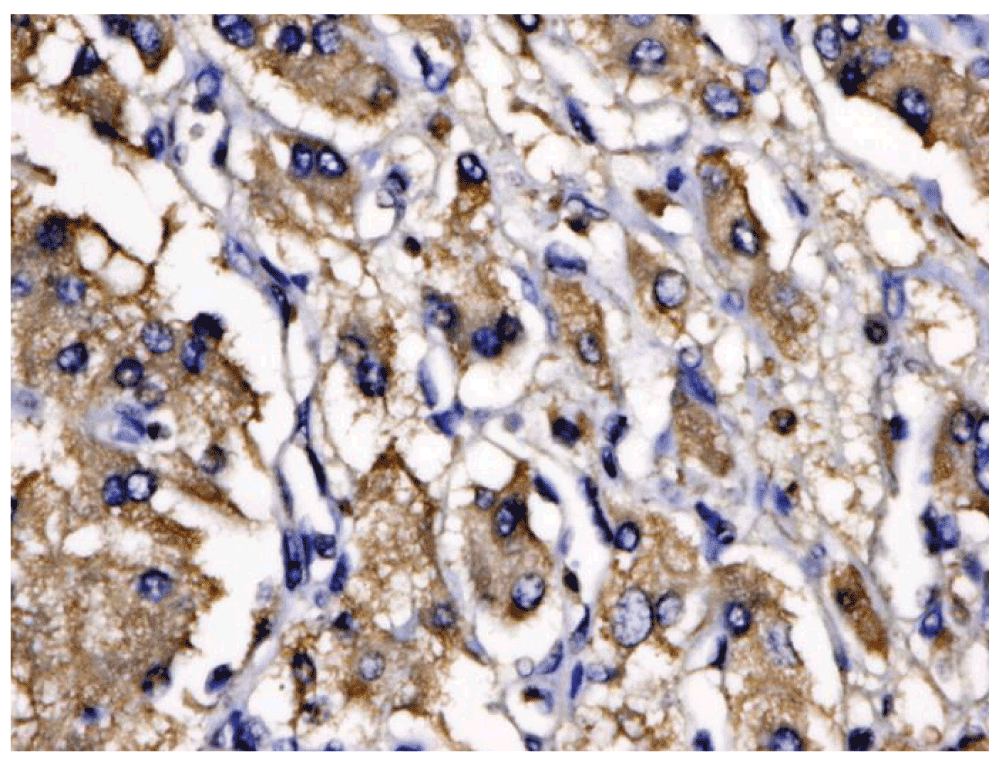
He began treatment with phenoxybenzamine 10 mg twice daily, metyrosine 240 mg four times daily and carvedilol 6.25 mg twice daily. A week later, phenoxybenzamine and carvedilol was withheld due to the development of hypotension. He subsequently developed ischemic hepatitis (shock liver) with coagulopathy and renal failure. On day 11 of hospital stay, he experienced a catecholamine release episode with right shoulder pain, tachycardia, diaphoresis, and acrocyanosis of distal extremities. Because his cardiac index had decreased to 1.4 l/min/m2, he began treatment with labetalol drip at a infusion rate of 1mg/min, an intra-aortic balloon pump was placed and he was intubated. With improvement of cardiac indices he was thought to be ready for surgery. On day 15 he underwent right adrenalectomy, which revealed an 8.2 × 8.1 × 4.2 cm retroperitoneal pheochromocytoma with benign pathology, with chromogranin- and synaptophysin-positive cells (Figure 1). He was given fluid and blood resuscitation, and placed on infusions of norepinephrine 0.1 mcg/kg/min, vasopressin 0.1 units/min, epinephrine 1 mcg/min and milrinone 0.75 mcg/kg/min, which were titrated as needed. His ejection fraction improved to 15–20% and cardiac output improved to 5 l/min, allowing removal of the intra-aortic balloon pump and extubation. The post-operative course was complicated by hypotension and respiratory distress. When his ejection fraction had improved to 20–25% (Table 2), he was classified as New York Heart Association Stage IV, class D, his medical management was planned and he was discharged 34 days after initial presentation.
There was no evidence of any multiple endocrine neoplasia syndromes, and he was negative for the Ret proto-oncogene mutation. Initial endocrine work up done during the first week of his hospital stay indicated that he had mild secondary hyperparathyroidism with a PTH level of 80 pg/ml (12–65 pg/ml), which was treated with cholecalciferol 1000 IU once daily with the first week of his stay and the PTH level normalized when rechecked before discharge. The calcitonin level was normal. The chromogranin level was 40 nmol/l (0–5 nmol/l). The urine-free cortisol level was 297 µg per 24 hours, with a detectible ACTH level of 8 pg/ml. Plasma and urine dopamine levels were normal and there was no elevation of urine 5-hydroxyindole acetic acid (which would indicate a serotonin-secreting tumor).
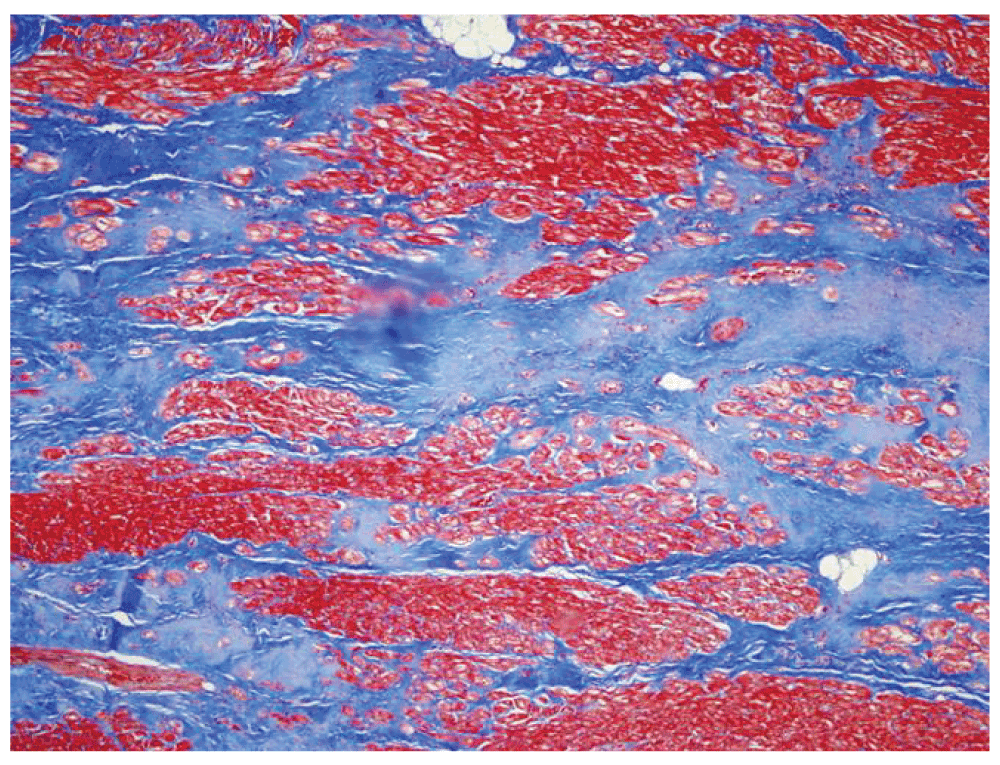
The patient was readmitted 16 days after discharge with symptoms of cough, lethargy and oliguria. He received intravenous fluids for vomiting and diarrhea. He was immediately intubated for respiratory distress and placed on dopamine infusion. Temperature was 36.7°C, heart rate was 104 beats/min, blood pressure was 112/72 mmHg and oxygen saturation was 100% on Fraction of inspired oxygen (FiO2) 80%. On examination, he was tachycardic with grade III/VI systolic ejection murmur, hepatomegaly and diminished peripheral pulses. Chest X-ray showed right-sided pleural effusion. Ejection fraction was 20% (see Table 2) and cardiac index was decreased to 1.36 l/min/m2 (Table 3). He was treated with milrinone infusion at a rate of 0.75 mcg/kg/min which was titrated and the patient was weaned from mechanical ventilation. However, he decompensated in the following days, requiring re-intubation and placement of an intra-aortic balloon pump, and was listed as status 1A for heart transplant. Approximately 3 weeks after the second admission a donor heart became available. He tolerated the procedure and was placed on low-dose epinephrine, vasopressin and milrinone. The patient’s explanted heart showed cardiomegaly (mass 380 g), with biventricular cardiac myocyte hypertrophy (Figure 2) and dilation. Septal and left ventricular white-tan fibrous scarring consistent with prior ischemic injury was evident (Figure 3). Most coronary vessels showed various degrees of eccentric atherosclerotic stenosis (Figure 4). No occlusive or thromboembolic lesions were discovered. Post-operative echocardiogram showed normal systolic function, with ejection fraction 65% (Table 2). He has been clinically stable since discharge in October 2008, with an ejection fraction of 60% at 2.5 years (Table 2) and normal cardiac pressures at three-year follow-up (Table 3). Seven years after the initial event, there has been no recurrence of the pheochromocytoma and the transplanted heart remains at optimal function.
During each cardiac catheterization, the cardiac index, pulmonary artery pressure and pulmonary capillary wedge pressure was obtained.
To the authors’ knowledge there are no reports to date of a catecholamine-induced cardiomyopathy that has failed to improve following appropriate treatment and surgical removal of a pheochromocytoma. Our case is unique in that the patient had successful surgical excision of the catecholamine-secreting tumor without reversal of left ventricular failure, and because the presence of atherosclerosis and fibrosis in the coronary vessels was substantial. This is the first (to the authors’ knowledge) reported incident of catecholamine-induced cardiomyopathy requiring orthotopic heart transplant following adequate treatment of a known pheochromocytoma. In three previous cases, the diagnosis of pheochromocytoma was not made until after the patients had undergone an orthotopic heart transplant9,10. Two additional reports involved a left ventricular assist device and intra-aortic balloon pump with extracorporeal membrane oxygenation as a bridge to myocardial recovery11,12.
The patient reported here had a significant degree of atherosclerosis of the coronary arteries, particularly given his age of 28 years. This brings to light the importance of considering the effects of coronary artery disease both independently and in the setting of catecholamine-induced vasospasm. The persistent elevation of catecholamines may accelerate the progression of atherosclerosis and development of fibrosis in the coronary vessels. Additionally, ischemic damage to the myocardium caused by coronary artery disease may worsen the prognosis of catecholamine-induced cardiomyopathy and decrease the chances of improving left ventricular function following resection of a pheochromocytoma.
Consideration of possible residual tumor burden or the presence of metastases is appropriate in this circumstance that left ventricular function fails to improve following surgical resection. However, this patient has had no clinical evidence of elevated catecholamines and there has been no clinically significant elevation of follow-up catecholamine levels.
We conclude that catecholamine-induced cardiomyopathy may be irreversible if there is structural damage to myocytes despite adequate medical and surgical treatment of a pheochromocytoma. In such cases patients may have a positive long-term outcome with orthotopic heart transplant and sustain normal left ventricular function following transplant. It is also important to consider other contributing factors to myocardial damage, including pre-existing atherosclerosis and how the presence of persistently elevated catecholamines may exacerbate known coronary artery disease.
The patient has provided written informed consent for the publication of his clinical details and clinical images.
JU wrote the manuscript, revised and edited the final version; WO wrote the manuscript; DR revised and edited the manuscript; MLS provided images of the pathology and the narrative for the images; CNB wrote the manuscript, reviewed the charts for details of the case; REM wrote the manuscript, reviewed the charts for details of the case.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 04 Aug 15 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)