Keywords
Bumble bee, Bombus impatiens, parasites, Crithidia bombi, plant secondary metabolites, nicotine, alkaloids, tritrophic interactions
Bumble bee, Bombus impatiens, parasites, Crithidia bombi, plant secondary metabolites, nicotine, alkaloids, tritrophic interactions
We have revised Version 1 in response to the Reviewers’ comments, for which we are grateful. The major changes are as follows:
See the authors' detailed response to the review by David Baracchi
See the authors' detailed response to the review by Michael Simone-Finstrom and Margarita Lopez-Uribe
Throughout the past two decades, many wild and managed bee species have experienced severe declines (Allen-Wardell et al., 1998; Cameron et al., 2011; Potts et al., 2010). In many cases of bee decline, parasitism has been implicated as a potential cause (reviewed in Goulson et al., 2015 and Potts et al., 2010). Secondary metabolites – plant compounds that do not play a role in the plant’s primary metabolism – frequently have antimicrobial properties (Schmidt et al., 2012), and could offer a means of natural parasite control. Secondary metabolites are found in the floral nectar of many plant species (Heil, 2011).
The effects of secondary metabolites on insects, including bees and other pollinators, are context-dependent. A wide range of secondary metabolites, including terpenes, alkaloids, and phenolics, are toxic to insects (Detzel & Wink, 1993; Kumrungsee et al., 2014; Raffa et al., 1985; Singaravelan et al., 2006; Wink & Theile, 2002). Interaction with other stressors, such as infection or climatic stress, can exacerbate these toxic effects (Goulson et al., 2015; Holmstrup et al., 2010; Köhler et al., 2012a). However, under some circumstances, the antimicrobial properties of secondary metabolites can provide health benefits to infected insects. Insects have been shown to self-medicate with secondary metabolites in response to parasite infection (reviewed in Abbott, 2014). For example, Grammia incorrupta (wooly bear) caterpillars exhibited self-medication behavior in response to tachinid fly parasitism by increasing their consumption of pyrrolizidine alkaloids, which decreased the survival of unparasitized caterpillars but increased the survival of parasitized caterpillars (Singer et al., 2009).
Several recent studies have indicated that plant secondary metabolites, including those found in nectar, can benefit infected pollinators as well. Honey bees self-medicated in response to parasitism through increased foraging for resins, which are used in hive construction and have antimicrobial properties (Simone-Finstrom & Spivak, 2012), and through preferentially feeding on certain types of honey, such as sunflower honey, which reduced pathogen load (Gherman et al., 2014). Bumble bees (Bombus terrestris) infected with the intestinal parasite Crithidia bombi displayed increased preference for nicotine-containing artificial nectar, which also reduced parasite load (Baracchi et al., 2015). In another bumble bee species (B. impatiens), consumption of the alkaloid gelsemine significantly reduced C. bombi infection intensity (Manson et al., 2010), and in a separate study, four other nectar secondary compounds had significant medicinal effects, with an additional four compounds causing non-significant decreases in infection severity (Richardson et al., 2015a).
Previous studies of the effects of nectar secondary metabolites on pollinators have focused primarily on single compounds in isolation. Under natural conditions, however, pollinators would likely encounter several compounds at once, since many plant species produce multiple secondary metabolites. For example, many Nicotiana species contain both nicotine and anabasine in nectar (Adler et al., 2012), and Chelone glabra contains the iridoid glycocides aucubin and catalpol in nectar (Richardson et al., 2015b). This raises the possibility of interactions between nectar secondary metabolites.
Synergistic interactions between secondary metabolites from other plant tissues are well established. Among herbivores, the iridoid glycosides aucubin and catalpol had synergistic effects on the survival of common buckeye (Junonia coenia Hübner) caterpillars that specialize on plants with these compounds; caterpillars that consumed both iridoid glycosides had an increased rate of survival relative to caterpillars that consumed either glycoside alone (Richards et al., 2012). Amides in plants in the Piper genus had synergistic deterrent effects on herbivorous ants, while the same compounds were neutral or attractive in isolation (Dyer et al., 2003). Synergy between secondary metabolites can also alter antimicrobial effects. Carvacrol and thymol, for example, inhibited the growth of the bacterium Listeria innocua more effectively in combination than alone (García-García et al., 2011). Carvacrol was also more effective against the bacterium Vibrio cholerae when combined with cymene, although cymene alone had no antimicrobial activity (Rattanachaikunsopon & Phumkhachorn, 2010).
Antagonism between secondary metabolites has also been demonstrated. The deterrent effect of the amide piperine on the hemipteran Sibaria englemani is significantly reduced when piperine is combined with the amide piplartine, although piplartine alone had no effect on S. englemani feeding preference (Whitehead & Bowers, 2014). The linear furanocoumarins psoralen, bergapten, and xanthotoxin exhibited antagonistic interactions in their effects on insect mortality; the toxicity of psoralen combined with either or both of the other two compounds was significantly lower than would be predicted based on their toxicities in isolation (Diawara et al., 1993). If similar interactions, either synergistic or antagonistic, are present between secondary metabolites in nectar, they could exacerbate or ameliorate the effects of single compounds found in previous studies.
To evaluate interactions between secondary metabolites from the nectar of a single plant, we tested the effects of nicotine and anabasine alone and in combination on bumble bee resistance to the gut parasite Crithidia bombi. Nicotine and anabasine co-occur in the nectar of several species in the genus Nicotiana, which includes cultivated tobacco (Nicotiana tabacum) as well as several ornamental species (Adler et al., 2012). The effects of nicotine and anabasine in combination on bee disease have not previously been studied. In addition, this is the first study to our knowledge that explicitly tests for interactive effects of multiple secondary compounds on bumble bee disease.
We tested the effects of these compounds in two environmental contexts, variable and controlled conditions. Bumble bees in the wild encounter a wide range of environmental conditions, which could alter the effects of diet and parasitism. In general, temperature can decrease tolerance to environmental toxins, including secondary metabolites (Holmstrup et al., 2010), and exert unpredictable effects on insect-parasite interactions through modulation of host survival, host immune function, and parasite viability (Thomas & Blanford, 2003). Variable temperatures impose exceptional energetic costs on bumble bees by forcing them to actively regulate body temperature in order to fly (Heinrich, 1972). These costs might create caloric deficits that increase parasite virulence in Bombus (Brown et al., 2000). Alternatively, heightened energy needs could lead to increased consumption of plant foods, thereby elevating exposure to secondary metabolites. Globally, responses to environmental variability have implications for conservation: Bumble bee species with narrow climatic ranges are particularly vulnerable to decline (Williams et al., 2007; Williams et al., 2009), and projected climate change may further restrict these species’ distributions through increases in mean temperature and the frequency of extreme events (Diffenbaugh & Field, 2013).
Bombus impatiens is the most common bumble bee species in eastern North America, with a range extending from Ontario and Maine to southern Florida (Balaban et al., 2014). It is an important pollinator in agriculture, and commercial distribution of B. impatiens is becoming increasingly common (Colla et al., 2006).
Crithidia bombi is a common trypanosome parasite of bumble bees in Europe and North America (Colla et al., 2006; Lipa & Triggiani, 1988). Its range has been expanding within North America and into parts of South America, potentially due to spillover from commercial to wild bumble bee populations (Colla et al., 2006; Schmid-Hempel et al., 2014; but see Whitehorn et al., 2013). C. bombi is known to increase mortality in bumble bees under food stress conditions (Brown et al., 2000), and to reduce bumble bee foraging rate (Otterstatter & Thomson, 2006).
Nicotine is an agonist of the nicotinic acetylcholine receptor (nAChR), and therefore acts as both a stimulant drug and a toxin to many organisms (Benowitz, 1998). Nicotine is toxic to many insects, and has been historically used as an insecticide (Ujváry, 1999). Honey bees are deterred by nicotine in nectar (Köhler et al., 2012b), and both honey bees (Köhler et al., 2012b; Singaravelan et al., 2006) and bumble bees (Baracchi et al., 2015) are adversely affected by nicotine consumption when they are not infected by parasites. However, nicotine also has antimicrobial properties (Pavia et al., 2000), and recent studies have suggested that it can reduce parasite load in bumble bees infected with C. bombi (Baracchi et al., 2015; Richardson et al., 2015a), and may improve survival of diseased honey bee colonies (Köhler et al., 2012b). Anabasine, like nicotine, is a nAChR agonist, and has been used as an insecticide (MacBean, 2012). The behavioral effects of anabasine are similar to those of nicotine, although anabasine, unlike nicotine, does not have addictive effects (Caine et al., 2014). Anabasine in nectar deterred honey bees (Singaravelan et al., 2005), and reduced C. bombi load in infected bumble bees (Richardson et al., 2015a).
We inoculated bumble bees with C. bombi, and assessed the differences in pathogen load and mortality between adult bees fed nicotine (yes/no) and anabasine (yes/no) in a factorial design, resulting in four diet treatments: 2 ppm nicotine, 5 ppm anabasine, 2 ppm nicotine and 5 ppm anabasine together, or a control alkaloid-free solution. All diet treatments also contained 30% sucrose in distilled water. Chemicals ((-)-nicotine, cat. no. N3876; (+/-)-anabasine, cat. no. 284599) were purchased from Sigma-Aldrich (St. Louis, MO). Alkaloid concentrations were chosen to mimic the highest concentrations that would be found in Nicotiana nectar under natural conditions (Adler et al., 2006; Tadmor-Melamed et al., 2004).
We conducted two experiments under different environmental conditions. The first experiment (‘Variable’, conducted 26 February 2014 to 20 March 2014, Dataset 1) had a smaller sample size (n = 178 bees) and variable environmental conditions. In ‘Variable’, experimental bees and pupae were kept on the lab bench in a room where temperatures fluctuated between 10 and 35°C. This fluctuation was the result of a building steam leak. During most of the daytime when experimenters were present, the temperature was near 35°C, although on several weekends the heating system was shut down entirely for repairs, and temperatures as low as 10°C were reached. Bees in ‘Variable’ were also exposed to everyday stimuli and ambient fluorescent and window lighting (approximately 12 h photoperiod).
The second experiment (‘Stable’, conducted 20 May 2014 to 14 July 2014, Dataset 2) had a larger sample size (n = 339 bees) and carefully controlled environmental conditions (see sample sizes in Table S1). In ‘Stable’, experimental bees as well as pupae were kept in an incubator at 27°C in constant darkness to reduce mortality and more closely mimic conditions in a bumble bee hive.
Experimental bees were obtained from pupal clumps of commercial B. impatiens (Biobest, Leamington, Ontario, Canada). Pupal clumps were removed from colonies weekly and kept in 500 mL plastic containers, with each container containing the pupal clumps from a single colony that were collected on a specific date. In ‘Variable’, pupal clumps were incubated on the lab bench for the majority of the experiment until 2 d before the emergence of the final experimental bees, at which time the clumps were moved to a 30°C incubator (Percival Scientific, Perry, IA) due to excessive pre-experiment mortality under the variable lab conditions. Hence, all bees would have spent the majority of the pupation period under variable conditions. In ‘Stable’, pupal clumps were incubated at 27°C in darkness throughout the experiment. In both experiments, callow bees (newly emerged worker bees less than one day old) were collected upon emergence from pupal clumps. They were weighed and their mass at emergence, date of emergence, and colony of origin were recorded. Bees were then isolated in individual 20 mL vials. The lid of each vial was equipped with a 2 mL microcentrifuge tube with a cotton wick containing 500 μL artificial nectar (30% sucrose solution). Each day, bees were transferred to clean vials and given 500 μL fresh artificial nectar and a 10 mg piece of multifloral pollen (Koppert Biological Systems, Howell, MI) on which they fed ad libitum. For two days after emergence, bees were fed pollen and control nectar (30% sucrose solution), then were inoculated with C. bombi. They were starved for several hours to ensure that they would consume the inoculum, then fed 10 μL of C. bombi inoculum containing 6,000 C. bombi cells (see below). Following inoculation, bees were fed the appropriate nectar treatment and pollen ad libitum for 7 days. Bees were assigned systematically to secondary compound treatments in blocks of four, such that each block contained a bee in each treatment.
To inoculate experimental bees, inoculum (C. bombi cells in sucrose solution) was prepared from the gut tracts of bees taken from colonies infected with C. bombi. These colonies were obtained from the same supplier as the experimental colonies, and were infected with C. bombi from wild bees collected in Amherst, Massachusetts (September 2013). Infected bees were dissected and their gut tracts were macerated with a plastic pestle in microcentrifuge tubes containing 300 μL distilled water. Samples were incubated for 5 hours at room temperature to allow gut tissue to settle. C. bombi cell density was then assessed using a hemocytometer, and inoculum was prepared from the supernatant of the samples with sufficient concentrations of C. bombi cells. The supernatant was diluted to a concentration of 1200 cells/μL and had an equal volume of 50% sucrose solution added to result in a 25% sucrose solution. Each bee was fed 10 μL of inoculum, containing 6,000 C. bombi cells, using a 20 μL micropipette.
Seven days after inoculation, bees were dissected to assess parasite loads. Gut tracts were extracted and crushed with a pestle in microcentrifuge tubes containing 300 μL distilled water. Samples were allowed to sit for 5 hours to allow gut tissue to settle. C. bombi cell concentrations in the gut extract were measured using a hemocytometer. C. bombi cells were counted in five cells of the hemocytometer and summed (0.004 µL each; 0.02 µL total).
To determine the effects of temperature on nectar consumption, we measured 24 h nectar consumption of bees incubated at 2 different temperatures, 27 or 33°C. Bees were removed from their natal colonies and starved in snap-cap vials 2 h before the experiment began. To begin the experiment, bees were given access to a 2 mL microcentrifuge feeding tube for 24 h. The feeding tube was filled with 1 mL artificial nectar (30% w/w sucrose) and punctured in the center of the lid with a 0.8 mm diameter sewing needle to allow bees access to the nectar. The upper half of the feeding tube was then inserted into a hole punched in the vial cap. Vials were incubated on their sides at a 10° angle in constant darkness. The feeding tube was weighed at the beginning and end of the 24 h experiment to estimate nectar consumption. The average mass loss of 6 control tubes, which were incubated identically in vials without bees, was subtracted from each bee’s estimated consumption to correct for leakage and evaporation. Bees were weighed post-experiment to allow use of bee mass as a model covariate. At each temperature, we ran 3 one-day trials of 24 bees (12 from each of 2 colonies), alternating between days of 27 and 33°C.
For ‘Variable’, for which exact dates of death were not recorded, mortality was analyzed using a generalized linear mixed model with binomial error distribution (Pinheiro et al., 2015). Probability of death was used as the response variable with nicotine treatment, anabasine treatment, and their interaction as predictor variables. Bee colony was included as a fixed predictor, and date of inoculation was included as a random factor. Wald tests (Lesnoff & Lancelot, 2012) were used to test the marginal significance of individual predictor variables (see Supplementary material script 1). Mortality data for ‘Stable’, in which we recorded time from inoculation to death to the nearest day, were analyzed using a Cox proportional hazards mixed-effects model (Therneau, 2015). Death hazard rate was used as the response variable; nicotine, anabasine, and their interaction as predictor variables; colony as a fixed predictor; and date of inoculation as a random factor (see Supplementary material script 3). To facilitate direct comparison between results of ‘Variable’ and ‘Stable’, the results of ‘Stable’ were also analyzed with the same binomial mixed model used for the ‘Variable’ experiment.
Parasite counts were found to best fit the log-normal distribution and were analyzed using generalized linear mixed models (Bates et al., 2015) with penalized quasi-likelihood parameter estimation (Venables & Ripley, 2002). Parasite counts were (x+1)-transformed for use as the response variable. Nicotine, anabasine, and their interaction were used as predictor variables. Bee colony was included as a fixed predictor, mass (at emergence from pupations) as a model covariate, and date of inoculation as a random factor. Marginal significance of individual terms was evaluated using Wald tests (Lesnoff & Lancelot, 2012). Code for analysis is given in Supplementary material script 2 (‘Variable’ experiment) and Supplementary material script 4 (‘Stable’ experiment).
In variable temperature conditions, the nicotine treatment significantly increased mortality (Table 1). Nearly half of bees fed nicotine-containing nectar died within 7 days of inoculation, which was nearly double the frequency of death in treatments without nicotine (Figure 1). Anabasine did not affect mortality, and there was no significant interaction between the two alkaloid treatments (Figure 1, Table 1).
Table shows binomial mixed model results of χ2 tests for effects of predictor variables on probability of death during the 7 d experiment.
| Source | χ2 | Df | P |
|---|---|---|---|
| Nicotine | 4.1749 | 1 | 0.041 |
| Anabasine | 0.0374 | 1 | 0.85 |
| Nicotine*Anabasine | 0.0256 | 1 | 0.87 |
| Colony | 0.911 | 4 | 0.92 |
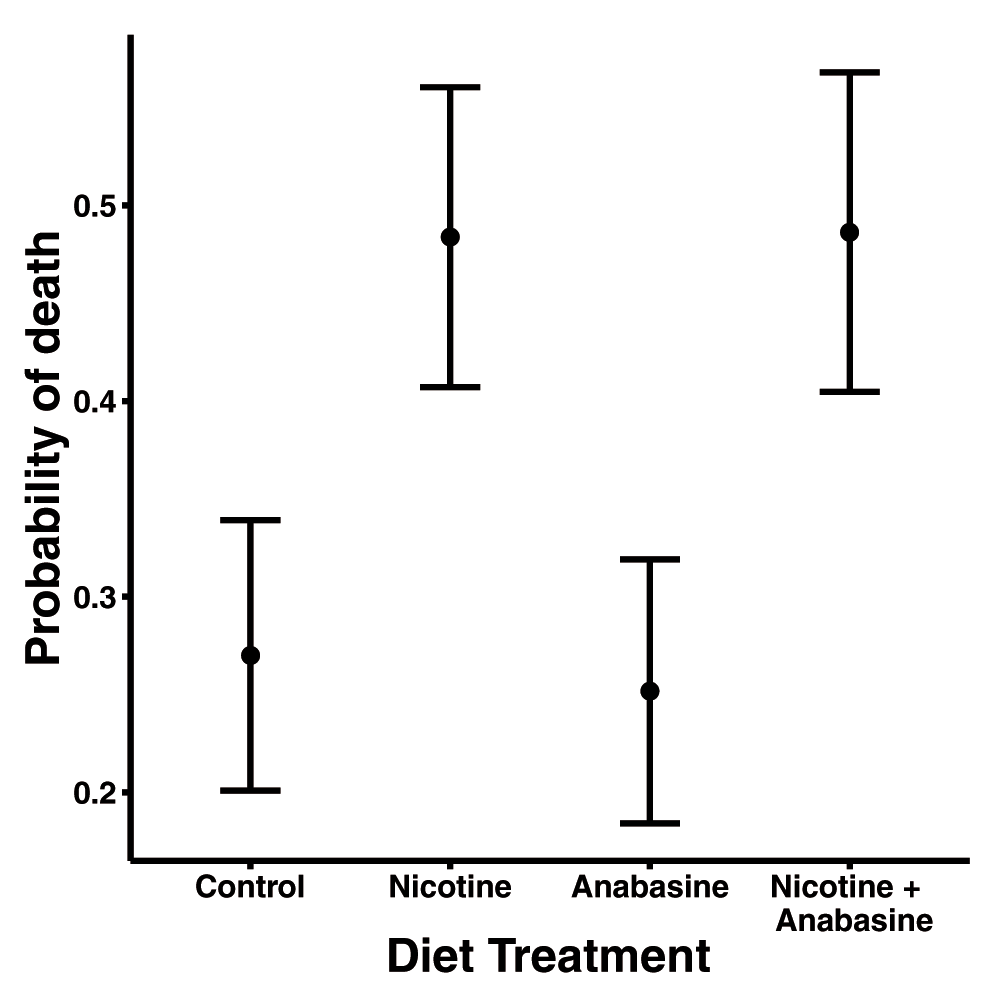
Points show adjusted mean probability of death in each treatment group. Error bars represent ±1 standard error. Sample sizes: n=45 (Control), n=46 (Nicotine), n=46 (Anabasine), n=41 (Nicotine + Anabasine).
Nicotine (linear model β = -1.01 ± 0.295 standard error) and anabasine (β = -0.94 ± 0.31 S.E.) each significantly decreased parasite loads. However, nicotine and anabasine displayed antagonistic effects (Nicotine * Anabasine β = 1.96 ± 0.44 S.E.), such that bees consuming both alkaloids did not realize the medicinal effects of either compound (Figure 2, Table 2). Parasite load had a significant negative relationship with bee mass (β = -11.17 ± 3.60 S.E., Table 2), indicating that larger bees had lower parasite loads after controlling for other factors.
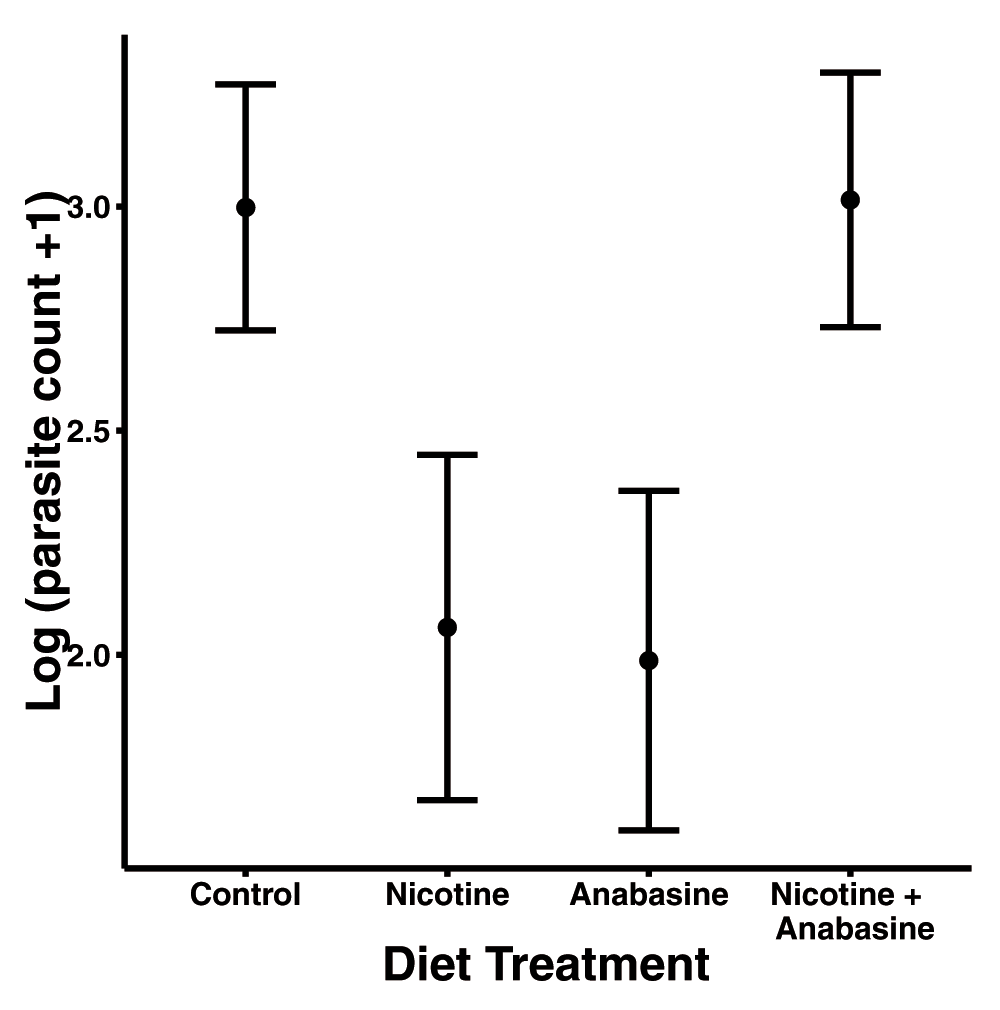
Points show adjusted mean parasite count in each treatment group. Error bars represent ±1 standard error. Sample sizes: n=31 (Control), n=24 (Nicotine), n=33 (Anabasine), n=20 (Nicotine + Anabasine).
Results of Wald tests for marginal significance of terms in a generalized linear mixed model with penalized quasi-likelihood parameter estimation. “Mass” refers to bee mass at time of emergence.
| Source | χ2 | Df | P |
|---|---|---|---|
| Nicotine | 10.054 | 1 | 0.0025 |
| Anabasine | 12.843 | 1 | <0.001 |
| Nicotine*Anabasine | 22.045 | 1 | <0.001 |
| Colony | 15.48 | 4 | 0.0038 |
| Mass | 10.517 | 1 | 0.0012 |
Under controlled conditions (27°C with constant darkness), neither alkaloid nor their interaction significantly affected mortality (Figure 3, Table 3, Table S2). Although the death hazard ratio was lowest in the Control group (estimate = 0.82, 95% CI = (0.56, 1.19)) and highest in the group that received both alkaloids (estimate = 1.19, 95% CI = (0.90, 1.78)), this difference did not approach statistical significance (pairwise comparison: z = 1.49, P = 0.45). However, nicotine significantly increased parasite loads (β = 0.28 ± 0.12 S.E., Table 4), while the effects of anabasine (β = 0.20 ± 0.12 S.E.) were also positive but not significant (Figure 4, Table 4). This was the opposite result of that observed in ‘Variable’, in which alkaloid ingestion decreased the severity of Crithidia infection. Although much weaker than in ‘Variable’, we found the same pattern of antagonistic interaction between the two alkaloids (Nicotine * Anabasine β = -0.26 ± 0.16 S.E., Figure 4), indicating that the deleterious effects of each compound were reduced in bees consuming the nicotine + anabasine combination (Figure 4). However, this interaction was not statistically significant (Table 4). Overall parasite loads in ‘Stable’ were much higher, with median parasite counts (37.5 cells * 0.02 µL-1) more than triple those observed in ‘Variable’ (11.0 cells * 0.02 µL-1). As in ‘Variable’, there was a significant negative relationship between mass at emergence and parasite count (β = -3.41 ± 1.32 S.E., Table 4). Average bee mass in ‘Stable’ (121 mg) was significantly lower than in ‘Variable’ (160 mg) (t(290) = -9.17, P<0.001), possibly reflecting elevated pre-experiment mortality of smaller bees under the fluctuating conditions of ‘Variable’. These bees might have been more likely to die during pupation or prior to inoculation.

Lines show survival curves for bees each treatment group. There were no significant effects of diet treatments on survival. Sample sizes: n=83 (Control), n=79 (Nicotine), n=91 (Anabasine), n=86 (Nicotine + Anabasine).
Table shows marginal significance of individual terms in Cox proportional hazards test for effects of predictor variables on mortality hazard rate.
| Source | χ2 | Df | P |
|---|---|---|---|
| Nicotine | 0.14 | 1 | 0.71 |
| Anabasine | 0.21 | 1 | 0.65 |
| Nicotine*Anabasine | 0.19 | 1 | 0.66 |
| Colony | 7.6 | 3 | 0.054 |
Results of Wald tests for marginal significance of terms in a generalized linear mixed model with penalized quasi-likelihood parameter estimation. “Mass” refers to bee mass at time of emergence.
| Source | χ2 | Df | P |
|---|---|---|---|
| Nicotine | 5.84 | 1 | 0.026 |
| Anabasine | 2.78 | 1 | 0.095 |
| Nicotine*Anabasine | 2.59 | 1 | 0.11 |
| Colony | 6.76 | 3 | 0.080 |
| Mass | 6.91 | 1 | 0.0086 |
Contrary to our expectation that increased temperature would increase nectar consumption, bees drank significantly less when incubated at 33°C than at 27°C (Table 5). Mass-adjusted mean consumption at 33°C (117 mg) was less than half that at 27°C (256 mg) (Figure 5). Bee mass was also a significant predictor of consumption, but colony of origin was not (Table 5).
| Source | SS | Df | F | P |
|---|---|---|---|---|
| Temperature | 0.64 | 1 | 64.04 | <0.001 |
| Colony | 0.01 | 1 | 0.98 | 0.33 |
| Mass | 0.24 | 1 | 23.61 | <0.001 |
| Residuals | 1.38 | 138 |

Points show adjusted mean nectar consumption at each incubation temperature. Error bars represent ±1 standard error. Sample sizes: n=69 (27°C), n=74 (33°C).
Nicotine consumption increased mortality in ‘Variable’, but did not affect mortality in ‘Stable’. The climatic differences between the two experiments may be responsible for this context-dependent response. In ‘Variable’, the incubation temperature of the experimental bees was not controlled: Experimental bees, pupal clumps, and colonies were kept in a room with temperatures that ranged from 10 to 35°C, with the temperature usually at the high end of that range during the day. The stressful nature of the conditions was evidenced the overall higher masses of bees in ‘Variable’, which we believe reflects pre-experiment mortality of many smaller and weaker bees. In ‘Stable’, by contrast, bees were incubated at a constant temperature of 27°C.
We hypothesize that the toxic effects of nicotine in the ‘Variable’ experiment may have been exacerbated by exposure to heat and fluctuating temperatures. Interaction between heat stress and secondary metabolites has been documented in several other species (reviewed in Holmstrup et al., 2010), including some insects and related arthropods. For example, Li et al. (2014) found synergistic interaction between heat stress and avermectin toxicity in the western flower thrips (Frankliniella occidentalis), which led to reduced survival and increased upregulation of heat shock proteins. Mercury exposure reduced heat tolerance in springtails (Folmosia candidia) (Slotsbo et al., 2009), and high temperature increased uptake and toxicity of organophosphate insecticides to the midge Chironomus tentans (Lydy et al., 1999). Our results suggest that interaction between heat stress and toxins may occur in B. impatiens as well. An experiment in which temperature and secondary metabolite consumption are manipulated in a factorial design would more definitively test for such interaction.
Our results indicate that nicotine can be toxic to bumble bees even at very low concentrations when bees are parasitized. Another recent study (Baracchi et al., 2015) also found that chronic consumption of low concentrations (2.5 ppm) of nicotine increased mortality in uninfected B. terrestris, although not in those infected with Crithidia. These findings contrast with previous studies of honey bees, which did not find significant effects of naturally occurring nicotine concentrations on mortality in bees of unknown parasite status. Detzel & Wink (1993) determined the honey bee LD50 for nicotine to be 2000 ppm, far higher than any concentration that occurs in nectar. Singaravelan et al. (2006) found that larval survival of honey bees was not affected by naturally occurring concentrations of nicotine (up to 5 ppm), even when consumed consistently for several days, although a much higher concentration of nicotine (50 ppm) did significantly reduce survival. The discrepancy between their results and ours may be due to bumble bees having a greater sensitivity to nicotine than do honey bees. Further study is needed to compare the tolerance of a range of pollinator species to secondary metabolites consumed alone and in combination.
Nicotine had stronger effects on mortality in the ‘Variable’ than in the ‘Stable’ experiment. This result suggests that sensitivity of bumble bees to nicotine is further increased under temperature-stressed conditions that are nonetheless realistic in many habitats. Drastic temperature variation similar to that experienced by bees in ‘Variable’ is common in continental climates, where bumble bees are abundant. For example, in Amherst, MA, where this study was conducted, daily temperature swings of over 15°C are common, and temperatures as low as 10°C and as high as 30°C are frequently experienced within a few days of each other, or even within a single day (Menne et al., 2012a; Menne et al., 2012b). Wild bees, therefore, are likely to experience temperature conditions under which nicotine could be significantly toxic.
To our surprise, the ‘Variable’ conditions under which nicotine consumption increased mortality were also the conditions under which alkaloid consumption reduced parasitism. In ‘Variable’, bees that consumed either alkaloid alone had significantly lower parasite counts than control bees; in contrast, bees that consumed both alkaloids had similar parasite counts to controls. Our results are consistent with the results of recent studies that found reduced parasite loads under nicotine and anabasine consumption (Baracchi et al., 2015; Richardson et al., 2015a). The reduction in parasite load may be due to alkaloid-induced increases in gut motility. Both nicotine and anabasine have been demonstrated to reduce gut transit time in the Palestinian sunbird Nectarinia osea (Tadmor-Melamed et al., 2004). Although their effect on gut transit time in insects has not been studied, rapid excretion is known to be part of some insects’ physiological response to alkaloids (Wink & Theile, 2002). It is therefore plausible that consumption of nicotine and anabasine could cause an increased rate of excretion in bees, thus clearing C. bombi cells from the gut and leading to the observed reduction in parasite load.
The lack of effect of the combined alkaloids on parasite load is more puzzling. The concentrations of the individual alkaloids may have been within the medicinal window of concentration at which antiparasitic effects were dominant. However, the combined effects of both alkaloids may have weakened bees’ ability to fight infection through excessive stimulatory, laxative, and/or immunosuppressive effects. These combined toxic effects could have offset the medicinal effects realized at lower concentrations in the single-alkaloid treatments.
When bees were kept under constant conditions in ‘Stable’, alkaloid ingestion had different effects on mortality and parasitism from those found in ‘Variable’. In contrast with the antiparasitic effects of alkaloids in ‘Variable’, in ‘Stable’, nicotine consumption significantly increased parasite counts, while anabasine also increased parasite loads, although not significantly. This result is consistent with a growing body of research demonstrating that neonicotinoids, a class of insecticides chemically similar to nicotine, have immunosuppressant effects on bees (reviewed in Goulson et al., 2015). While the effects of nicotine are not necessarily the same as those of neonicotinoids, both nicotine and neonicotinoids function as nAChR agonists, (Jeschke et al., 2011), suggesting similar pharmacological activity.
The immunosuppressant effects of neonicotinoids have been most well studied in honey bees. Under lab conditions, sub-lethal colony-level exposure to imidacloprid increased levels of Nosema infection (Pettis et al., 2012). In the field, colonies that had foraged on corn treated with thiabendazole had significantly elevated levels of black queen cell virus and Varroa mites (Alburaki et al., 2015). At the molecular level, clothianidin and imidacloprid induced increased transcription of a gene coding for a negative modulator of NF-Kβ immune signaling in honey bees, causing decreased immune function and increased viral replication (Di Prisco et al., 2013). Additional studies on bumble bees are needed to compare the effects of neonicotinoids and naturally occurring alkaloids, as well as to evaluate how the effects of these compounds vary across species, genera, and dietary contexts.
We hypothesized that the stronger medicinal and toxic effects of the alkaloids in ‘Variable’ may have resulted from increased total alkaloid consumption due to elevated nectar intake under the generally hotter conditions of ‘Variable’. However, our Consumption experiment showed that increasing incubator temperature from that used in ‘Stable’ (27°C) to one characteristic of ‘Variable’ (33°C) significantly decreased the volume of nectar consumed. Hence, it appears that the warmer temperatures of ‘Variable’ would have curtailed nectar consumption rather than promoting it. Still, we cannot exclude the possibility that warmth-related decreases in consumption would have been offset by other factors that promoted nectar intake, including increased activity, energy expenditure, and stress-related defecation in the comparatively stimulating experimental environment. In addition, the Consumption experiment used uninfected bees, whereas bees in both ‘Variable’ and ‘Stable’ were inoculated with parasites. Further investigation is needed to define how the thermal environment, external stimuli, nectar alkaloid content, and infection interact to influence patterns of consumption.
The stronger effects of alkaloids in ‘Variable’ may therefore reflect complex interactions between alkaloids, temperature stress, environmental stimuli, and immunity rather than simple differences in alkaloid consumption. Under the fluctuating conditions of ‘Variable’, bees may have been more susceptible to the effects of the alkaloids, both in the form of increased toxicity and increased gut motility, accounting for both the higher mortality and decreased C. bombi counts in ‘Variable’. For example, the hot conditions of ‘Variable’ would appear to have increased evaporative moisture losses while decreasing nectar intake, leaving bees more susceptible to dehydration. Nicotine's agonistic effects on intestinal peristalsis may have helped to clear parasites from the gut, but also have contributed to further dehydration. In addition, bees in ‘Variable’ were exposed to external stimuli in the lab environment, including light and vibration, which may have synergized with stimulatory effects of the alkaloids to increase defecation. In the lab, bees often defecate explosively when startled—sometimes tens of centimeters into the air (ECPY, personal observation). Bees on the lab bench in ‘Variable’ would have been agitated more frequently than bees in the incubator in ‘Stable’, and their sensitivity to agitation may have been increased by alkaloid consumption.
The higher temperatures of ‘Variable’ may have additionally functioned as an externally imposed fever that reversed the immunosuppressive effects of nicotine. Febrile amelioration of infection has been shown in many animals (reviewed in Kluger, 1978), including honey bees (Campbell et al., 2010) and other insects (Stahlschmidt & Adamo, 2013). The lower absolute parasite counts relative to ‘Stable’ may reflect heat-related inhibition of C. bombi, which grows best at 27°C (Salathé et al., 2012). Stimulatory effects of nicotine and anabasine, enhanced by exposure to everyday disturbance in ‘Variable’, could have increased activity level and metabolic rate, thereby further raising body temperature and slowing parasite growth, albeit at the expense of increasing heat stress on bees. The effects of a given increase in body temperature would have been more pronounced under the hot conditions of ‘Variable’, which may have approached the parasite’s thermal tolerance limit.
Our results in ‘Stable’ contrast with those of a recent study (Baracchi et al., 2015), which showed that nicotine consumption reduced parasite loads in Bombus. There were a number of differences between that study and ours that may explain the differing results. First, the two experiments used different bee species: Baracchi and colleagues used the larger B. terrestris, which may be more resistant to potential immunosuppressive effects of nicotine. Even within our experiments, which used bees of a single species from a single supplier, we found highly significant effects of mass and colony, indicating strong effects of both size and genetic variation on parasitism that are consistent with previous findings (Otterstatter & Thompson, 2006). Interspecific variability could be tested by comparing effects of secondary metabolites on parasitism in a range of Bombus species. Second, Baracchi et al. employed different bee housing materials. Bees were housed in petri dishes, which may have been less confining than our snap-cap vials, as suggested by the overall lower rates of mortality in the experiments of Baracchi et al. compared to ours. This extra space could have allowed bees to take advantage of alkaloid-induced increases in gut motility by expelling Crithidia-rich feces in less-frequented parts of their arenas, whereas our bees would have had close and persistent contact with their own waste. It would be intriguing to investigate how the effects of alkaloids differ depending on levels of crowding, including in densely populated hive environments. A third explanation could be that the antiparasitic effects of alkaloids depend on other environmental cues, including daylight. Both our study and that of Baracchi et al. showed medicinal effects of nicotine when bees were housed on the lab bench (as in ‘Variable’), whereas nicotine increased parasitism for bees housed in constant darkness in ‘Stable’. Future experiments are needed to explicitly test the roles of environmental conditions and stimuli on bee and parasite tolerance to alkaloids.
Our results also contrast with those of Richardson et al. (2015a), which employed the same bee species and unlit rearing conditions as our ‘Stable’ experiment, but found that both nicotine and anabasine significantly reduced C. bombi parasite load in B. impatiens. The differing effects of nicotine may be due to our use of the (-)-enantiomer of nicotine, whereas Richardson et al. (2015a) used a +/- enantiomeric mixture (Sigma N0267, personal communications). (-)-Nicotine, which is far more common in nature (Armstrong et al., 1998), appears to be more pharmacologically active than (+)-nicotine in vertebrates, aquatic invertebrates (Barlow & Hamilton, 1965; Gause & Smaragdova, 1939), and insects including honey bees (Tomizawa & Yamamoto, 1992). Interestingly, Gause & Smaragdova (1939) found the two enantiomers to be isotoxic to protozoa. If (-)-nicotine is more toxic to bees than is (+)-nicotine, but both enantiomers are equally toxic to C. bombi, then (-)-nicotine might have lesser medicinal value.
Another possible explanation for our differing results relates to the C. bombi itself. C. bombi is known to be genetically diverse; Salathé & Schmid-Hempel (2011) identified 213 strains infecting bumble bees in Switzerland. Multiple strains are often present in a single host. Tognazzo et al. (2012) found that 67% of infected workers and 54% of infected queens carried mixed-genotype infections, with queens harboring up to 29 different genotypes. In addition, it is possible that not all supposed C. bombi infections in fact represent a single Crithidia species. Schmid-Hempel & Tognazzo (2010) identified two genetically and morphologically distinct lineages within the C. bombi complex, which they classified as cryptic species. They retained the name C. bombi for the lineage which more closely matches Lipa & Triggiani’s (1988) original description of C. bombi, and proposed the name C. expoeki for the other lineage. Both lineages are present in both Europe and North America, suggesting an old divergence. If our C. bombi cultures and those used by Richardson et al. (2015a) and (Baracchi et al., 2015) represent different strains, or different species, it is possible that they vary in alkaloid tolerance. In addition, these strains might vary in virulence and pathogenicity. The medicinal effects of alkaloids in ‘Variable’, where absolute parasite loads were lower overall, suggests that alkaloid ingestion might be most therapeutic against relatively mild infections.
Our results represent an important first step towards understanding the interactive effects of multiple secondary metabolites on pollinators. We did not find evidence for synergy between Nicotiana nectar alkaloids, although we did find some evidence for antagonism. To elucidate the potential role of interactions between compounds in the plant-pollinator-parasite system, it will be necessary to test for interactions between other sets of compounds. Within Nicotiana, the wild tobacco N. attenuata contains at least 35 nectar secondary compounds, including sesquiterpenes (Kessler & Baldwin, 2007); many terpenoids have potent trypanocidal activity, yet are relatively benign to animal cells (Otoguro et al., 2011). Among other plant families, Asclepias species are pollinated by bumble bees and contain several cardenolides in their nectar (Manson et al., 2012) that could be tested for interactive effects. Another plant species to investigate is Chelone glabra, which has high concentrations of the iridoid glycosides aucubin and catalpol in its nectar (Richardson et al., 2015b). Synergy between these glycosides has been demonstrated in their effect on Junonia coenia caterpillars (Richards et al., 2012).
Overall, the effects of nectar alkaloids on parasitized pollinators may represent a tradeoff between toxicity to the parasite and toxicity to the host. In the case of nicotine, bees appear to be more sensitive to alkaloid toxicity than parasites are. While nicotine inhibits the growth of many microbial pathogens, significant antimicrobial effects require concentrations between 100 and 250 ppm (Pavia et al., 2000). By contrast, Singaravelan et al. (2006) found that nicotine was toxic to bees at 50 ppm, and our own results suggest that nicotine can have toxic effects at concentrations as low as 2 ppm. However, the studies establishing toxicity of nicotine in bees have all focused on chronic consumption of a diet high in nicotine. In nature, bumble bees are generalist pollinators, and are known to forage on several plant species within a narrow time frame and even within a single foraging trip (Free, 1970). In landscapes with varied floral resources, they might be able to avoid chronic nicotine toxicity by exploiting a range of plant species.
Our results emphasize the importance of interactions between stressors in pollinator health, and demonstrate that the effect of any single factor can vary greatly depending on the other factors involved. Research on pollinator health often focuses on single factors in isolation; however, in natural conditions, pollinators are often exposed to several stressors simultaneously (Goulson et al., 2015). Previous research has demonstrated both medicinal and toxic effects of secondary metabolites such as nicotine and anabasine. Our results suggest that the predominant effect can vary with environmental and dietary context. In order to better elucidate the role of secondary metabolites in pollinator health, future research should explicitly address the role of these complex interactions.
F1000Research: Dataset 1. Data for ‘Variable’ experiment, 10.5256/f1000research.6870.d101937 (Thorburn et al., 2015a).
F1000Research: Dataset 2. Data for ‘Stable’ experiment, 10.5256/f1000research.6870.d101940 (Thorburn et al., 2015b).
F1000Research: Dataset 3. Data for Consumption experiment, 10.5256/f1000research.6870.d109062 (Thorburn et al., 2015c).
All authors conceived the study. ECPY and LPT designed and conducted the experiments using methods developed by REI. ECPY analyzed the data. LPT and ECPY prepared the first draft of the manuscript. All authors revised the manuscript and have agreed upon the final content.
This research was funded by the National Science Foundation under NSFDEB-1258096, NSF GRFP (Grant DGE-0907995 to ECPY), and NSF DDIG (Grant NSFDEB-1501907 to ECPY and LSA); by the National Research Initiative (NRI) Arthropod and Nematode Biology and Management Program of the USDA Cooperative State Research, Education, and Extension Service (CSREES) Grant no. USDA-AFRI 2013-02536; and by the Garden Club of America Centennial Pollinator Fellowship (ECPY).
I confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors are grateful to Biobest for supplying bee colonies; and to Ali Hogeboom, Melissa Ha, Caitlin McAllister, and lab assistants for help with ‘Stable’ experiment. We thank our reviewers Drs David Baracchi, Michael Simone-Finstrom, and Margarita Lopez-Uribe for their improvement of the paper.
Script 1. R script for analysis of C. bombi parasite loads in ‘Variable’ experiment.
Click here to access the data.
http://dx.doi.org/10.5256/f1000research.6870.s101933
Script 2. R script for analysis of mortality in ‘Variable’ experiment.
Click here to access the data.
http://dx.doi.org/10.5256/f1000research.6870.s101934
Script 3. R script for analysis of C. bombi parasite loads in ‘Stable’ experiment.
Click here to access the data.
http://dx.doi.org/10.5256/f1000research.6870.s101935
Script 4. R script for analysis of mortality in ‘Stable’ experiment.
Click here to access the data.
http://dx.doi.org/10.5256/f1000research.6870.s101936
Script 5. R script for analysis of ‘Consumption experiment’.
Click here to access the data.
http://dx.doi.org/10.5256/f1000research.6870.s109063
| Treatment | Experiment | |
|---|---|---|
| Variable | Stable | |
| Control | 45 | 83 |
| Nicotine | 46 | 79 |
| Anabasine | 46 | 91 |
| Nicotine + Anabasine | 41 | 86 |
| Total | 178 | 339 |
Table shows binomial mixed model results of χ2 tests for effects of predictor variables on probability of death during the 7 d experiment.
| Source | χ2 | Df | P |
|---|---|---|---|
| Nicotine | 0.117 | 1 | 0.7323 |
| Anabasine | 0.0444 | 1 | 0.8331 |
| Nicotine*Anabasine | 0.5456 | 1 | 0.4601 |
| Colony | 6.8536 | 3 | 0.0767 |
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
References
1. Baracchi D, Brown MJ, Chittka L: Behavioural evidence for self-medication in bumblebees?. F1000Res. 2015; 4: 73 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Competing Interests: I am one of the authors of a paper that is cited prominently by Thorburn et al.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 16 Dec 15 |
read | read |
|
Version 1 21 Sep 15 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)