Keywords
Bombus terrestris, Crithidia bombi, foraging, nicotine, pathogens, pollinators, pollinator-plant interactions, secondary metabolites
This article is included in the Agriculture, Food and Nutrition gateway.
Bombus terrestris, Crithidia bombi, foraging, nicotine, pathogens, pollinators, pollinator-plant interactions, secondary metabolites
Parasites can have a dramatic impact on their hosts, and consequently provide a powerful selective force for host defence mechanisms. Molecular mechanisms (e.g. the innate and adaptive immune system) are traditionally considered the major anti-parasite defences in the animal kingdom. However, hosts can rely on a range of alternative defence mechanisms, such as morphological barriers (St Leger, 1991), changes in life-history traits (Michalakis, 2009), symbiont-mediated defences (Oliver et al., 2010) and altered behaviours (de Roode & Lefèvre, 2012; Moore, 2002).
Behavioural immunity is an important modality of defence against diseases (de Roode & Lefèvre, 2012), and medication behaviour is a key immune mechanism in some animals (Clayton & Wolfe, 1993; de Roode et al., 2013). Medication behaviour has been defined as the use of anti-pathogenic substances found in the environment or produced by other species or individuals (Lozano, 1998). In therapeutic medication, sick individuals alter their behaviour to medicate themselves in response to parasites (Singer et al., 2009), while prophylaxis is displayed by healthy individuals in response to parasite risk rather than infection (Castella et al., 2008). For example, wood ants, bees and wasps behave prophylactically to incorporate conifer resin, propolis, or venom containing antimicrobial compounds into their nest, which inhibits the growth of bacteria and fungi (Baracchi & Turillazzi, 2010; Baracchi et al., 2011; Chapuisat et al., 2007; Castella et al., 2008; Simone et al., 2009), while, from a therapeutic perspective, ants apply antimicrobial venomous secretion to the cuticle of contaminated larvae to medicate their brood (Tragust et al., 2013). So far, most evidence for animal self-medication comes from the consumption of curative plants by vertebrates (Rodriguez & Wrangham, 1993). For example, chimpanzees, Pan troglodytes, alter their foraging to include medicinal substances (particular plant species) in their diets to cure helminth infections (Wrangham, 1995). Plants are good candidates for prophylactic or therapeutic foods as they often contain metabolites that display a wide range of biological activities (Cowan, 1999) which were originally evolved to combat herbivores or plant-parasites (Hadacek, 2002). This preferential ingestion of “non-nutritive” food and chemicals to self-medicate is known as pharmacophagy or zoopharmacognosy. Despite numerous studies investigating feeding plasticity with respect to plant nutrients and medicinal metabolites (reviewed in Mooney & Agrawal, 2008), investigations of potential pharmacophagy are rare, especially in insects. Exceptions concern self-medication behaviour described in two species of woolly bear caterpillars, which increase their preference for pyrrolizidine alkaloids or iridoid glycosides when parasitized, improving their chances of surviving parasitoid infection (Bernays & Singer, 2005; Singer et al., 2009; Smilanich et al., 2011). Similarly, wasp-infected fruit fly larvae preferentially consumed high-ethanol fly food as a medicine against their parasitoid wasp larvae, again increasing their survival (Milan et al., 2012), while no evidence for self-medication to nematode parasitism has been found in the fly Drosophila putrida (Debban & Dyer, 2013). Trans-generational medication, but not self-medication, has been described in the monarch butterfly (Lefevre et al., 2010) and self-medication has been hypothesized for honeybees that increase plant resin collection in response to a fungal infection (Simone-Finstrom & Spivak, 2012).
Animal societies arguably face the most intense pressure from pathogens. This pressure is enhanced in insect societies due to a suite of traits, including the high number of individuals living in high densities, relatively low genetic variability, and the relatively stable, high levels of humidity and temperatures of their nests (Schmid-Hempel, 1998). In addition, social pollinators, such as bumblebees and honeybees, are often exposed to an increased risk of infection via flowers (reviewed in McArt et al., 2014), which represent a shared “public place” where homo- and hetero-colonial conspecifics and other heterospecific pollinators feed repeatedly every day. Given the potential importance of parasites and disease in driving declines of managed honeybees (de Miranda & Genersch, 2010; Rosenkranz et al., 2010) and wild bumblebees (Cameron et al., 2011; Fürst et al., 2014; Schmid-Hempel et al., 2014), understanding the potential relevance of pharmacophagy to social pollinators may be a key to understanding and managing these declines.
Here we use an important natural and managed pollinator, the bumblebee Bombus terrestris, and its parasite Crithidia bombi to investigate the potential for pharmacophagy in social pollinators. C. bombi, a trypanosome gut parasite, is the most prevalent parasite of bumblebees (Shykoff & Schmid-Hempel, 1991). The parasite, transmitted either vertically or horizontally (Durrer & Schmid-Hempel, 1994; Otterstatter & Thomson, 2007), infects adults per os, and two-three days post infection, infective cells are released through the faeces of bees (Schmid-Hempel & Schmid-Hempel, 1993). Queens infected by C. bombi have a reduced success in colony founding (Brown et al., 2003), and produce fewer reproductive offspring (Brown et al., 2003), while infected workers experience a higher mortality rate under stressful conditions (Brown et al., 2000). Moreover, infection impairs foraging success and learning abilities, inducing additional costs to the colony (Alghamdi et al., 2008; Gegear et al., 2006). Recent research (Manson et al., 2010; Richardson et al., 2015) has shown that several secondary metabolites such as alkaloids (including nicotine) and glycosides, reduce the C. bombi load after consumption by the bumblebee species Bombus impatiens, suggesting that these pollinators might exploit nectar toxins or other metabolites to self-medicate.
To test whether bumblebees are able to self-medicate using naturally occurring nectar secondary metabolites we conducted a series of toxicological, microbiological and behavioural experiments using a different species of Bombus (B. terrestris) and C. bombi as models and nicotine as a natural nectar alkaloid. Nicotine is encountered by pollinators at variable concentrations between 0.1 ng/μl and 3 ng/μl in floral nectar of Nicotiana species (native of South America and naturalised worldwide by humans) and Tilia species (native throughout most of the temperate Northern Hemisphere) (Detzel & Wink, 1993; Naef et al., 2004; Tadmor-Melamed et al., 2004).
All experiments were performed with worker bumblebees (B. terrestris) obtained from a continuous rearing program (provided by Koppert B.V., The Netherlands) and conducted under standardized laboratory conditions. The insects were provided ad libitum with commercial pollen (provided by Koppert B.V., The Netherlands) and 30% sucrose solution as protein source and energy respectively. The parasites (the protozoan flagellates C. bombi) used for the experimental infections were taken from several naturally infected colonies that we started in the laboratory from infected queens.
To determine whether the nectar alkaloid nicotine influences the severity of C. bombi infections in bumblebees, we designed two experiments following (Manson et al., 2010). In the “Continuous Exposure” test, bees were first inoculated with C. bombi and then fed on a daily diet of a nicotine solution or sucrose solution (Control), simulating the continual ingestion of nectar constituents by an infected foraging bee. In the “Delayed Exposure” test, we first exposed directly C. bombi cells to nicotine or control solutions for two hours before inoculating bees, and then we fed them on a sucrose-only solution. We subsequently compared the parasite load in inoculated bumblebees.
A mixture of different parasite strains was prepared by collecting faeces from 30 workers from three infected colonies. The faeces were mixed for one minute with a vortex mixer and the C. bombi cocktail was allowed to settle at room temperature for two hours, after which the supernatant was removed and mixed thoroughly. Cell counts were made using a haemocytometer. The faeces were then mixed with sugar water to produce an inoculum concentration of 2,000 parasite cells/μl. Prior to inoculation, bees were deprived of all food for two hours to facilitate infection. Bees derived from two different healthy colonies were screened to make sure that the bees were parasite-free. Each bee was then presented with a 10 μl drop of inoculum and observed until the inoculum was drunk. Thus, each bee ingested a total of 20,000 parasite cells. This dose falls within the range of C. bombi cells present in faeces from infected workers (Logan et al., 2005), and therefore simulates cells available for transmission to healthy bees.
Post inoculation, in the “Continuous Exposure” test, bees from three colonies were kept individually in Petri dishes and received either a 0.5 ml solution of 2.5 ng/μl nicotine (nectar concentration in the natural range of this alkaloid) in 30% sucrose (Experimental bees, n = 20) or 0.5 ml of 30% sucrose only (Control bees, n = 20) along with a 1g pollen lump daily for 10 days. In the “Delayed Exposure” test the C. bombi inoculum was exposed to nicotine in the dark for two hours prior to host ingestion, simulating direct exposure of the pathogen to nectar in a flower. C. bombi cells were placed in a solution of 2.5 ng/μl nicotine in 30% sucrose (Experimental treatment), and in a solution of 30% sucrose only (Control treatment). Two hours later 20 Experimental bees and 20 Control bees were inoculated (for inoculum preparation see above). The treatment simulates the period between the deposition of Crithidia cells by infected bees and the next flower visit by a naïve bee. Post inoculation, bees of both groups were kept in individual Petri dishes and given 0.5 ml of 30% sucrose solution and a fresh pollen lump daily.
In both experiments, the infection levels were checked at day 7 and day 10 post inoculation (the period of time in which pathogen load is saturated (Schmid-Hempel & Schmid-Hempel, 1993)). Each bee was removed from its Petri dish and put into a small glass tube until it defecated. In cases when no or too little rectal fluid was obtained, the procedure was repeated for that bee a few hours later. Faeces were transferred to a haemocytometer to count the number of parasite cells.
In order to determine the impact of nicotine consumption on bumblebee survival and any possible interactive effects of dietary toxin consumption and physiological stress (for which we used starvation, as Crithidia has its biggest detrimental impacts on starved bees (Brown et al., 2000)), we conducted a series of experiments in which we exposed bumblebees to artificial nectars enriched with nicotine maintained either starved or provided with ad libitum food. “Starved bees” were moved individually from their nest into Petri dishes, starved for two hours and fed either with ad libitum 30% sucrose solution food for 30 minutes (Starved, Control) or 2.5 ng/μl nicotine in 30% sucrose (Starved, Nicotine). Survival censuses were conducted every hour until all bees were dead. “Ad libitum food bees” were kept individually in Petri dishes, and given 0.5 ml of 30% sucrose solution and a fresh pollen lump daily (Control ad libitum food), 2.5 ng/μl nicotine in 30% sucrose solution and a fresh pollen lump daily (Nicotine ad libitum food), 2.5 ng/μl nicotine in 30% sucrose solution on day 0 and 0.5 ml of 30% sucrose solution and a fresh pollen lump daily (Nicotine-once ad libitum food). Survival censuses were conducted daily until all bees had died. For each of the five treatments we chose bees from three different young healthy colonies and we randomised bees across treatment groups. Each treatment group was composed of 60 bees (20 bees per colony). Comparisons of the survival parameters of bumblebees in all treatments allowed us to evaluate the effect of nicotine, starvation, and colony membership on survival. Dead bees were immediately weighed using a microscale (Navigator N30330, Ohaus, Pine Brook, USA).
In order to evaluate whether infected bees benefit from the consumption of nicotine in terms of survival and/or parasite load we conducted two additional experiments in which infected bumblebees received artificial nectars enriched with nicotine or not and were maintained either starved (three groups of 30 bees, 10 bees from three different colonies, 90 bees in total) or provided with ad libitum food (three groups of 45 bees, 15 bees from three different colonies, 135 bees in total). In both experiments the three groups of bees were inoculated with C. bombi as described above and individually kept in Petri dishes under three types of diet (each diet consisted of two solutions dispensed by two different Eppendorf tubes): Control Group: 30% sucrose only in both dispensers (Suc-Suc group); Exp. Group 1: 2.5 ng/μl nicotine in 30% sucrose in both dispensers (Nic-Nic group); Exp. Group 2: 30% sucrose only in one dispenser and 2.5 ng/μl nicotine in 30% sucrose in the other one (Suc-Nic group). “Starved bees” were fed for 12 days and then starved until all bees were dead. The infection levels were checked at day 7 and day 10 post inoculation. Survival censuses were conducted every hour (starved bees) and every day (ad libitum food bees) until all bees were dead. At the end of the experiment we quantified total consumption of artificial nectars in each dispenser for each bee. Comparison of the survival parameters of bumblebees in all treatments allowed us to evaluate the effect of nicotine and starvation on survival.
For testing, each bee colony was housed in a wooden nest box (28 × 20 × 11 cm) connected to a wooden flight arena with a transparent, UV-transmitting Plexiglas lid (120 × 100 × 35 cm), by means of a transparent Plexiglas tube. Shutters along the length of this tube enabled control of the traffic of bees between nest boxes and flight arena (Chittka, 1998). Each bumblebee was individually marked with a coloured numbered disk.
Bees were pre-trained to forage on 12 square transparent plastic flowers of 24 × 24 mm (Perspex® Neutral) organized in two patches equidistant from the entrance of the nest. Plastic chips were placed on vertical transparent glass cylinders to raise them above the green floor of the flight arena. During the pre-training all flowers were rewarding with a 15 μl droplet of 30% sucrose solution, placed in a well in the centre of the flower (Raine & Chittka, 2008). This provided bees with an equal chance to associate both these patches (left and right) with reward during the pre-training period. Bees were allowed to forage freely on these flowers which were refilled as soon as the bees moved on a different artificial flower. In this way bees never experienced an empty flower with the exception of the last visited one. The number of foraging trips (bouts) made in the flight arena by each bee was observed to ensure only strongly motivated foragers visiting both patches (bees that did at least five consecutive foraging bouts) were selected for the experiment (Raine et al., 2006).
After pre-training, the preference of both healthy and infected pre-trained bees was tested for blue plastic flowers (Perspex® 727) containing nicotine (one patch reward: 2.5 ng/μl nicotine in 30% sucrose solution; one patch reward: only 30% sucrose solution). Each bee (n = 31 infected bees; n = 28 healthy bees) was tested individually and one hundred consecutive choices were recorded after the first bout was initiated. Bees were regarded as choosing a flower when they landed and fed from it. Bees approaching or just briefly landing on a flower were not considered as choosing that flower. As in the pre-training, flowers were refilled after the bee moved to a different one so that bees never experienced an empty flower with the exception of the last visited one. Flowers were washed between subsequent bees in order to remove possible scent marks (Saleh & Chittka, 2006). The patch formed by nicotine-containing flowers was swapped from left to right for half the bees of each group (healthy and infected bees). Controlled illumination was provided by high frequency fluorescent lighting [(TMS 24F) lamp with HF-B 236 TLD (4.3 Khz) ballasts, Phillips, Netherlands fitted with Activa daylight fluorescent tubes, Osram] which simulated natural daylight (Dyer & Chittka, 2004). At the end of the experiment all the bees were sacrificed and the concentration of C. bombi in their hind gut was determined (see above).
In the infection experiments 10 out of 80 of bees died before day 10 for unknown causes. In total, we analysed the infection intensities of 40 (day 7) and 36 (day 10) bees in the “Continuous Exposure” experiment, and 37 (day 7) and 34 (day 10) bees in the “Delayed Exposure” experiment. To compare differences in parasite load between control and experimental bees at day 7 and day 10 post inoculation in both experiments we used a generalized linear mixed model (GLMM), with pathogen counts as the within-subject variable and C. bombi exposure to nicotine, time (day 7 and day 10), colony of origin, and bee body weight as explanatory factors. As the data were not normally distributed and homogeneity of variances and sphericity could not be assumed in several cases, we performed corrections according to Huynh-Feldt epsilon (Field, 2009). For the statistical evaluations in the survival experiments, we used the classical survival parameters including the survival distribution, percent survival at the end of the census period, median survival time (LT50), and the hazard ratio of death, using the Cox Proportional Regression analysis to generate the Wald Statistic. The hazard function characterized the instantaneous rate of death at a particular time while controlling for the effect of the other variables on survival. The following variables were entered in the regression model: colony of origin, body weight, nicotine treatment. The survival distributions for all treatments were computed and analysed with the Breslow Statistic (Mantel–Cox Test). For the behavioural experiment, a T test was used to examine differences between preferences for nicotine-rich nectar and control nectar in healthy and infected bees. Spearman rank correlation tests were used to correlate parasite load and nicotine preference. All statistical analyses were done on SPSS 13® for Windows.
In the “Continuous Exposure” test, a diet enriched with nicotine reduced the intensity of C. bombi infections in bumble bees (Dataset 1). GLMM analysis revealed significant main effects of nicotine and time since inoculation on infection intensity, but not colony of origin or bee body weight (Table 1). At both 7 days and 10 days post-inoculation, bees exposed to nicotine had infections that were, on average, 1.11 and 0.56 times respectively less intense than control bees (t test, day 7: n = 20-20, t = 5.2, df = 38, P < 0.001; day 10 n = 18-18, t = 3.47, df = 34, P = 0.001; Figure 1). Infection intensities increased significantly from day 7 to day 10, independently of nicotine treatment (no-significant Nicotine and Colony x Time effect; Table 1).
| Factor | F-value | Df | P-value |
|---|---|---|---|
| Nicotine | 35.3 | 1,61 | 0.001 |
| Time | 16.2 | 1,61 | 0.001 |
| Bee body weight | 1.07 | 1,61 | 0.3 |
| Colony | 0.46 | 1,65 | 0.8 |
| Interactions | - | - | N.S. |
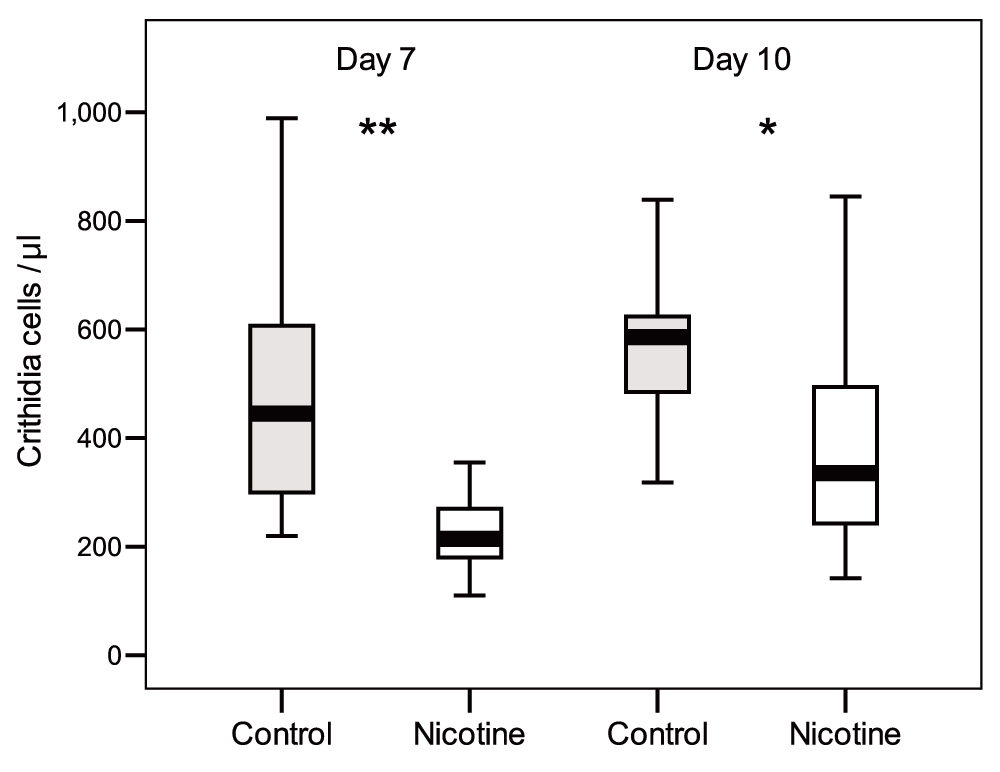
Faeces were checked after 7 days and 10 days post inoculation. Box plots show medians, 25th and 75th percentiles (** P < 0.001; *P = 0.001).
In the “Delayed Exposure” test, exposing C. bombi to nicotine for two hours before inoculation had no effect on parasite load (Table 2) (Dataset 1). At 7 days and 10 days post-inoculation, bees exposed to nicotine had infections that on average were as intense as those of control bees (t test, day 7: n = 19-18, t = 0.16, df = 35, P = 0.87; day 10: n = 17-17, t = -0.69, df = 32, P = 0.5; Figure 2). Infection intensities increased significantly from day 7 to day 10, independently of nicotine treatment (there was no-significant Nicotine x Time and Colony x Time effects; Table 2). Taken together, these findings prove the antimicrobial activity of nicotine against the pathogen when ingested by bumblebees, but also indicate that when pathogens are exposed to the alkaloid prior to host ingestion the protozoan’s viability is not directly affected.
| Factor | F-value | Df | P-value |
|---|---|---|---|
| Nicotine | 0.02 | 1,62 | 0.8 |
| Time | 27.1 | 1,60 | 0.001 |
| Bee body weight | 0.52 | 1,62 | 0.4 |
| Colony | 2.9 | 1,62 | 0.1 |
| Interactions | - | - | N.S. |
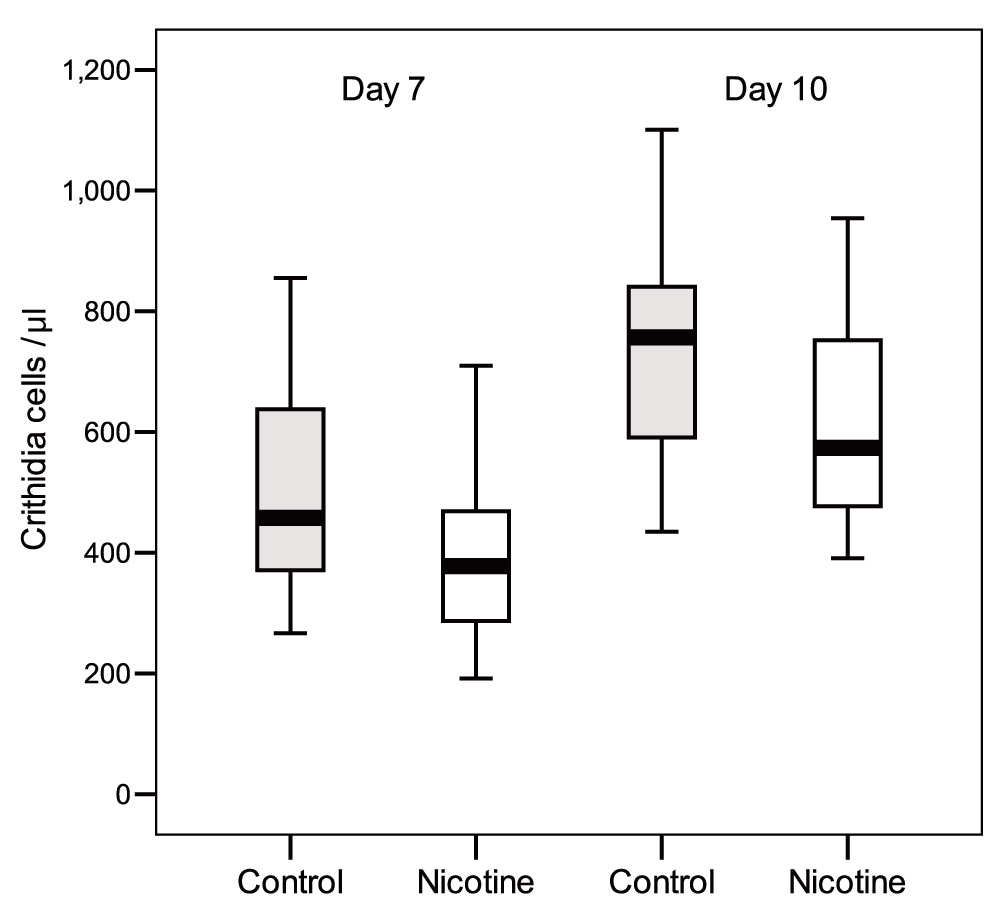
Faeces were checked after 7 days and 10 days post inoculation. Box plots show medians, 25th and 75th percentiles (P = N.S.).
In the “Starved” test, statistical evaluation of the survivorship of control and experimental bumblebees revealed that a nicotine diet was not a significant predictor of mortality (Log-rank Mantel Cox test χ2 = 0.21, df = 1, P = 0.88; Figure 3A) (Dataset 2). Furthermore no effect of colony of origin and bee body weight on mortality was found (GLM, treatments: F = 1.1, df = 1, P = 0.29; Colony F = 0.46, df = 2, P = 0.63; body weight: F = 0.19, df = 1, P = 0.66). The median lethal time (LT50) for the two groups did not differ (control LT50: 39 hours, exp. bees LT50 = 37 hours).
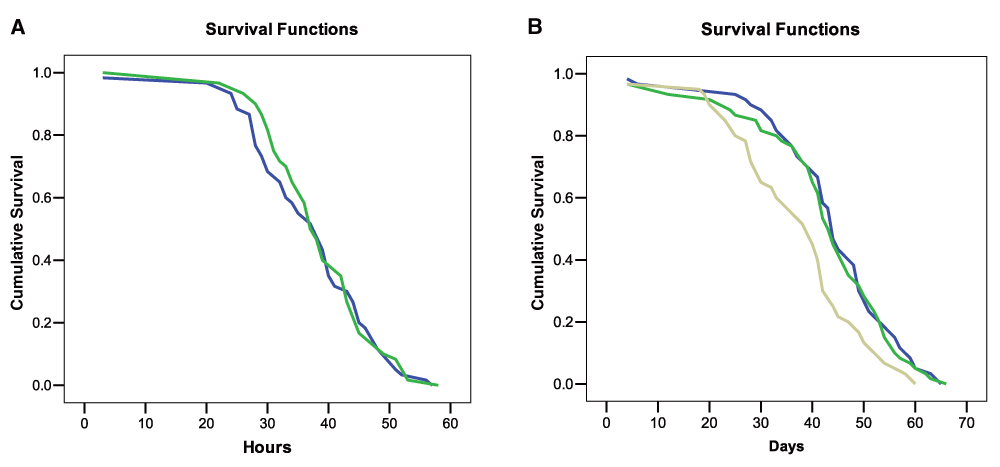
A: Cumulative survival of bees fed with a sucrose solution with (blue line) or without (green line) nicotine and starved. B: Cumulative survival of bees that received a daily diet of sucrose solution with (beige line), or without nicotine (blue line), or a single dose of nicotine on day one (green line).
In the “ad libitum food” test a Log-rank Mantel Cox test showed that a daily diet including nicotine was a significant predictor of mortality (χ2 = 11.56, df = 2, n = 180, P = 0.003; Figure 3B) (Dataset 2). Pairwise statistical comparisons revealed that bees fed consistently with nicotine had significantly lower survivorship than ‘Nicotine-once’ and ‘Control bumblebees’ (P = 0.001), while the latter two experimental groups did not differ (P = 0.86). LT50 of bees fed daily with nicotine was 39 days while ‘Nicotine-once’ bumblebees and control bees had a LT50 of 44 and 43 days respectively. Colony of origin and body weight did not affect bee mortality (GLM, Colony: F = 0.35, df = 2, P = 0.71; body weight: F = 1.90, df = 1, P = 0.16), but we found a significant interaction between body weight and treatment (larger bees were less susceptible to nicotine, GLM, F = 5.12, df = 1, P = 0.025). Taken together, these findings indicate that nicotine has some detrimental effects on healthy bumblebees if consistently consumed for weeks but also that these effects are possibly quite weak.
In both “ad libitum food bees” and “starved bees” tests, a nicotine diet was not a significant predictor of survival (Log-rank Mantel Cox test: “ad libitum food bees”: n = 135, Nic-Nic vs Nic-Suc χ2 = 0.3, P = 0.6; Nic-Nic vs Suc-Suc χ2 = 0.01, P = 0.9; Nic-Suc vs Suc-Suc χ2 = 0.7, P = 0.4; “Starved bees”, n = 76; Nic-Nic vs Nic-Suc χ2 = 0.4, P = 0.5; Nic-Nic vs Suc-Suc χ2 = 0.1, P = 0.7; Nic-Suc vs Suc-Suc χ2 = 0.01, P = 0.9) (Dataset 3). Furthermore no effect of colony of origin on mortality was found (GLM, “ad libitum food bees”: F = 1.4, df = 2, P = 0.24; “Starved bees”: GLM, F = 2.02, df = 2, P = 0.14). The median lethal time LT50 for the three groups did not differ (“ad libitum food bees”: Suc-Suc LT50: 22 days, Nic-Suc LT50 = 23, days, Nic-Nic LT50 = 22; “Starved bees”: Suc-Suc LT50: 25 hours, Nic-Suc LT50 = 28 hours, Nic-Nic LT50 = 31 hours).
GLMM analysis revealed significant main effects of treatment (df = 2, F = 3.46, P = 0.03) and time since inoculation (df = 1, F = 57.3, P < 0.001) on infection intensity, but not colony of origin (df = 2, F = 1.64, P = 1.96). No interaction between diet, time and colony was significant. Overall bees caged in Petri dishes consumed less food over the entire duration of the experiment if exposed to nicotine (Anova test: F = 9.68, n = 90, df = 2, 87, P = 0.001; Dunnett T3 post hoc test: Suc-Suc vs Nic-Nic and Suc-Suc vs Nic-Suc P < 0.001) (Dataset 4). Infected bees showed a slight preference (54 ± 17 %) for sucrose solution laced with nicotine (Paired samples t test, t = 2.14, df = 29, n = 30, P = 0.04).
Overall these findings indicate that, even though nicotine reduces the parasite load in infected bees, and such bees have a slight preference for sucrose solution laced with the alkaloid, there is no net benefit in term of survival for infected bees.
Infected bumblebees allowed to forage on plastic flowers showed a significantly increased propensity to visit nicotine rewarding flowers when compared to healthy bees (t test, n = 31, 28, t = -2.4, df = 57, P = 0.016; Figure 4) (Dataset 5). Indeed on 100 consecutive choices infected bees visited the nicotine flowers on average 64.5 ± 13.8 (s.d.) times while healthy bees visited them 54.8 ± 19.4 (s.d.) times. Since test bees were introducing nicotine into the colony throughout testing, we controlled for prior exposure to nicotine effect on nicotine preference. Bees tested later in the experiment did not show a higher or lower nicotine preference (Spearman test, Infected bees: ρ = -0.21, n = 31, P = 0.3; Control bees n = 28, ρ = 0.041, P = 0.8). There was no correlation between pathogen load and the propensity of infected bees to visit flowers with nicotine-rich artificial nectar (Spearman test: n = 31, ρ = 0.19, df = 29, P = 0.28).
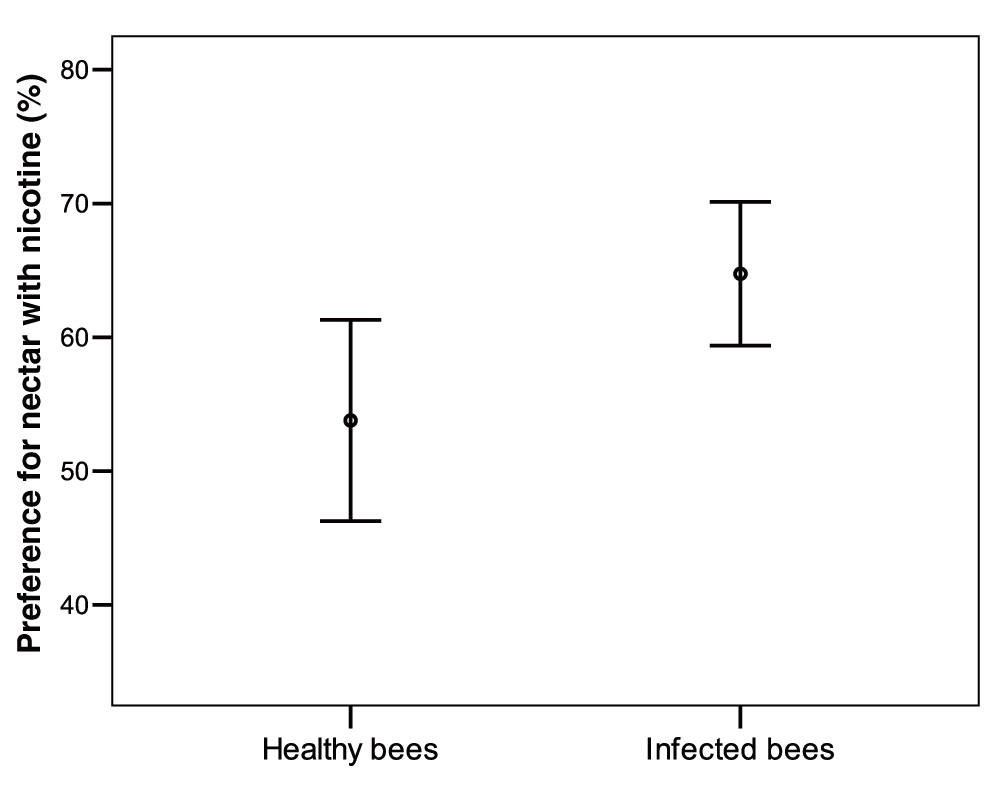
Infected bees visited nicotine-containing flowers 64.5 ± 13.8 (s.d.) times while healthy bees visited them 54.8 ± 19.4 (s.d.) times.
Here we demonstrate that parasitized bumblebees modify their diet preference and foraging behaviour to delay the development of an infection. In our experimental setup the parasite infection induced an increased consumption of nicotine both in individually caged as well as in foraging bumblebees. Despite this preferential ingestion of a “non-nutritive” antimicrobial alkaloid by infected bees, the self-medication behaviour is not efficient since dietary nicotine does not fully cure C. bombi infection. Nonetheless bumblebees exhibited a reduced C. bombi load after daily consumption of the alkaloid. In nature, infection entails an array of costs (Alghamdi et al., 2008; Brown et al., 2000; Brown et al., 2003; Gegear et al., 2006). As a consequence, any reduction in the severity or progression of infection in bees, induced by mechanisms such as the consumption of nectar containing curative alkaloids (e.g. gelsemine (Manson et al., 2010), anabasine and nicotine (Richardson et al., 2015)), might be beneficial in terms of fitness for both bees and colonies.
In the same way as bumblebees have adapted their foraging behaviour to reduce the uptake of parasites (Fouks & Lattorff, 2011), bumblebees may be adapted to modify their diet with curative nectars once infected. The recent demonstration that honeybee nurse bees, infected with the microsporidian gut parasite Nosema ceranae, show different preferences for various types of honeys in a simultaneous choice test, preferring honeys with a higher antibiotic activity (Gherman et al., 2014), suggests that such behaviours may be widespread in social pollinators. However, our results suggest that a description of this behaviour as pharmacophagy may require further evidence. Indeed, although dietary nicotine slows the progression of infection by a few days, this effect does not induce any benefit in terms of life expectancy of infected bees. Even if we cannot completely exclude that the weak effect of nicotine is due to the initial challenge being too strong for the nicotine to have a measurable influence on life expectancy, both nicotine concentration and Crithidia inocula used in our study simulated natural doses. Additional field and mesocosm tests are thus needed to clarify the actual benefits of ingestion.
Nicotine also has a costly effect on uninfected individuals, as shown by our toxicological assays. A daily diet containing nicotine, lasting more than two months, reduced the life expectancy of bumblebees, and this effect was stronger in smaller bees. This might possibly be aggravated in the wild, where bees are exposed to other stressors and do not have access to ad libitum food. However, we note that differences in mortality rate between controls and nicotine-treated bees started to be evident only after 20 days from the first exposure suggesting that in nature this detrimental effect may be mitigated due to the relatively short lifespan of foragers in the wild (da Silva-Matos & Garófalo, 2000). Moreover, in nature, bees may not forage on a single nectar source continuously for weeks as we simulated in our experiments, further reducing the negative effect of nicotine intake. In infected bumblebees the detrimental effect of nicotine is no longer evident suggesting that detoxification costs might be counterbalanced by the advantages in slowing the progression of the infection. However, contrary to our prediction, we found no trade-off between costs and benefits in terms of survival, and infected bumblebee lifespan was not affected by the consumption on nicotine. Similar results have recently been found for the antimicrobial alkaloid anabasine that did not induce a significant fitness benefit in the bumblebee species B. impatiens despite its effectiveness in reducing the parasite load by up to 80 percent (Richardson et al., 2015).
The cost imposed by the consumption of nicotine in our experiments may explain why healthy bees did not constantly consume high doses of nicotine (Tiedeken et al., 2014). Similarly, infected bees kept in Petri dishes reduced the overall uptake of food if exposed to nicotine. This is surprising given that those bumblebees also had a slight preference for sucrose solution laced with the alkaloid, and free-flying healthy bumblebees were not repelled by artificial nectar laced with nicotine. While these behavioural preferences may be explained by the impact that some nectar alkaloids, including nicotine, have on learning and memory in bees (Chittka & Peng, 2013; Thany & Gauthier, 2005; Wright et al., 2013), the mechanism behind the overall reduced consumption caused by nicotine remains unexplained. In humans at least, it is well established that nicotine has appetite-reducing effects (Jessen et al., 2005).
Currently it is unclear how nicotine acts on C. bombi. Nicotine is a highly toxic molecule (Benowitz, 1998) that acts against a wide spectrum of bacterial and fungal pathogens (Pavia et al., 2000). House sparrows and several finch species, for example, add smoked cigarette butts retaining substantial amounts of nicotine to their nests to reduce mite infestations (Suárez-Rodríguez et al., 2013). While our in vivo microbiological experiments prove the antimicrobial activity of nicotine against the pathogen when ingested, they also suggest that nicotine does not directly interfere with the protozoan’s viability, at least when measured as infectivity. As suggested by Manson et al. (2010), who found similar effects of the natural alkaloid gelsemine, an alkaloid-rich diet might increase a bee’s excretion rate, as occurs for nectarivorous bird (Tadmor-Melamed et al., 2004), effectively “flushing” C. bombi cells from the gut. Another possibility might be that nicotine, or perhaps its metabolites, directly modify the mid-gut epithelium or the environment of its lumen, making it less suitable for the parasite.
In conclusion, we believe that our results suggest that a more careful approach to interpreting impacts of plant metabolites on insect parasites is warranted. Recent findings have suggested that the preferential ingestion of natural nectar secondary metabolites in pollinators might play a key role in mediating pathogen transmission within and between colonies (Richardson et al., 2015) or interactions among pollinators and their parasites (Manson et al., 2010). Similarly, our results and other recent studies (Gherman et al., 2014; Richardson et al., 2015) have suggested that bees may self-medicate by consuming plant secondary metabolites when they are infected with parasites. However, our study suggests that the conditions under which nicotine consumption provides benefits to either bees or plants remain to be identified. The contention that secondary metabolites in nectar may be under selection from pollinators, or used by plants to enhance their own reproductive success (Chittka & Peng, 2013; Thomson et al., 2014; Wright et al., 2013), should ideally be confirmed with further studies, which examine the impacts of these metabolites on both bee and plant fitness under field-realistic conditions.
F1000Research: Dataset 1. Infection experiments, 10.5256/f1000research.6262.d44610 (Baracchi et al., 2015a).
F1000Research: Dataset 2. Laboratory toxicity bioassays, 10.5256/f1000research.6262.d44612 (Baracchi et al., 2015b).
F1000Research: Dataset 3. Trade-off between detrimental and beneficial effects of nicotine, 10.5256/f1000research.6262.d44613 (Baracchi et al., 2015c).
F1000Research: Dataset 4. Diet preference of caged bees, 10.5256/f1000research.6262.d44614 (Baracchi et al., 2015d).
F1000Research: Dataset 5. Behavioural test, 10.5256/f1000research.6262.d44615 (Baracchi et al., 2015e).
D.B. conceived the study and carried out the experiments. D.B. and M.J.F.B. designed the experiments. LC made significant contributions to the interpretation of the data. All authors equally contributed in writing and revising the draft of the manuscript and have agreed the final content.
D.B. was supported by a Marie Curie Intra European Fellowship within the 7th European Community Framework Programme.
The authors confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors thank Anna Woodhouse for helping in behavioural experiments, Dr. Gemma Baron for providing Crithidia samples, Dr. Caroline Brennan for nicotine solution, and Thomas Ingraham (an employee of F1000Research) for comments on the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: Statement regarding conflict. I have two connections to this research. First, Lars Chittka did a stint of postdoctoral work in my lab at Stony Brook; we collaborated on several papers in the 1990s, and co-edited a book in 2001. We correspond occasionally but have not had an active collaboration since then. Second, I later supervised postdoctoral research on bumble bee disease and nectar chemistry by Robert Gegear, dissertations by Michael Otterstatter and Jessamyn Manson, and undergraduate research by Miruna Draguleasa. I share authorship on several of their papers that are cited prominently by Baracchi et al.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 3 (revision) 29 Oct 15 |
|||
|
Version 2 (revision) 26 May 15 |
read | read | |
|
Version 1 19 Mar 15 |
read | read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)