Keywords
DAMPs, PAMPs, Sterile inflammation, Sepsis, SIRS, TLRs
DAMPs, PAMPs, Sterile inflammation, Sepsis, SIRS, TLRs
TNF, tumour necrosis factor; IL, interleukin; MIP, macrophage inflammatory protein; HLA-DR, human leukocyte antigen-DR; TLR, toll-like receptor; PBMC, peripheral blood mononuclear cells; SIRS, systemic inflammatory response syndrome.
Sepsis and multiorgan failure (MOF) are the leading causes of death in surgical patients1–3. Several studies have described surgery induced systemic inflammatory response syndrome (SIRS)4,5 and compensatory anti-inflammatory response syndrome (CARS)6–9 as prevalent causes of these complications. A causal relationship of events such as induction of cytokine storm10–12, immune cell activation and infiltration of activated immune cells13 with development of SIRS following surgery has been reported in a cohort of patients. Upregulated expression of toll-like receptors (TLRs) by activated monocytes/macrophages and hyper-reactivity of peripheral blood mononuclear cells to pathogen associated molecular patterns (PAMPs) have further been shown to be associated with this overwhelming inflammatory response post-injury14. Paradoxically there are also reports claiming downregulated expression of TLRs on circulatory monocytes as well as impaired responses to PAMPs in patients post-surgery15,16.
Existing literature does not offer clarity on factors that contribute to these diagonally opposite biological outcomes in patients post-surgery. We hypothesised that the pre-surgery status of patients could determine post-surgery consequences – while previous studies have demonstrated immunodynamics and inflammation profiles post-surgery17 very little has been understood about the correlation between pre-operative inflammatory status and outcome post-injury. An association between lower pre-operative plasma IL-6 and early allograft dysfunction due to high systemic inflammation18 and higher pre-operative systemic inflammation has been demonstrated with increased risk of infection post-surgery19,20. These reports however suffer from lack of robust and comprehensive measurement of cellular and molecular parameters of inflammation and the current study was designed to fill this lacuna.
Here we report the quantification of plasma cytokines, expression of monocyte surface receptors such as TLRs, scavenger receptors, HLA-DR and other co-stimulatory molecules in patients before and after elective surgery. Our results revealed downregulation of specific inflammatory mediators in 6 hrs post-surgery plasma in most of the patients while the rest displayed distinct increased levels of plasma mediators. Increased as well as decreased levels of plasma inflammatory molecules correlated with surface expression of monocyte receptors such as TLRs, scavenger receptor CD36, HLA-DR and co-stimulatory molecules. Further, our findings demonstrate an inverse association between pre-surgical status and inflammation profile post-surgery viz., patients with relatively higher basal levels of plasma cytokines and monocyte surface receptors developed a hypo-inflammatory response while patients with relatively lower basal levels of plasma cytokines and monocyte surface receptors developed a hyper-inflammatory response post-surgery.
Lipopolysaccharide from Escherichia coli serotype O55:B5 (cat. No. L2880-100MG) and PAM3CSK4 (cat. No. IMG-2201) were purchased from Sigma-Aldrich and Imgenex India Pvt. Ltd. respectively. RBC lysis (cat. No. 00-4333-57), cell fixation (cat. No. 00-8222-49) and permeabilization (cat. No. 00-8333-56) buffers were purchased from eBiosciences. Bio-Plex Pro Assays-27 plex kit was purchased from Bio-Rad (cat. No. M500KCAF0Y). DNA isolation kit (QIAamp DNA Mini kit [250]; cat. No. 51306) was purchased from Qiagen. Q-PCR SYBR mix (2X Brilliant III STBR Green QPCR Master Mix, cat. No. 600882-51) was purchased from Agilent Technologies.
Patients admitted for gastrointestinal and general surgery in the Department of General & Laparoscopic surgery, Neelachal Hospital Pvt. Ltd. Bhubaneswar between September 2013 and October 2014 were recruited for this study. All of the surgeries included in this study were cases of elective surgery. A total of 19 patients between the age of 18 years and 75 years were included. Inclusion criterion was surgical interventions with a minimum incision of 3 inches. Pregnant women, terminally ill patients and patients admitted for emergency surgery or accidental trauma cases were excluded from the study. Details of patients, type of anaesthesia used, duration of surgery, duration of hospital stay, pre-surgical total blood cell counts etc. are shown in Table 1. Total 23 healthy volunteers who were not on any medication since two weeks prior to blood collection participated in this study. The project protocol was approved by the human ethics committee of Institute of Life Sciences (no. 28/HEC/13) and the IRB committee. Written informed consent was obtained from each of the patients for voluntary participation in the study.
Data are presented as mean of 23 healthy controls and 19 surgery patients. Patients were categorized into two groups on the basis of post-operative inflammatory responses viz., ‘hypo-responsive’ and ‘hyper-responsive’. Values represent the mean ± SEM, with median and middle quartiles indicated in parentheses. RBCs, red blood cells.
Blood was collected in ACD 15% (V/V) containing tubes. Sampling was done in two batches - initially 8 subjects were selected and sampled twice, immediately before anaesthesia (0hps) and 6 hrs after completion of surgery (6hps). After analyzing the data, 11 more patients were included and sampling was done thrice for this cohort; immediately before anaesthesia (0hps), 6 hrs and 24 hrs after completion of surgery (6hps and 24hps respectively). Whole blood was aliquoted and used immediately for complete blood count (CBC), for conducting ex vivo stimulations and analysis by flow-cytometry. Plasma was isolated from the rest of the sample by centrifugation and was stored at -80°C for conducting further assays.
Two hundred microliters of whole blood was used for complete blood count (CBC) in haematology analyzer (Sysmex XS800i).
Fifty microliters of freshly collected blood samples were stimulated with TLR ligands; PAM3CSK4 and LPS (10ng/ml for both) for 2 hrs at 37°C. Cells were fixed, permeabilized (following manufacturer’s instructions) and stained with antibodies to CD14-(FITC), CD66b-(APC) and TNF-α-(PE-Cy7) (antibody panel-1). Another aliquot (50ul) was used for multicolour immune staining with antibodies to CD14-(APC-Cy7), TLR2-(PE-Cy7), TLR4-(APC) and CD36-(FITC) (antibody panel-2) and CD14-(APC-Cy7), HLA-DR-(PE-Cy7), CD40-(APC) and CD80-(FITC) (antibody panel-3). After staining RBCs were lysed with RBC lysis buffer following manufacturer’s protocol. Stained cells (with antibody panels -1, 2 and 3) were acquired and analyzed by flow cytometry using BD FACS LSR Fortessa; data were analysed using BD FACS Diva software (version 7.0). To nullify day to day variations flow-cytometer settings were maintained uniform by performing bead based instrument settings following the protocol provided by BD Biosciences. To get rid of false positive or false negative events, gating was done using fluorescence minus one (FMO) controls. Monocyte expression of intracellular cytokines was shown as mean fluorescence intensity (MFI) whereas expression of surface markers was scored as percentage of positive cells.
Plasma cytokines were measured by Bio-plex Pro Assays-27 plex following the manufacturer’s instructions and the final reading was taken in Bio-plex 200 system from Bio-rad.
Isolation of plasma DNA. 100μl of plasma was mixed with 100μl of PBS and centrifuged at 700×g for 5 minutes at 4°C. Upper 190μl volume was collected without agitating the lower portion and centrifuged at 18000×g for 15 minutes at 4°C. From this upper 170μl was transferred into a new tube and plasma DNA was eluted using QIAamp DNA Mini kit following manufacturer’s instructions. Preparation of standard curve: mitochondrial cytochrome-b gene (mtCyt-b) was amplified from eluted DNA by end-point PCR using following primer set; forward 5’CCACCCCATCCAACATCTCC3’ and reverse 5’CTCGAGTGATGTGGGCGATT3’. Concentration of PCR product (copy number/ng of DNA) was calculated and standards (109 to 1 copy number/μl) were prepared by log10 dilution. Standards were amplified for the same gene (mtcyt-b) by real-time quantitative PCR (RT qPCR) and standard curve was prepared by plotting DNA copy number and cT value in X and Y-axis respectively. Calculation of mitochondrial DNA copy number: cT values of mtCyt-b for plasma DNA samples were obtained by RT qPCR. Real copy number of mtDNA was calculated from reference standard curve by using observed cT values. Protocol was adapted from Kiichi Nakahira et al.21.
GraphPad Prism (version 5.01) software was used for statistical analysis and results of all experiments were expressed as mean±SEM. Comparisons between groups were made by nonparametric unpaired Student’s t-test (Mann-Whitney test) for Table 1, nonparametric paired Student’s t-test (Wilcoxon matched pairs test) for Supplementary Figure 2 and Supplementary Figure 3 and one way ANOVA choosing nonparametric paired test (Friedman test) for rest of the figures. P values were analysed by two-tailed test and P<0.05 was considered as significant (at 95% confidence intervals).
Effect of surgery on systemic inflammatory responses was studied by estimating 27 different plasma biomarkers in patients pre- and post-surgery. Eighteen out of 27 mediators tested revealed levels detectable by the assay (Supplementary Table 1). Four of the 19 patients displayed hyper-inflammation as shown by increased plasma levels of TNF-α, IL-1β, IL-ra, IL-7, IL-8 and MIP1a in comparison to pre-surgery levels while in the remaining 15 patients all the 6 inflammatory molecules decreased consistently within 6 hrs post-surgery (Figure 1). Comparison of plasma parameters pre-surgery with post-surgery levels allowed us to differentiate bimodal inflammation consequences in patients after surgery. The dichotomy of inflammatory cytokine response between the two groups persisted at 24 hrs post-surgery also (Supplementary Figure 1). For conceptual clarity the expression ‘hypo-responsive’ and ‘hyper-response’ will be used in the manuscript to classify the former and later groups of patients. Other plasma molecules did not show persistent bimodal inflammatory response post-surgery (Supplementary Table 1). Age, type of anaesthesia used, duration of surgery or hospital stay and several blood cell parameters were comparable between the two groups (Table 1).

Cytokine contents (pg/ml) in plasma collected from patients at 0 and 6 hrs post-surgery were measured by bead based multiplex immunoassay. Percent changes in 6hps plasma cytokines (with respect to 0hps) were calculated and values were plotted individually. Data points above and below the X-axis indicate increased and decreased levels of cytokines respectively. Results are presented as separate data points for 19 patients among whom 15 showed decreased and 4 showed increased levels of TNF-α, IL-1β, IL-1Ra, IL-7, IL-8 and MIP-1a at 6hps plasma.
The following receptors on monocytes were scored by multicolour flow-cytometry pre- and post-surgery in all patients: toll-like receptors TLR2 and TLR4, CD-36 a scavenger receptor, co-stimulatory molecules CD40 and CD80, and HLA-DR. Expression levels of all the above listed receptors were significantly decreased (P<0.05 to P<0.01) in hypo-responsive patients at 6 hrs post-surgery, very similar to inflammatory plasma cytokine levels in this group (Figure 2). There was however no significant change in any of the receptor levels in hyper-responsive patients (Figure 2).
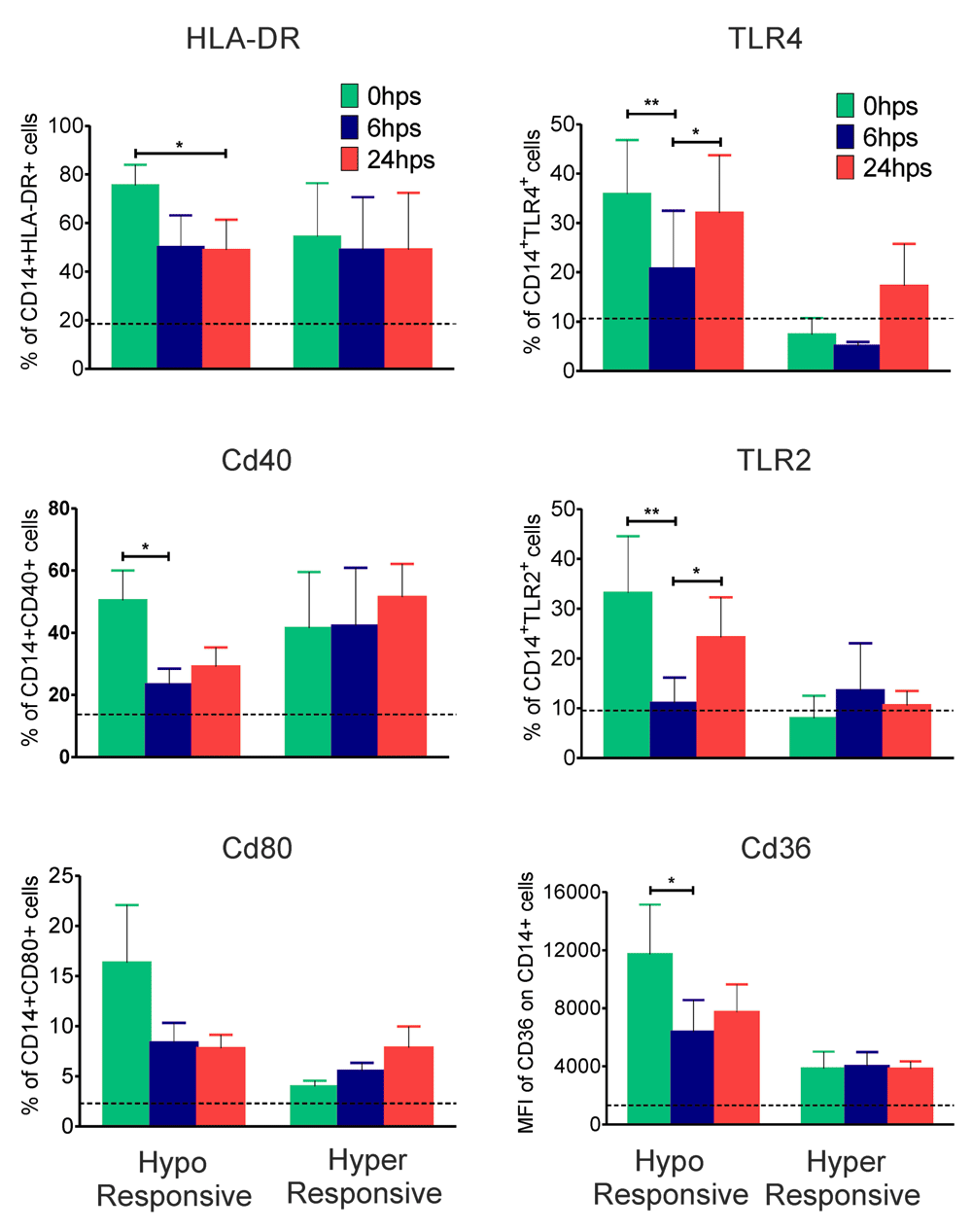
Whole blood collected from patients at 0, 6 and 24 hrs post-surgery was stained with fluorescent conjugated anti-human antibodies for CD14, HLA-DR, CD40, CD80, TLR4, TLR2 and CD36 in two different panels as mentioned in materials and methods section. Percentage of HLA-DR+, CD40+, CD80+, TLR4+, TLR2+ and MFI of CD36 on CD14+ monocytes derived by flow-cytometric analysis is shown. Values are presented as mean±SEM of 7 hypo-responsive and 4 hyper-responsive individuals. Dotted black lines indicate mean values of respective parameters for healthy controls (n=16). Statistical comparisons were performed among all time points by one way ANOVA (*P<0.05 and **P<0.01).
Decreased plasma cytokines as well as receptors on monocytes 6 hrs post-surgery in hypo-responsive patients as shown above indicated an intrinsic defect in responding to TLR agonists. This was experimentally tested by stimulating whole blood with LPS, a TLR4 agonist and PAM3CSK4, a TLR2 agonist at different time points post-surgery and scoring intracellular TNFα in circulating monocytes (Ly6G-CD14+). The results are shown in Figure 3 – circulatory monocytes of hypo-responsive patients tested 6 hrs post-surgery responded significantly less to LPS (P<0.05) as well as PAM3CSK4 (non significant) when compared with stimulation of their cells pre-surgery –the decreased activation was more prominent to LPS than to PAM3CSK4 (Figure 3). The impaired responses recovered to pre-surgery levels at 24 hrs post-surgery. There was no effect in hyper-responsive patients in terms of response to TLR ligands. The response to both TLR2 and TLR4 agonists were comparable pre- and post-surgery in these patients.

Whole blood samples collected from patients at 0, 6 and 24 hrs post-surgery were stimulated with LPS (10ng/ml) (A) and PAM3CSK4 (10ng/ml) (B) for 2 hrs in presence of brefeldin-A (1X). Cells were surface stained for CD14 and CD66b followed by fixation, permeabilization and intracellular staining for TNF-α. MFI of TNF-α in CD14+CD66b- gated monocytes was measured by flow-cytometry and values were presented as mean±SEM of hypo-responsive (n=7, left panel) and hyper-responsive (n=4, right panel) individuals separately. Statistical comparisons were performed among all time points using one way ANOVA (*P<0.05).
Plasma levels of 27 host molecules in normal healthy controls were compared with patients before undergoing surgery. Figure 4a reveals that levels of IL-1β, IL-8, MIP-1a and TNF-α are significantly higher (P<0.05 to P<0.001) in hypo-responsive patients when compared with healthy controls. The levels between hyper-responsive group and healthy controls were however comparable. Similarly pre-surgery expression of CD36, CD40, CD80 and HLA-DR on circulating monocytes were significantly more (P<0.05 to P<0.001) on hypo-responsive patients when compared with controls and there was no significant difference between healthy controls and hyper-responsive cases. These observations suggest that hyper- or hypo-inflammation observed post-elective surgery is determined by pre-existing plasma levels of inflammatory molecules and pathogen responsive surface receptors on monocytes.

Plasma levels (pg/ml) of TNF-α, IL-1β, IL-1Ra, IL-7, IL-8 and MIP-1a in healthy controls (n=23) and both hypo (n=15) and hyper-responsive (n=4) patients pre-surgery measured by bead based multiplex immunoassay are shown (A). Surface expression of HLA-DR, CD40, CD80, TLR4, TLR2 (percent of positive cells) and CD36 (MFI) on CD14+ peripheral blood monocytes collected from healthy controls (n=16) and both hypo (n=15) and hyper-responsive (n=4) patients pre-surgery was scored by flow-cytometry (B). Values are presented as mean±SEM and statistical significance for hypo and hyper-responsive individuals with respect to healthy controls was tested by t-Test (*P<0.05, **p<0.01 and ***P<0.001).
Absolute copy number of mtDNA in 0 and 6 hrs post-operative plasma were scored to check role of endogenous danger molecules ‘DAMPs’ post-surgery. DNA was isolated from equal volume of plasma samples and copy number of mitochondrial cytochrome-b DNA was scored by real-time quantitative PCR. Results showed significant decrease (P<0.01) in copy number of mtcyt-b DNA in 6 hrs post-surgery plasma of hypo-responsive individuals. Although not statistically significant hyper-responsive individuals also followed the same trend (Supplementary Figure 3).
Inflammation status post-surgery has been a contentious issue - some patients display features of high inflammation and signs of SIRS and others display hypo-responsive or immune paralysis phenotypes. The current study was undertaken to investigate if pre-operative inflammation status would contribute and determine post-operative inflammatory responses in patients undergoing elective surgery. The study design excluded patients undergoing surgery post trauma which could by itself contribute to induction of inflammation before surgery. The results revealed a characteristic bimodal host inflammatory response following elective surgery. The majority of patients with relatively higher pre-existing systemic inflammation displayed lower inflammation parameters post-surgery and, conversely, a small cohort of patients with decreased levels of inflammation before surgery responded vigorously with significantly elevated inflammatory molecules. A comparative analysis of 27 plasma inflammatory biomarkers revealed downregulation of cytokines such as TNF-α, IL-1β, IL-7 and IL-8, chemokines MIP-1a and the antagonist of IL-1 cytokine, IL-1Ra in 79% of subjects whereas the same mediators were upregulated in 21% of subjects at 6 hrs post-surgery. In the former category of patients decreased levels of plasma mediators persisted at 24 hrs also while in the latter the levels increased further at the same time point. On the basis of these initial findings we designated patients with downregulated plasma inflammatory biomarkers as hypo-responsive and those with upregulated plasma inflammatory biomarkers as hyper-responsive individuals. Earlier investigators in our view may have missed such bimodal distribution of inflammation due to faulty analysis of data - most studies compute mean and deviation of each of the parameters pre- and post-surgery without taking cognisance of shift in inflammation parameters in each of the patients.
Factors such as age, sex, type of anaesthesia used, duration of surgery, degree of surgical injury etc. were all comparable between hypo-responsive and hyper-responsive patients. However TLRs that contribute significantly to induction of inflammation by PAMPs and DAMPs were significantly decreased on circulating monocytes in hypo-responsive patients and were either unaltered or marginally increased on monocytes of hyper-responsive individuals at different time points post-surgery. Expression of HLA-DR and co-stimulatory molecules (CD80 and CD86) on monocytes were downregulated in hypo-responsive patients, an observation similar to other reports22,23. Expression of monocyte CD36, a dominant scavenger receptor involved in phagocytosis was significantly downregulated only in hypo-responsive patients. The study of receptors on monocytes and plasma levels of cytokines are only suggestive of hypo- or hyper-inflammation status and its validation would depend on demonstration of response of immune cells to stimulation by PAMPs and DAMPs tested ex vivo – stimulation of whole blood with TLR-2 and TLR-4 agonists revealed significantly decreased induction of TNF-α by monocytes collected from hypo-responsive individuals indicating immunoparalysis.
Further, although previous studies have revealed a positive association of elevated plasma mtDNA with excessive inflammation in critically ill surgery patients21,24,25 we observed reduced copy number of plasma mtDNA in both hypo- and hyper-responsive individuals following surgery. This may be indicative of the fact that increased plasma cytokines along with high copy number of mtDNA are responsible for post-surgical complications while in uncomplicated situations although the plasma cytokines increase the host system takes control over excessive inflammation by reducing the number of plasma mtDNA.
Taken together our data revealed that basal pre-surgery levels of plasma TNF-α, IL-1β, IL-7, IL-8, MIP-1a and monocyte expression of TLR-2, TLR-4 and CD80 etc. are significantly elevated in hypo-responsive patients as compared to hyper-responsive counterparts. These findings also explain several earlier reports on immuneparalysis as well as hyper-inflammation – a paradoxically opposite scenario in cohorts of patients post-surgery18–20.
Our observations of bimodal response in patients post-surgery also provides us a model to categorise patients undergoing elective surgery as ‘immune paralysis’ prone vs ‘hyper-Inflammation’ prone. We propose that surgical trauma predominantly and essentially leads to hypo-responsiveness and tolerance critical for regulating innate immune activation for uneventful recovery post-surgery and that failure to do so in a small cohort of patients could result in persistent hyper-inflammation leading to susceptibility to SIRS or sepsis and that pre-existing inflammation status before surgery could play a critical role in determining the clinical outcome. It is however not clear currently from this study if patients who displayed hyper-inflammation phenotype post-surgery would have developed SIRS and/or sepsis since the patients were not followed beyond 24 hrs. It is a major limitation of this study in our view but we are currently addressing this issue and its validation could result in development of robust biomarkers for predicting outcome of surgery. The results of this study hopefully will lead to similar analysis in different surgical cohorts by other investigators. Further, the ability to predict inflammation outcome could also assist in decision making before administering immunosuppressive drugs post-surgery.
F1000Research: Dataset 1. Complete blood count raw data, 10.5256/f1000research.6991.d10160326
F1000Research: Dataset 2. Mitochondrial cytochrome-b raw data, 10.5256/f1000research.6991.d10160427
F1000Research: Dataset 3. Flow-cytometry .FCS files and analyzed data files, 10.5256/f1000research.6991.d10161228
PB conducted most of the laboratory assays, analysed the data and wrote the draft manuscript, RM performed analysis of multi-colour flowcytometry assays, JM conducted the surgery on patients and clinically evaluated each of the patients who participated in the study and BR conceived and designed the project, interpreted the data and finalysed the manuscript.
This research was supported by the core grants to Institute of Life Sciences, Bhubaneswar, India from Department of Biotechnology, Government of India. PB was supported with a fellowship from Council of Industrial and Scientific Research, New Delhi.
I confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The Institute of Life Sciences is fully funded by Department of Biotechnology, Government of India. The authors acknowledge with thanks all the patients and volunteers who participated in the study and the staff of Neelachal Hospitals for their co-operation.
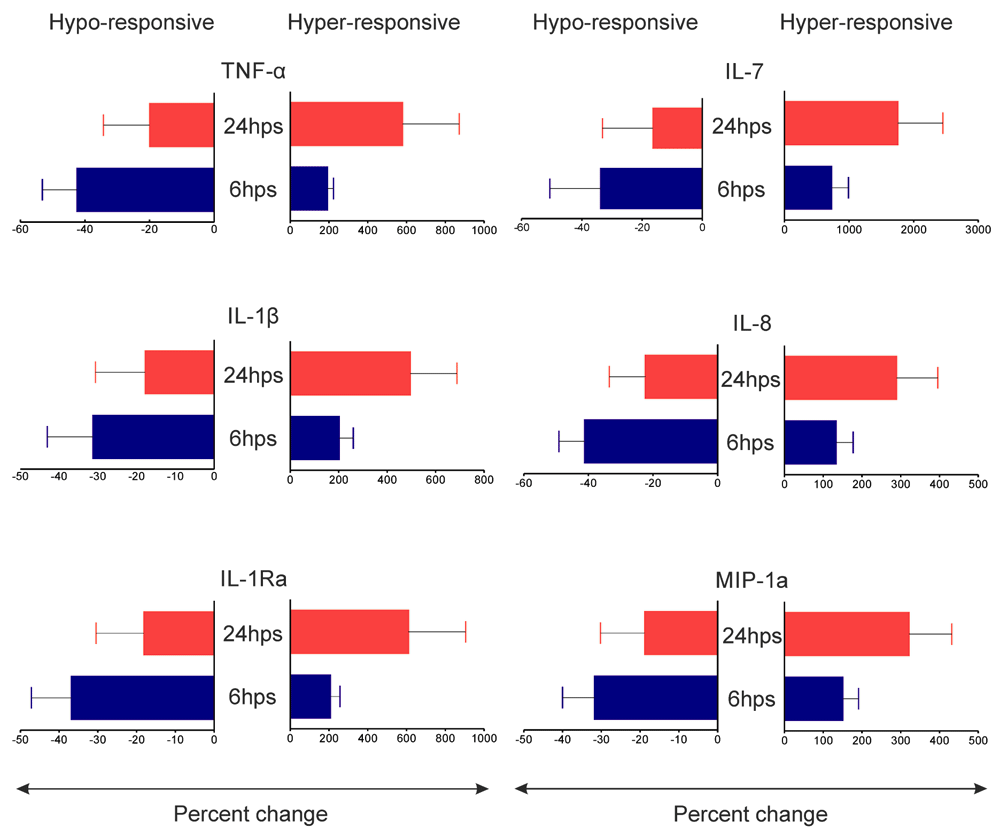
Percent changes of TNF-α, IL-1β, IL-1Ra, IL-7, IL-8 and MIP-1a in 6hps and 24hps plasma (with respect to 0hps) were plotted separately for hypo and hyper-responsive groups. Data represent mean of 7 hypo-responsive (left panels) and 4 hyper-responsive (right panels) individuals. Although there were quantitative differences none of the mediators showed statistical significance between 6hps and 24hps time points in any group.
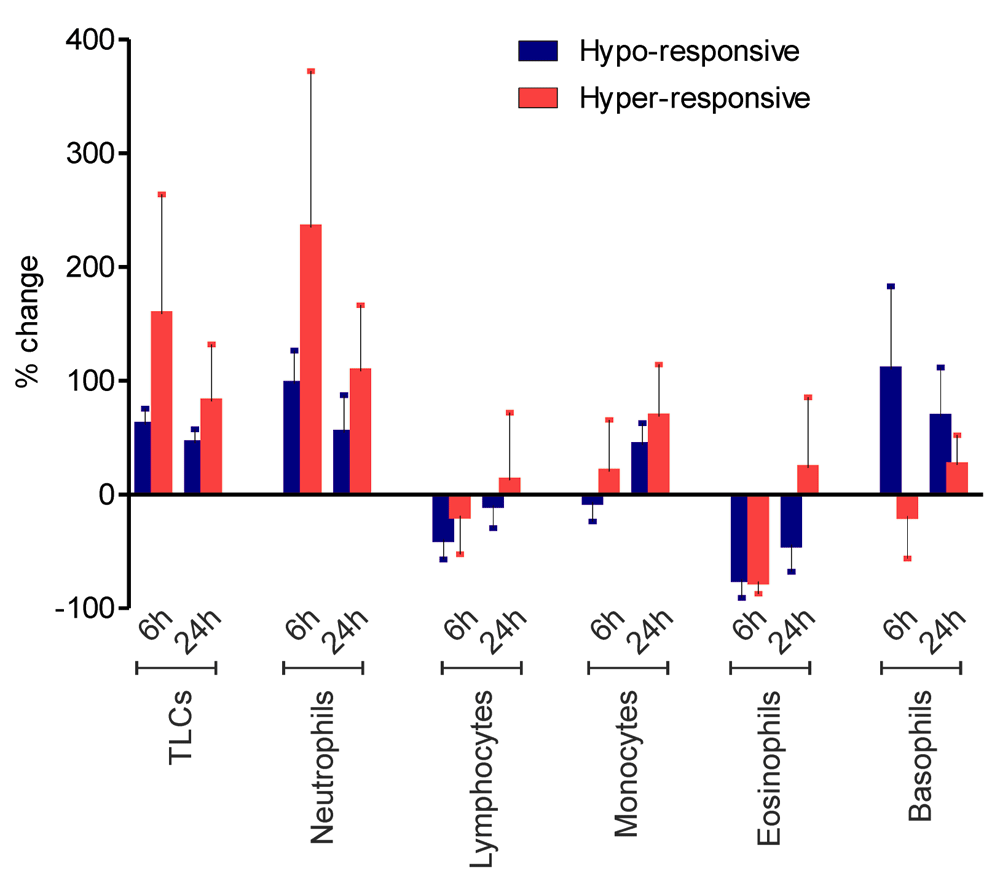
Absolute numbers of immune cells in peripheral blood of patients at 0, 6 and 24 hrs post-surgery were scored. Percent changes of total leukocyte counts (TLCs), neutrophils, lymphocytes, monocytes, eosinophils and basophils at 6hps and 24hps are presented as mean±SEM of hypo-responsive (n=7, blue bars) and hyper-responsive (n=4, red bars) patients. Statistical analysis showed no significant difference between two groups for any parameter at any time point.
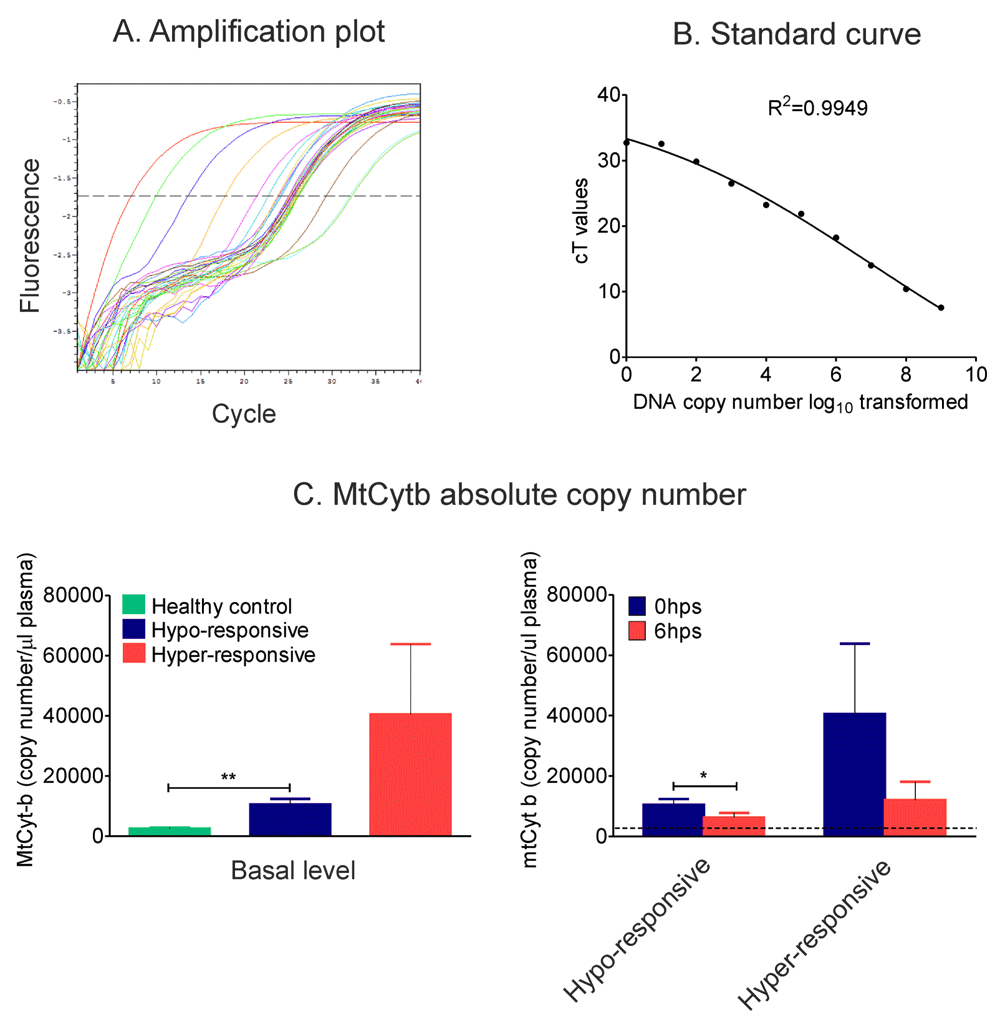
A) Amplification plot shows regular increase in cT values of serially diluted (1:10) cytochrome-b DNA standards and the samples falling within the range of standards. B) The standard curve derived by plotting log10 transformed copy number of cytochrome-b DNA and cT values shows a linearity over a high range (R2=0.9949). C) Absolute copy numbers of plasma mitochondrial DNA derived from standard curve were plotted for healthy controls and patients pre-surgery (C, left panel) as well as for patients at 0 and 6 hrs post-surgery (C, right panel). Dotted black line indicates mean of healthy controls. Data showed mean±SEM of 23 healthy controls, 15 hypo-responsive and 4 hyper-responsive individuals. Statistical analysis was done by using t-test and/or one way ANOVA (*P<0.05 and **p<0.01).
Cytokine contents (pg/ml) in plasma collected from healthy controls (n=23) and patients at 0, 6 and 24 hrs post-surgery (n=19) measured by bead based multiplex immunoassay. Sur, surgery; hps, hours post-surgery; HC, healthy control.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 10 Sep 15 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)