Keywords
Hypoxia, Critical care, Sherpa, High altitude, Microcirculation, Nitric Oxide, Mitochondria
This article is included in the UCL Child Health gateway.
This article is included in the University College London collection.
This article is included in the Sports cardiology collection.
Hypoxia, Critical care, Sherpa, High altitude, Microcirculation, Nitric Oxide, Mitochondria
A wide range of pathologies can lead to deterioration of a patient such that they require admission to a critical care unit. Critically ill patients are therefore a heterogeneous group of severely ill individuals, in whom hypoxaemia (a lack of oxygen in arterial blood) is common and may subsequently lead to cellular hypoxia1. The resulting cellular dysfunction may cause organ damage, and in some cases death2. Traditional management of established critical illness, based on efforts to increase convective oxygen delivery through augmented cardiac output, haemoglobin concentration and oxygenation, does not appear to improve outcomes3. Additionally excessive oxygen therapy may cause harm4,5. Consequently the role of alternative potential therapeutic targets such as the microcirculation, mitochondrial activity and nitric oxide (NO) are becoming increasingly more popular as alternative strategies6–9. Permissive hypoxaemia has also been proposed as a viable option for critically ill patients, to avoid the harms associated with invasive methods of restoring normoxaemia in the critically ill10.
Understanding the mechanisms of human hypoxic adaptation in the context of critical illness is difficult. The wide range of underlying diseases and the complexity of treatment interactions with physiology make structured experiments challenging. The study of human responses to hypoxia occurring as a consequence of hypobaria at high altitude may be used as an alternative method of exploring elements of the pathophysiology of critical illness1,11,12. Studying healthy individuals progressively exposed to hypobaric hypoxia defines beneficial adaptive responses and may identify candidate pathways in the critically ill. Animal models, often proposed as being a suitable alternative to large-scale field studies, fail to match the complexity of human physiology in the critically ill patient13, and discordance between multiple models has been described14.
In 2007, the University College London (UCL) Centre for Altitude, Space and Extreme Environment Medicine (CASE Medicine) conducted the largest study of human volunteers at high altitude, Caudwell Xtreme Everest (CXE)12,15. Resulting data have emphasized the need for studying the microcirculation16–18, NO formation and metabolism19, and mitochondrial biology20–22. A further research programme (Xtreme Everest 2 (XE2)) was therefore proposed to address this23. XE2 was designed to study the physiological (especially microcirculatory, mitochondrial, NO-related metabolic and epigenetic) responses to hypobaric hypoxia in native lowlanders, and compare them to those in Sherpas23. Sherpas are descended from an ancestral high altitude population resident on the Tibetan plateau for over 500 generations24. Such high altitude populations show evidence of genetic selection25–29 that may underpin their anecdotally reported extraordinary tolerance to hypoxia. Their phenotype may therefore hold the key to successful hypoxic adaptation in humans.
We describe the design and conduct of XE2, our approach to high altitude translational research, and discuss the strengths and weaknesses of this programme of investigation.
XE2 (December 2012 to May 2013) was undertaken by the Xtreme Everest Oxygen Research Consortium (XE-ORC), which comprises a partnership between the UK’s UCL CASE Medicine, the University of Southampton Centre for Human Integrative Physiology (CHiP), and Duke University Medical Centre (DUMC) in the USA.
The study design, risk management plan and protocols were approved (in accordance with the declaration of Helsinki) both by the UCL Research Ethics Committee and the Nepal Health Research Council (NHRC).
All potential participants, recruited through word of mouth and advertisement, were given written information about the study. Our Nepali Research Leader (RKBC) translated this locally in Nepal. Opportunities for questions, in person or over the telephone, were offered at multiple stages in both countries, and all participants submitted written consent for participation in the studies. At all stages throughout the expedition, independent translators were present to allow for communication between Sherpas and investigators.
Eligible participants were lowland children aged 8 to 17 years, or adults (aged 18 years or above) of either lowland or Sherpa origin. For the lowland participants, an independent expert experienced in mountain medicine identified those fit to travel to altitude by reviewing a health-questionnaire (supplementary material) and telephone interviews as required. The forms of those selected were then screened by the expedition Chief Medical Officer (GGK) to confirm fitness both to travel to altitude, and to participate in research. Potential participants considered ‘at risk’ were either telephoned or reviewed in person by GGK. Where appropriate, and with permission, the participant’s medical practitioner was contacted. Those with significant cardiac or respiratory disease (e.g. severe chronic obstructive airway disease, ischemic heart disease with angina, or symptomatic heart failure) were excluded. For the Sherpa subjects, two doctors performed a pre-recruitment screening interview in Nepali based on the health-questionnaire. One doctor was a local Nepali (RKBC) and one an expert experienced in mountain medicine and XE2 investigator (DL/MG) who confirmed fitness for travel to altitude and research. As all participants would be undergoing cardiopulmonary exercise testing (CPET), additional exclusion criteria based on the American Thoracic Society/American College of Chest Physicians guidelines for clinical exercise testing were also adhered to30.
At each laboratory, an independent Medical Officer (MO) was responsible for decisions relating to the participants involvement with research protocols, the administration of medication and ascent or descent from their current altitude. Participants were recommended not to self-medicate, but to consult either the site MO, or their trek leader when between laboratory sites. In order to standardize medical care, clear guidelines for common altitude and non-altitude related conditions were formulated prior to departure, and adhered to. Medication use was recorded by the individual (in a standardized daily diary) and by the trek leader or MO.
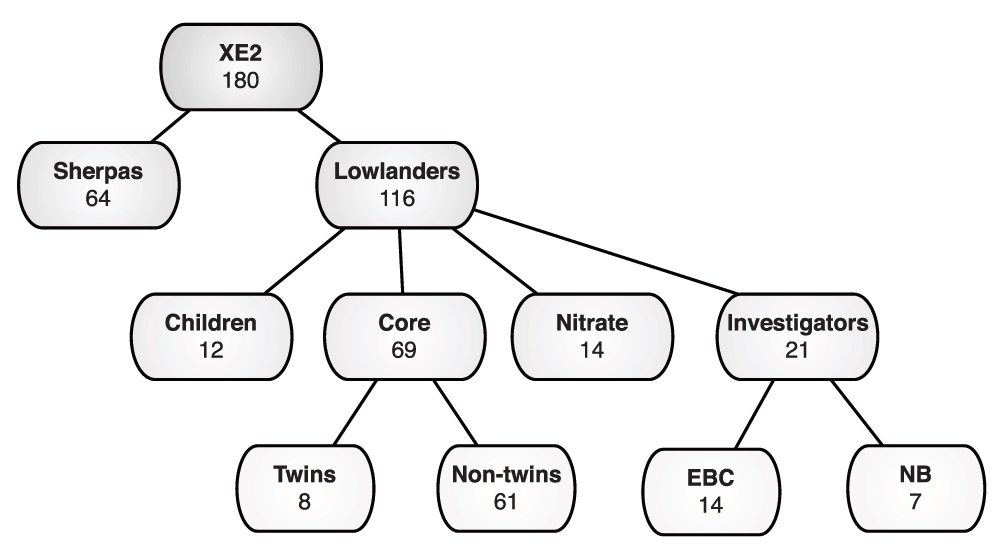
In total, 187 participants were selected for inclusion in the study and underwent baseline testing. Sherpas were defined as direct descendants (for two generations) of Nepali Sherpas, drawn from communities in the Solukhumbu and Rowaling valleys. Sherpa participants were recruited by word of mouth through local community contacts and were required to have evidence of two generations of all Sherpa ancestors (parents and grand-parents). A detailed altitude history was then taken from all Sherpa participants including their altitude in utero, at birth, during childhood, in adulthood, and for the 12 months preceding XE2. Lowlanders were recruited in the UK; all were born and lived below 1000m, and were not from a native high altitude population (e.g. Tibetan, Andean, Ethiopian). They included European, American and South African participants. Lowlanders were divided into four cohorts:
Core - eligible for all studies; eight of these participants were monozygotic twins (four pairs), for a pilot epigenetics study.
Nitrate metabolism - allocated only to be involved in a study of whole body NO production.
Investigators - who conducted the studies and remained at altitude for the duration of the expedition permitting studies of exposure to chronic hypoxia.
Children - who took part in the Young Everest 2 Study (YES2) expedition linked to XE2 in which children ascended to Namche Bazaar (NB) to participate in a programme of non-invasive studies. YES2 will not be discussed further in this manuscript.
Potential investigators were sourced through word of mouth at CASE Medicine events and interviewed prior to their appointment (EGK, DM, KM).
All participants were required to provide baseline information that included details of previous altitude exposure and the occurrence of any altitude-related illnesses. Importantly, within the Core and Investigator cohorts, some selected participants had previously taken part in CXE (2007), thereby allowing the evaluation of consistency in the individual response to an identical high altitude exposure.
Of the 187 participants that were selected for the study and underwent baseline testing, 180 departed for high altitude investigations (Figure 2). Of the seven participants that withdrew from the study prior to ascent to altitude, six lowlanders withdrew in London (LDN) (three for medical reasons), and one Sherpa withdrew in Kathmandu (KTM). Additionally, in LDN one person did not meet the American Thoracic Society/American College of Chest Physicians guidelines for clinical exercise testing, and was withdrawn from CPET prior to departure. Baseline characteristics for each cohort are listed in Table 1.
Of the 104 lowlanders (children excluded), and 64 Sherpas leaving KTM, 102 (98%) and 64 (100%) reached Everest base camp (EBC) respectively. Of the two who did not reach EBC, one dropped out at NB, and the other at Pheriche due to gastrointestinal and cardiovascular problems respectively. All the Investigators reached their allocated laboratories, however, one left EBC early for medical reasons, and two for personal reasons. Two investigators left NB early for personal reasons, and one investigator left NB early to move to EBC. One investigator also arrived late at EBC.
Baseline ‘normoxic’ (35m) data for lowlander Core and Investigator groups were collected at The London Clinic Hospital, from 3rd December 2012 to 25th January 2013. Sherpa baseline testing was performed in KTM (1300m; 4th March to 16th April 2013). Between baseline testing and trek ascent, participants remained below 3000m in order to avoid hypoxic exposure prior to the expedition.
Participants trekked in groups of up to 14. All lowlanders flew to KTM and spent one night there prior to flying to Lukla (2800m). Similarly, Sherpas flew from KTM to Lukla at the beginning of the trek. In order to ensure that study participants were exposed to an identical pattern of hypoxia exposure, all participants followed an identical ascent and descent profile (Figure 1). High altitude field laboratories were situated at NB (3500m) and EBC (5300m). The KTM laboratory was also used for descent testing for participants following their return from EBC. Barometric pressure, temperature, and humidity data were recorded twice daily at each laboratory and are summarized in Table 2.
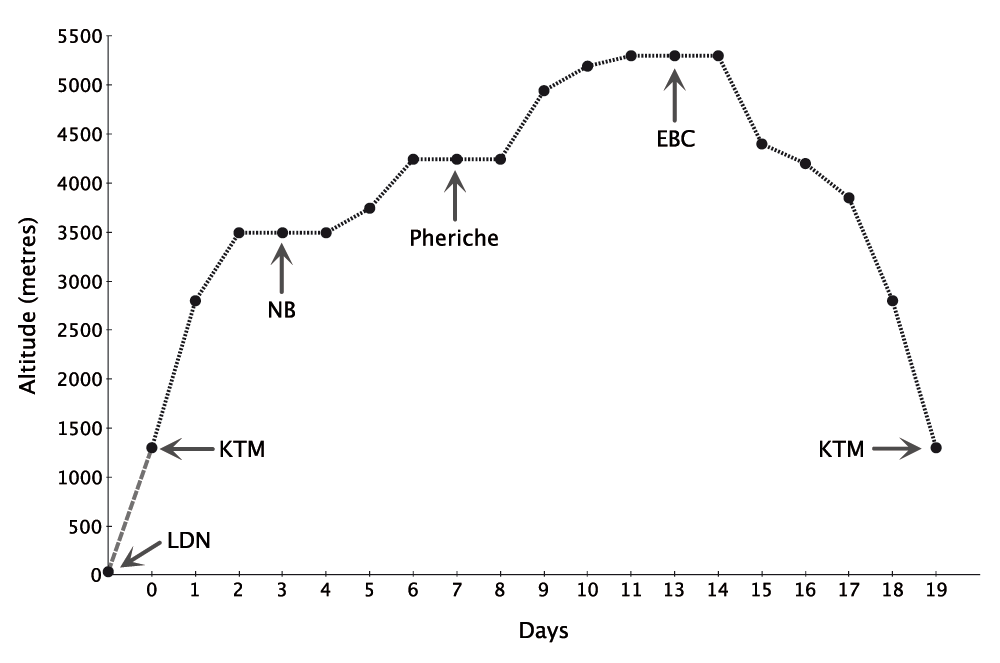
Key: LDN = London, KTM = Kathmandu, NB = Namche Bazaar, EBC = Everest Base Camp.
Key: NB = Namche Bazaar, EBC = Everest Base Camp.
Key: NB = Namche Bazaar, EBC = Everest Base Camp.
Key: LDN = London, KTM = Kathmandu, NB = Namche Bazaar, EBC = Everest Base Camp.
The ascent to EBC from KTM was completed over 11 days with incorporated rest days built in to the schedule to reduce the likelihood of acute mountain sickness (AMS). This ascent profile was identical to the CXE profile both because it was proven safe with minimal participant dropouts and because it would allow comparison of data between the two expeditions12.
The Investigator cohort underwent a similar ascent profile to the rest of the participants, but then remained in situ at their relevant laboratories for six weeks prior to descent. By using repeated testing at serial intervals, we were able to study the effects of both acute and chronic hypoxia on these participants. It should also be noted that upon their arrival at EBC, the Investigators were required to construct the laboratory and set up equipment, and consequently testing was started two days after arrival.
Upon arrival at each laboratory participants all were given a camp safety and science brief, and individualized timetables. Within these, in an attempt to minimize diurnal variations in physiological responses, participants were tested for each study at the same time at each site. Furthermore as many studies required abstinence from caffeine and food for specific periods, fasting periods and meal times were clearly highlighted for each individual. Within each laboratory, the timetable was adhered to and subjects were tested for particular studies on either day one or day two. These specific days were kept constant throughout the trek so as to control for the effects of acute acclimatization and responses over time. Because of the potential interaction between some studies, not every participant underwent all of the studies. The XE2 research portfolio, detailing core studies, additional studies and those in common with CXE, are highlighted in Table 3 and Table 4.
Blood, urine, saliva and exhaled breath condensate samples were all kept in -40°C freezers at each site. Muscle biopsy specimens (LDN, KTM and EBC) were stored in liquid nitrogen (-196°C), and then shipped to the UK on dry ice. All samples were brought from the field to KTM by helicopter and then repatriated to the UK on dry ice (-78°C).
Data analysis will follow a predetermined strategy of:
i) Description of phenotypes for each of the studies as outlined in Table 3 and Table 4 including plasma biomarkers and metabolomic analyses. This will include comparison with data obtained during a matched ascent in the CXE 2007 study (e.g. intra-individual comparison of the phenotypes from individuals who were participants in XE2 and CXE) as well as sub-group analyses (e.g. twins).
ii) Comparison of phenotypic adaptations between Sherpas and lowlanders during ascent and descent. We hypothesise that Sherpas will have a phenotype at baseline that is better adapted to hypoxia in comparison to lowlanders, that lowlanders ascending to altitude will tend to converge on the Sherpa phenotype, and that the Sherpa baseline phenotype will be less perturbed by altitude exposure than the lowlander baseline phenotype.
iii) Integrative analysis of genotype-epigenome-transcriptome-phenome across multiple datasets (XE2, CXE). The XE2 dataset will contribute to the accumulated Extreme Everest BioResource acquired during a number of high altitude research expeditions. Data will be incorporated into our bespoke comprehensive database, which enables linkage of all data elements with meta-data describing the provenance of each data item through the use of semantic web technology. Key questions will be raised in an iterative manner, driven both by a priori hypotheses and subsequently by data mining focused on the results obtained by unbiased outputs from individual ‘omics analyses.
Results from this study will be disseminated in peer-reviewed journals, on the Xtreme Everest website (www.xtreme-everest.co.uk) at scientific conferences and at public meetings.
We have characterized many features of the human response to progressive hypobaric hypoxia in a cohort of 180 individuals; 44 individual studies being safely conducted over four locations up to 5300m. In addition, the response to relative re-oxygenation was studied. The standardized ascent protocol ensured that differences between participants reflected inter-individual variability in hypoxic adaptation, as opposed to variability in hypoxic exposure. In matching the 2007 CXE ascent profile, the data from the two studies may also be combined to create a single cohort. The parallel study of lowlanders and highlanders will permit the identification of beneficial phenotypic adaptations and genetic alteration (and their relationships) in these groups. The study of investigators exposed to sustained (six weeks) hypoxia facilitated study of immediate and longer-term adaptive processes.
The slow ascent profile minimized symptoms of AMS, increasing the number of participants successfully reaching EBC and available for the study. Despite good medical care, gastrointestinal illness may have occasionally confounded results. However, the application of standardized medical protocols, with detailed documentation of illness and medication, will ensure this can be accounted for. Whilst the expedition was promoted through word of mouth and public advertisement, the very nature of the trek itself meant that participants were of a self-selected nature as enthused to undertake a rigorous trek. They may thus not be representative of the general population. Laboratories in Nepal were temporary as opposed to permanent structures. Temperature and pressure fluctuations, both having the potential to confound data obtained, were recorded on a daily basis (Table 2). Whilst we attempted to maintain a constant temperature between all laboratories through the use of heaters, we accept that any measured differences may confound the results.
We believe that the wealth of data obtained from XE2 will aid our understanding of the human adaptive response to hypoxia, offering insights that may be of value to patients suffering from sub-acute hypoxemia as a result of critical illness.
DM, MG, MM, KM and DL conceived the study. EGK, DM, TA, AS, MF, AM, MP, HM, DL and MG designed the experiments. EGK, AS, TA, KM, AM, MP, GGK, DL, RK, MM and DM carried out the research. EGK and DM prepared the first draft of the manuscript. All authors were involved in the revision of the draft manuscript and have agreed to the final content.
No grant funding supported this work; financial contributions were provided by the following organisations: Xtreme Everest 2 was supported by the Royal Free Hospital NHS Trust Charity, the Special Trustees of University College London Hospital NHS Foundation Trust, the Southampton University Hospital Charity, the UCL Institute of Sports Exercise and Health, The London Clinic, University College London, University of Southampton, Duke University Medical School, the United Kingdom Intensive Care Society, the National Institute of Academic Anaesthesia, the Rhinology and Laryngology Research Fund, The Physiological Society, Smiths Medical, Deltex Medical, Atlantic Customer Solutions and the Xtreme Everest 2 volunteer participants who trekked to Everest Base Camp.
Some of this work was undertaken at University College London Hospital- University College London Biomedical Research Centre, which received a proportion of funding from the United Kingdom Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme. Some of this work was undertaken at University Hospital Southampton-University of Southampton Respiratory Biomedical Research Unit, which received a proportion of funding from the United Kingdom Department of Health’s National Institute for Health Research Biomedical Research Units funding scheme.
I confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Members of the Xtreme Everest 2 Research Group are as follows: S Abraham, T Adams, W Anseeuw, R Astin, B Basnyat, O Burdall, J Carroll, A Cobb, J Coppel, O Couppis, J Court, A Cumptsey, T Davies, S Dhillon, N Diamond, C Dougall, T Geliot, E Gilbert-Kawai, G Gilbert-Kawai, E Gnaiger, M Grocott, C Haldane, P Hennis, J Horscroft, D Howard, S Jack, B Jarvis, W Jenner, G Jones, J van der Kaaij, J Kenth, A Kotwica, R Kumar BC, J Lacey, V Laner, D Levett, D Martin, P Meale, K Mitchell, Z Mahomed, J Moonie, A Murray, M Mythen, P Mythen, K O’Brien, I. Ruggles-Brice, K Salmon, A Sheperdigian, T Smedley, B Symons, C Tomlinson, A Vercueil, L Wandrag, S Ward, A Wight, C Wilkinson, S Wythe.
Members of the Xtreme Everest 2 Research Scientific Advisory Board: M Feelisch, E Gilbert-Kawai, M Grocott (chair), M Hanson, D Levett, D Martin, K Mitchell, H Montgomery, R Moon, A Murray, M Mythen, M Peters.
Xtreme Everest 2 is a research project coordinated by the Xtreme Everest Oxygen Research Consortium, collaboration between the UCL Centre for Altitude, Space, and Extreme Environment Medicine, the Centre for Human Integrative Physiology at the University of Southampton and the Duke University Medical Centre.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 10 Apr 15 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)