Keywords
Neurogenic bladder, Voiding dysfunction, Micturitional disturbance, Bladder dysfunction, Detrusor-sphincter-dyssynergia, Multiple sclerosis, Behçet’s disease, Multiple sclerosis
Neurogenic bladder, Voiding dysfunction, Micturitional disturbance, Bladder dysfunction, Detrusor-sphincter-dyssynergia, Multiple sclerosis, Behçet’s disease, Multiple sclerosis
Behçet’s disease (BD) was first described by the Turkish dermatologist Hulusi Behçet in 1937 as a triad of clinical symptoms consisting of recurrent uveitis and aphthous ulcers of the mouth and genitalia1. BD is now known to be a multi-system autoimmune inflammatory vasculitis with an unclear etiology and a strong link to the HLA-B51 haplotype2. Patients with Behçet’s disease may present with a variety of symptoms, most commonly with mucocutaneous and ocular involvement thought to be vasculitic sequelae. In addition, BD may also involve the joints as well as the gastrointestinal and nervous systems3.
Approximately 5–15% of patients with BD demonstrate neurological symptoms including dementia, psychiatric disturbances, cranial nerve palsy, cerebellar ataxia and pyramidal tract signs4. Micturitional disturbances have been found in 5–67% of patients with neurologic BD and these have been linked to pontine lesions affecting the pontine micturition center (PMC), pontine urine storage center (PUSC), and the nucleus reticularis pontis oralis (PoO)1,3,5. Generally, nervous system lesions in neurologic BD have been observed primarily in the brain stem and spinal cord1. Post-mortem examination has demonstrated degeneration of the pyramidal tracts as well as focal necrosis of the upper tegmentum pontis, subthalamic area, and internal nucleus of the basal ganglia6,7. Generally, neurological BD is progressive in 85% of patients with remission and relapse occurring in the remaining 15%8.
Given that the etiology of BD is incompletely elucidated, its diagnosis remains an inexact science and relies on magnetic resonance imaging (MRI), analysis of evoked potentials and cerebrospinal fluid (CSF), and clinical symptoms. CSF analysis typically yields pleocytosis with polymorphonuclear predominance in the absence of oligoclonal bands1. Clinical presentation is usually with an acute or subacute attack followed by remission with or without sequelae, or with progression after an initial attack. Clinically, patients can exhibit a variety of symptoms, many of which overlap with other neurological and autoimmune conditions. The spectrum of symptoms includes, but is not limited to, arthralgia and arthritis, gastrointestinal tract inflammation, deep venous thrombosis, superficial thrombophlebitis, inflammation within the cardiovascular system, inflammatory problems in chest and lungs, auditory disturbances, balance difficulties, fatigue, and psychiatric disturbances9,10. As a result of the lack of specificity of the above findings, serologic testing to narrow the spectrum of differential diagnoses is typically undertaken, but there is currently no sensitive or specific serologic test for diagnosis of BD itself.
Multiple sclerosis (MS), like BD, is regarded as an autoimmune disorder involving the central nervous system (CNS) and resulting in axonal demyelination. Symptoms of MS are variable in spectrum and presentation and can involve almost any aspect of the nervous system. As a result, clinical data alone may be insufficient to make the diagnosis, and supporting data come from neuroimaging, CSF analysis, and evoked potentials. MRI findings can demonstrate plaques, which, in contrast to those found in BD, can be located in white matter of the optic nerve, the basal ganglia, and close to the ventricles of the cerebellum in addition to in the brain stem and spinal cord11. CSF analysis may show pleocytosis, but in contrast with the polymorphonuclear nature of that seen in BD, pleocytosis in MS is typically lymphocytic. In addition, oligoclonal bands of IgG may be seen on electrophoresis of CSF in 75–85% of patients with MS12. Analysis of evoked potentials demonstrates decreased amplitudes secondary to demyelination13. In contrast to BD, which can either present with an initial attack with or without sequelae or in a secondary progressive manner, MS is always progressive, and can follow either a relapsing remitting, relapsing progressive, or primary or secondary progressive pattern.
We describe the case of a 52 year old female who was initially diagnosed with relapsing remitting MS. This diagnosis was amended to Behçet’s disease after further workup 10 years later. In the interim, the patient was diagnosed with a neurogenic detrusor overactivity and detrusor-sphincter-dyssynergia and was treated initially with anticholinergic medications, intermittent catheterization, and subsequently with ileocecal augmentation cystoplasty.
The patient is a 52 year old Hispanic female with a history of obsessive compulsive disorder and anorexia since adolescence who developed depression and insomnia while in her forties. In addition, she exhibited progressively worsening bilateral hand tremors, worsening dexterity, anorexia, and balance difficulties. A single-photon emission computed tomography (SPECT) scan of the brain on initial presentation in 1998 demonstrated hypoperfusion of the basal ganglia and frontal lobes. The patient was treated with steroids at the time, resulting in transient improvement of her insomnia. A MRI of the brain performed in June of 2000 demonstrated white matter lesions within the subcortical regions including the corpus callosum and periventricular areas, as well as posterior fossa atrophy. Visual and somatosensory evoked responses, as well as auditory brainstem responses, were normal. The patient declined a lumbar puncture, and thus cerebrospinal fluid was not available for analysis. Immune panels, anti-nuclear antibody (ANA), anti-neutrophil cytoplasmic antibody (ANCA), angiotensin converting enzyme (ACE), and chest X-ray were all within normal limits. Per report from the patient, an echocardiogram performed in Mexico in 2000 demonstrated minimal mitral valve prolapse, but neither the echocardiogram report nor images were available for review. At this time, her physical exam demonstrated myokymia in a cranial nerve VII distribution, right upper extremity pyramidal drift, postural and action tremors in bilateral upper extremities, and balance difficulties when asked to stand on one foot. In light of these findings, which met the Barkhoff and Tintoré criteria for diagnosis of MS, and despite the presence of a significant psychiatric component, a diagnosis of relapsing-remitting MS was made14,15. Notably, the literature supports a diagnosis of MS in the setting of psychiatric disease16.
As a result of her neurological symptoms and findings on imaging, the patient was started on glatiramer acetate and intermittently remained on this medication until 2002. She was not treated with interferons given their potential to worsen depression17. Initially, her symptoms improved markedly with near-resolution of her hand tremor. However, she continued to complain of balance difficulties and memory problems. In addition, in October 2000, she experienced vertigo and confusional episodes as well as worsening depressive symptoms, all of which responded to pulsed intravenous solumedrol. In January 2002, she demonstrated worsening right hand dexterity with no changes in memory and operational judgment when compared with her prior visit. Repeat MRI at that time demonstrated decreased burden of disease per report. She underwent neuropsychological evaluation at this time as well, which demonstrated organic memory deficits with intact intellect. In March 2002, the patient became mentally disorganized and was admitted to the hospital for treatment and observation. At this time, her glatiramer acetate was stopped and the diagnosis of MS questioned. Repeat MRI in June 2002 demonstrated no increase in disease burden. On physical examination at this time, she continued to exhibit minimal tremor in bilateral upper extremities but no longer had pyramidal drift in her right upper extremity. As a result of her symptomatic improvement, glatiramer acetate was not restarted.
In October 2003, a MRI of the brain evidenced an increase in disease burden and symptomatically the patient exhibited worsening memory deficits. At this time, however, the patient was complaining of urgency and urge incontinence and was evaluated by other urologists. She underwent a Marshall-Marchetti-Krantz bladder neck suspension in late 2003 and subsequently developed immediate urinary retention requiring placement of a Foley catheter. As a result, she was referred to our practice for evaluation and further management. Video urodynamic evaluation in January 2004 demonstrated neurogenic detrusor overactivity with some loss of compliance, detrusor-sphincter dyssynergia, elevated voiding pressures, poor flow, and a post-void residual of 350ml. Her first sensation occurred at 90ml bladder volume with first urge to void at 270ml and a functional bladder capacity of 340ml (Figure 1). She did not leak with cough or Valsalva maneuver. Fluoroscopy and electromyogram (EMG) demonstrated mid-urethral obstruction at the sphincter and no vesicoureteral reflux.
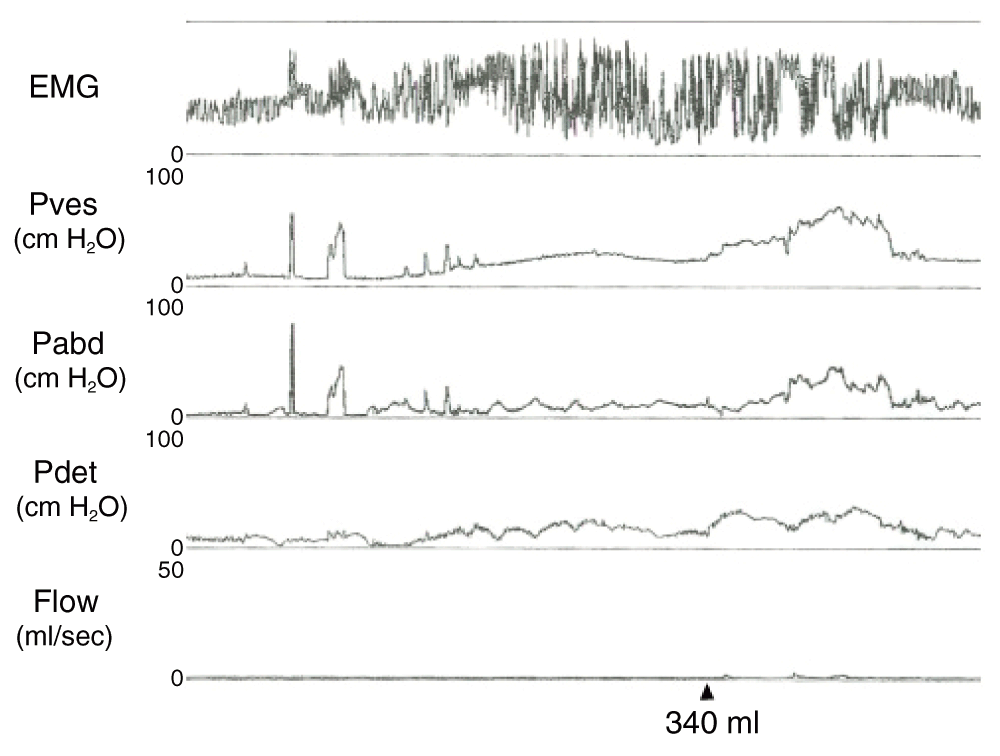
“EMG” – electromyogram “Pves” – intravesical pressure, measured in centimeters of water; “Pabd” – intra-abdominal pressure, measured in centimeters of water; “Pdet” – detrusor pressure, measured in centimeters of water; “Flow” – urinary flow, measured in milliliters per second. The arrow corresponds to the functional bladder capacity of 340 milliliters.
The patient desired to be free of urethral catheter drainage of her bladder, which was inconvenient and difficult given her limited manual dexterity. As a result, the patient underwent ileocecal augmentation cystoplasty with continent stoma in February 2004 without complications18. Her post-operative course was unremarkable and on subsequent office visits, she was doing well with much improved bladder symptoms consisting primarily of infrequent bladder spasms and occasional urinary tract infections with no urethral incontinence. These were successfully treated with intravesical anticholinergic medication and antibiotics. On subsequent video urodynamic evaluation performed in June 2010 an augmented bladder with normal compliance and 500ml capacity was demonstrated. There was no evidence of uninhibited contractions, urgency, vesicoureteral reflux, or leakage with cough or Valsalva.
In addition to the above symptoms, the patient was noted to be complaining of intermittent gastric upset, joint pain and swelling, muscle aches, oral, vaginal, and rectal ulcers, and intermittent fevers over the past several years. As a result, she was referred for rheumatologic workup in early 2010 which was remarkable for an elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), but with all other tests, including ANA, anti-cardiolipin antibody, rheumatoid factor, ACE, HLA-B27, antithrombin III, and complement studies within normal limits. Given the symptoms that she was exhibiting in addition to the neurological symptoms she initially presented with, the patient was diagnosed with fibromyalgia and Behçet’s disease and started on aziathioprine and vitamin D supplementation, on which she remains to this day with stable symptoms.
The above patient represents a diagnostic dilemma resulting in a change in diagnosis during the course of her treatment. Initially, the patient was diagnosed with MS on the basis of imaging findings as well as clinical symptoms that were consistent with the diagnosis, including CNS plaques in a distribution typically associated with MS, cranial nerve palsy, balance difficulties, and upper extremity tremors. However, CSF could not be obtained for evaluation, limiting the ability to differentiate between MS and other neurologic disorders. Furthermore, the patient initially presented with psychiatric symptoms that are atypical in the setting of MS, though an association between MS and psychiatric symptoms has been described16. Finally, additional serologies demonstrated no evidence for autoimmune disease or other findings that would suggest an alternate diagnosis. In addition, evaluation of evoked potentials was normal, which is unusual in the setting of MS, as 85% of MS patients have abnormal visual evoked potentials, 77% have abnormal somatosensory evoked potentials, and 67% have abnormal brainstem auditory evoked potentials. Nevertheless, it was not unreasonable to make an initial diagnosis of MS given the clinical and laboratory evidence at the time.
During the course of the patient’s treatment, additional symptoms became manifest that resulted in a change in diagnosis from MS to BD. These symptoms included oral and anogenital ulcers, gastrointestinal upset, and joint pain and swelling. Serologies continued to be unremarkable save for a transient increase in erythrocyte sedimentation rate and CRP. Together with the previously described symptoms and findings on imaging, this led to a change in diagnosis to neurologic BD. Lamentably, no evaluation of HLA-B51 haplotype was undertaken in this patient, as the Bw51 allele is closely associated with BD19. In addition, the absence of CSF for analysis further hindered an early, accurate diagnosis in this patient.
The patient’s initial treatment, based on the MS diagnosis, included pulsed steroids and glatiramer acetate. It is likely that the patient derived benefit from steroids, as these were used over short periods during symptom exacerbations and have demonstrated efficacy in treatment of both MS and BD symptoms20,21. In contrast, it is less clear whether the patient derived significant benefit from the glatiramer acetate, as there appear to be no studies evaluating its use in BD, and while its use in MS is established, the relapsing/remitting nature of the patient’s symptoms and the possibility of a wrong diagnosis calls into question the efficacy of the drug as administered to this patient.
From a urologic standpoint, the patient was not properly diagnosed by her urologists. She carried a clear neurologic diagnosis and her complaints were “overactive” in nature, not centered on stress urinary incontinence. The retropubic suspension aggravated her retention until she sought further medical care. She presented with neurogenic detrusor overactivity and detrusor-sphincter-dyssynergia along with symptoms that may be found in patients with both MS as well as BD22. Few studies exist examining micturitional disturbances in BD patients and the incidence of micturitional disturbances in patients with BD ranges between 5–67%23–25. In one study comprising eight patients with BD evaluated for lower urinary tract symptoms, seven demonstrated bladder dysfunction. The most common urodynamic finding was detrusor overactivity demonstrated in six patients, which is consistent with our findings. Bladder biopsies from these patients demonstrated blood vessel wall thickening and inflammatory infiltration of the lamina propria, consistent with prior findings by the same authors26, 27. Unfortunately, no tissue samples from our patient were available for pathologic analysis, preventing a comparison with the findings in the literature. This reported pathologic finding could result in loss of detrusor compliance.
A larger study evaluating 24 subjects with neurological BD demonstrated a 50% incidence of detrusor overactivity and 12.5% incidence of detrusor-sphincter-dyssynergia3. These patients generally had high post-void residuals, early first sensation, and low-normal bladder capacities, which mimic the findings in our patient. In addition, the authors mapped the presence of CNS lesions in their subjects, demonstrating the majority in the brain stem with a subset in the cerebrum, which is consistent with other studies1. Our review of the images revealed no lesions in areas typically affected by BD or in areas that may affect micturition as described above. The high incidence of overactive bladder symptoms demonstrated in our patient is also echoed in several other studies5,23,24,28, as are our findings on urodynamics1.
Our patient initially received anticholinergic medications for her overactive bladder symptoms with incomplete relief. Recommendations in the literature suggest an initial trial of anticholinergic therapy with the goals of preventing uninhibited contractions and lowering intravesical pressure, parameters which have been shown to improve with anticholinergic treatment in up to 75% of patients3,26. Our patient developed significant dry skin with oral anticholinergic medication and asked to be taken off the medication. While intravesical instillation of anticholinergic medications is an option that has been shown to be effective in patients with bladder symptoms due to neurological BD, this was not attempted in our patient24. Given the incomplete response to oral medications in our patient, as well as the desire to be free of an indwelling catheter in the setting of limited dexterity, ileocecal augmentation cystoplasty with a continent stoma was performed with a notable decrease in urgency symptoms and increase in bladder capacity postoperatively. Several studies have discussed the use of augmentation cystoplasty in patients with neurological BD resulting in improvements in bladder symptoms, continence status and renal function3,23,26. In agreement with these studies, we found our patient to have no uninhibited contractions as well as improved bladder compliance and increased bladder capacity after augmentation cystoplasty.
In summary, we describe a case of BD initially diagnosed as MS in a female with a neurogenic bladder secondary to her neurologic disease. The treatment of her neurologic disease was in line with the accepted standard of care for her diagnoses at the time they were made, as was the treatment of her neurogenic bladder. This case demonstrates the current challenges to accurate diagnosis of certain neurologic conditions and highlights the need for development of further sensitive and specific assays to facilitate these diagnoses, along with prudent use of urodynamic testing. Furthermore, this case demonstrates the efficacy of bladder augmentation with a continent catheterizable stoma in the setting of neurogenic detrusor overactivity in a patient with neurologic BD.
Written informed consent for publication of their clinical details and/or clinical images was obtained from the patient.
AWP reviewed all patient information, performed a literature review, participated in intellectual discussion pertinent to the conception of the report, and drafted the manuscript. TBB is the physician of the patient presented herein, provided all patient information, and participated in intellectual discussion pertinent to the conception of the report. Both authors have agreed to the final content of the manuscript.
A.W.P. is a K12 scholar supported by a Male Reproductive Health Research Career Development Physician-Scientist Award (grant HD073917-01) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Program (to Dolores J. Lamb).
I confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: urinary incontinence, neurourology
Is the background of the case’s history and progression described in sufficient detail?
No
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
No
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
No
Is the case presented with sufficient detail to be useful for other practitioners?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Behcet's Disease
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 10 Apr 15 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)