Keywords
Host immune response, Gram-positive, Lipoteichoic acid, D-alanylation, Glycolipid D-alanyl-phosphatidylglycerol, Surface charge, Lipoteichoic acid primer, Mass spectrometry, Lipidomics
Host immune response, Gram-positive, Lipoteichoic acid, D-alanylation, Glycolipid D-alanyl-phosphatidylglycerol, Surface charge, Lipoteichoic acid primer, Mass spectrometry, Lipidomics
All abbreviation “Pho” for phosphate are replaced with “P”.
All abbreviation “LTA” for lipoteichoic acid primer are replaced with “LTAP”.
The chemical structures in Figure 1 and 5 are redrawn to show double bond in the same thickness and glucosyl residues in their chair conformation.
Type I teichoic acids in B. subtilis as well as other four types of teichoic acids are mentioned. Type I and type IV teichoic acids undergo D-alanylation.
In the abstract, it becomes “in the identification of possible intermediate species”.
In the abstract, “would aid in” becomes “should aid in”.
In the introduction, lipid A and lipopolysaccharide as well as the outer membrane of Gram-negative bacteria has been mentioned.
In the discussion, abundance of D-alanine over L-alanine in the lipid lysate is discussed.
The word “adenylation” is replaced with “adenylylation”.
In the introduction, “DltB is predicted to be an integral membrane protein”.
The name “o-acyltransferase” becomes “O-acyltransferace”.
In figure legends, “aicd” has been corrected as “acid”.
“FA – fatty acid” has been added to the notes of Table 2, 3 and 4.
“The smaller 1017 amu anion” becomes “The 1017 amu anion”.
See the author's detailed response to the review by Katarzyna Duda
See the author's detailed response to the review by Christian Sohlenkamp
Phospholipids are the dominant cell membrane component in most bacteria1 which render bacterial cell surface negatively charged. This feature makes bacterial membrane the easy target of host immune molecules such as cationic antibiotic peptides2–4. Bacteria have been known to constantly modulate membrane components1,5. There are at least three pathways which may contribute to surface charge modulation: biosynthesis of phosphatidylethanolamine (PE), L-lysyl-phosphatidylglycerol (lysyl-PG), and D-alanylation of lipo- and wall-teichoic acids. In comparison with Gram-negative bacteria, Gram-positive bacteria typically have noticeably less PE1, but have an abundance of lysyl-PG or other aminoacylated PGs which most Gram-negative bacteria lack5,6. Besides, lipo- and wall-teichoic acids are only found in Gram-positive bacteria.
Gram-positive bacteria lack the outer membrane as well as phosphate-rich lipid A and lipopolysaccharide found in Gram-negative bacteria. Instead they have a diverse category of polymeric teichoic acids made of phosphate-containing repeating units. Peptidoglycan-attached wall-teichoic acids and glycolipid-anchored lipoteichoic acids were discovered six decades ago7. There are five types of lipoteichoic acids10 and four types of wall teichoic acids11. The biosynthesis pathways of the two types of teichoic acids have been characterized8–11. Glycerol or ribitol residues in the repeating units of type I and type IV lipoteichoic acids10 as well as type I wall teichoic acids11 are known to undergo D-alanine esterification7,9,12, which is known to be carried out by the four dlt operon-coded proteins DltABCD13. This surface charge modulation has been observed to significantly affect the antigenicity of the bacteria2. In the cytosol, DltA (~500 amino acid residues) catalyzes with the consumption of ATP first the adenylylation of D-alanine and then the thioester formation with D-alanyl-carrier protein DltC (~80 amino acids)13–15. Crystal structures of DltA16,17 have proven that DltA is homologous to adenylation domains (also called AMP-forming domains) found in modular nonribosomal peptide synthetases18 as well as fatty acyl-coenzyme A synthetases19 and firefly luciferases20. The functionally uncharacterized DltB (~400 amino acid residues) is predicted to be an integral membrane protein with multiple putative transmembrane helices with a low level of similarity to a putative group of membrane-bound O-acyltransferases21. DltD (~400 amino acid residues), with a single putative N-terminal transmembrane helix and a large globular domain, has been reported to bind DltC and possibly catalyzes the final D-alanyl transfer from DltC to teichoic acid22. We have recently characterized the presence of D- but not L-alanine in lipid lysate from Bacillus subtilis, implying the presence of D-alanyl-PG in the bacterial membrane23. Observation of other D-alanylated species in the bacterial membrane would help sketching a transfer route for the D-alanyl group from inside the cytosol to teichoic acids on the outer surface. Here I report profiling of B. subtilis lipids and identification of mono- and di-alanylated derivatives of nascent lipoteichoic acid primer with a single phosphoglycerol unit attached to the glycolipid anchor (chemical structures shown in Figure 1).
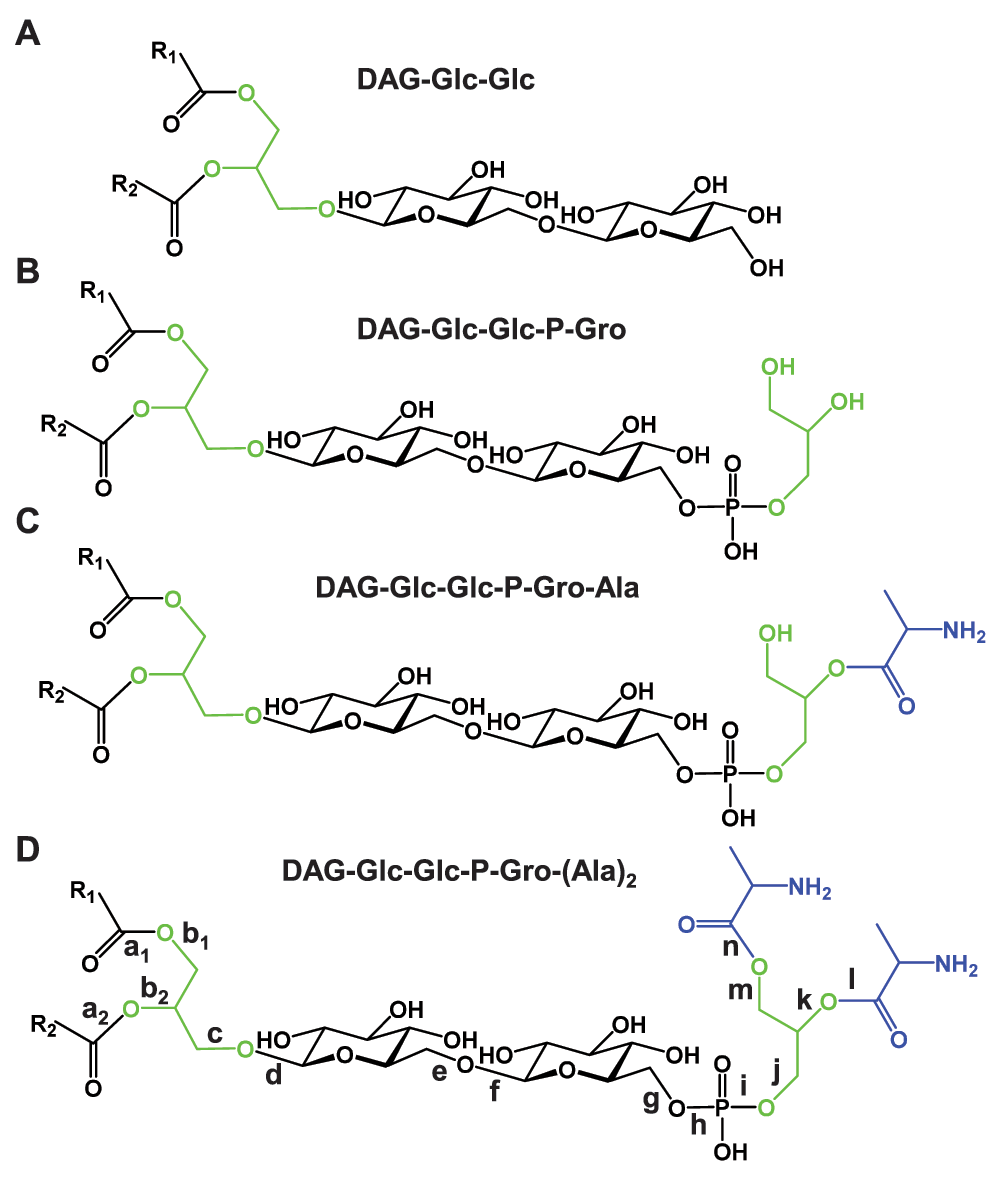
Scissile bonds are labeled alphabetically in D. DAG – diacylglycerol; P – phosphate; Gro – glycerol; Glc – glucose; Ala – alanine. A. The glycolipid anchor of lipoteichoic acid: DAG-Glc-Glc. B. The lipoteichoic acid primer: DAG-Glc-Glc-P-Gro. C. Mono-alanylated lipoteichoic acid primer: DAG-Glc-Glc-P-Gro-Ala. D. Bis-alanylated lipoteichoic acid primer: DAG-Glc-Glc-P-Gro-(Ala)2.
Bacterial strain and cell culture. The BL21 (DE3) strain of E. coli (Novagen) and B. subtilis strain 168 (Bacillus Genetic Stock Center) were first plated from freezer stock onto LB-agar media. A single colony was transferred into 100 ml of LB media. After incubation overnight at 37°C and 220 rpm in an environmental shaker, it was transferred to 1 liter of LB media. When the cell density reached ~1.0 at 600 nm, 200 ml cell culture supplemented with 2.0 ml of 1.0 M NaAc buffer at pH 4.6 was centrifuged at 5,500 rpm in a Beckman JLA-8.1 rotor for 16 minutes at 4°C. The wet cell pellet was used for lipid extraction.
Lipid extraction. HPLC-grade organic solvents (Fisher Scientific) and distilled and deionized water were used throughout the experiment. The lipid extraction procedure was following that of Bligh and Dyer24. Briefly, the wet cell pellet was re-suspended in a glass tube in 0.5 ml ice-chilled water and 2.0 ml of ice-chilled methanol. Then 1.0 ml of cold chloroform was added. The suspension was vortexed for 3 seconds every 5 minutes and incubated on ice for a duration of 10 minutes. After that, 2.0 ml cold chloroform was added followed by 1.5 ml of cold water. The tube was vortexed for 3 seconds and placed on a rocking platform at a room temperature of 21°C for 3 minutes. Phase separation was assisted by centrifugation at 1,300 rpm for 5 minutes with a Beckman Allegro X-22R centrifuge. The heavier chloroform-rich phase was transferred by a glass syringe to a second glass centrifuge tube. Another 2.0 ml cold chloroform was added to the first tube and vortexed for 3 seconds. Then the first tube was put back on the rocking platform at room temperature for 10 minutes. Centrifugation at 1,300 rpm for 5 minutes and transfer of the heavier chloroform-rich phase to the second glass tube followed. The combined chloroform-rich phase was mixed with 0.5 ml 0.5 M NaCl, vortexed for 3 seconds and gentle shaking by hand for 1 minute. After centrifugation at 1,300 rpm for 5 minutes, the chloroform-rich phase, 4.0–4.5 ml in volume, was collected in a third glass tube for storage at -80°C. Typically, the total lipid concentration was estimated as 0.5 mg/ml.
Lipid profiling by mass spectroscopy. The lipid samples were diluted by adding 2-fold volume of methanol to a concentration of ~0.15 mg/ml (or 150 ppm) for direct infusion at a rate of 0.6 ml/hour to a SCIEX 4000 QTRAP mass spectrometer. Electrospray ionization was achieved at a temperature of 500°C and a pressure of 20 psi for curtain gas as well as ion source gas 1 and 2. The collision energy in the ion trap was tested between 30 and 100 electronvolts for most efficient detection of target substructures in the lipids. The SCIEX Analyst 1.6 software was used to acquire and export averaged mass spectra with the 4000 QTRAP system. Agilent MassHunter B.06.00 was used to process mass spectra with an Agilent Q-TOF 6500 system. MS spectra in the figures were also analyzed with Mass++ 2.7.4 software25 and presented with Microsoft Excel.
Tandem mass spectroscopy. The targeted MS/MS spectra were first acquired using the SCIEX 4000 QTRAP system with multiple collision energy settings between 50 and 90 electronvolts. High-accuracy MS/MS spectra were acquired using the Agilent Q-TOF 6550 system with collision energy ranging from 30 to 80 electronvolts. Direct infusion was also employed but at a faster flow rate of 2.0 ml/hour for the Q-TOF 6550 system.
Polar lipid extraction on ice produced more species in the sample - Ice-chilled solvents instead of room-temperature ones were used during the well-established polar lipid extraction procedure devised by Bligh and Dyer24. The new lipid preparations did not show marked differences on thin-layer chromatograms. However, their mass spectra showed noticeable difference with the cold extraction producing more species than room-temperature extraction. The alanylated derivatives of lipoteichoic acid primer were not observed in lipids extracted at room temperature.
Profiling and tandem mass spectroscopy of polar lipids with dihexose head group - The sodiated form of the lipid anchor of lipoteichoic acid in B. subtilis has been identified by mass spectrometry previously26. Several mass spectrometric scans with the 4000 QTRAP system in search for the lipid anchor were experimented. The anchor has a common structure of DAG-dihexose, with the hexose being either glucose or galactose depending on the identity of the microbial organism27. The unbranched and typically glycerolphosphate polymer is attached to C-6 of the non-reducing hexopyranosyl end of the glycolipid anchor by a phosphodiester bond27. In B. subtilis, the head group is diglucose27 (Figure 1A). The sodiated dehydrated diglucose (342 – 18 + 23 = 347 amu) at a collision energy of +80 electronvolts revealed the two most intense peaks (887 and 915 amu) matching expected sizes of the lipid anchor with the two dominant fatty acyl compositions of (30:0) and (32:0), respectively26 (Figure 2A). Tandem mass spectra of the most abundant 915 amu species was then acquired with the SCIEX 4000 QTRAP system (Figure 2B) and the Agilent Q-TOF 6550 system (Table 1). The QTRAP system produced less noisy spectra which are shown in Figure 2–Figure 4. The m/z values obtained from the Q-TOF system were more accurate and are listed in Table 1–Table 4. The observed molecular mass 915.601 closely matched the calculated value of 915.603 for (32:0) [DAG-Glc-Glc + Na]+. All the observed fragment ions also had their m/z values within 0.002 amu of calculated monoisotopic masses. The molecular ion dissociated to form two most abundant fragments at 645 and 673 amu, corresponding to neutral loss of (17:0) fatty acid (270 amu) and (15:0) fatty acid (242 amu), respectively. The two fatty acids have been known as the dominant ones in B. subtilis lipids23. The 753 amu fragment ion corresponding to a neutral loss of a dehydrated glucose residue (162 amu) was less abundant than the twin peaks. Another set of twin peaks at 483 and 511 amu corresponded to neutral losses of the terminal glucose residue (162 amu) and either of the two fatty acids (270 and 242 amu respectively). The peak at 405 amu corresponded to the sodiated diglucose head group covalently linked with the didehydroxyl residue of glycerol CH2=CH-CH2-Glc-Glc (Table 1). The twin peaks at 365 and 347 amu corresponded to the sodiated diglucose head group and its dehydrated form, respectively. It is worth noting that the signature [DAG – OH]+ ion (551 amu) for glycerolphospholipids was missing. However, the two [MAG – OH]+ ions at 299 and 327 amu were observed at lower intensity. Even though the 405 amu ion was more intense than the 347 ion, lipid profiling by searching for precursors of the 405 amu cation was inferior to the precursor scan for the 347 amu cation.
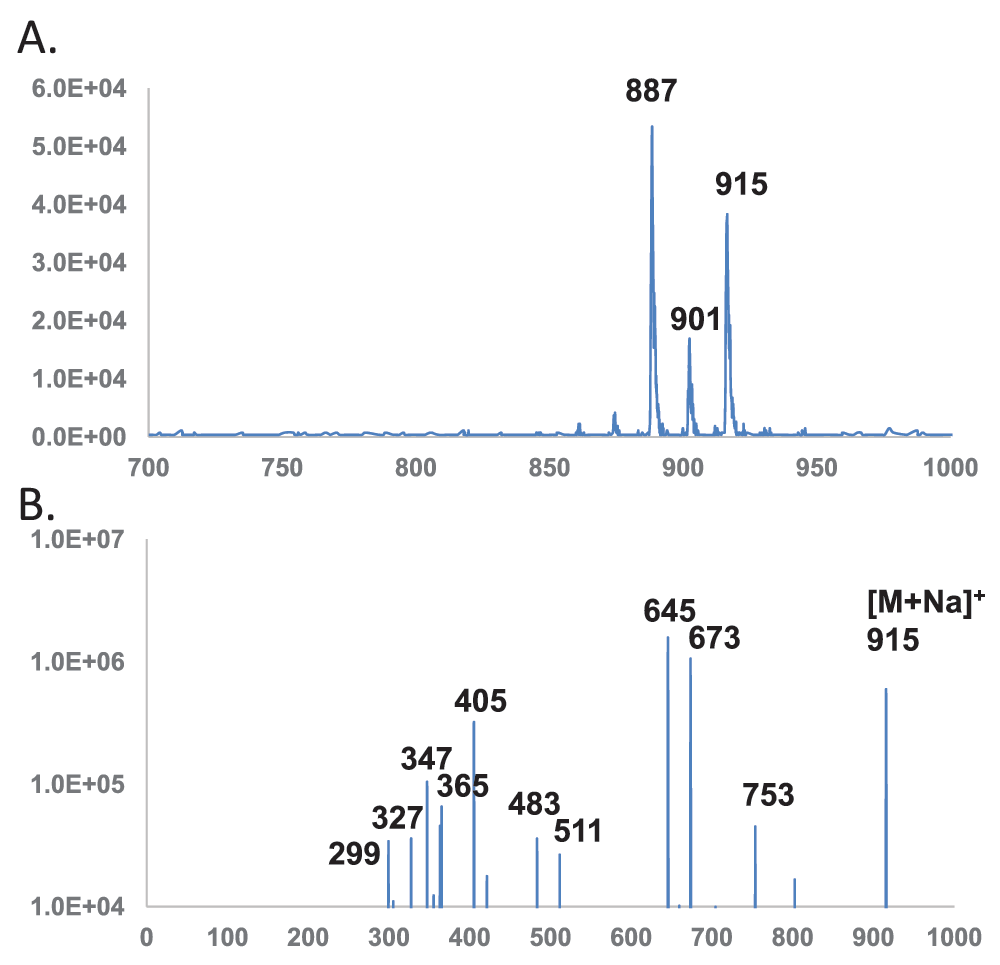
Horizontal axis denotes m/z values. Vertical axis denotes ion counts. DAG – diacylglycerol; Glc – glucose. A. Precursor scan for 347 amu sodiated diglucose dehydrate. B. MS/MS spectrum of sodiated (32:0) DAG-Glc-Glc (915 amu).
Note: The alphabetically labeled scissile bonds are shown in Figure 1D. P – phosphate; Gro – glycerol; MAG – monoacylglycerol; DAG – diacylglycerol; Glc – glucose. There are equivalent choices such as between a1 and a2, as well as between b1 and b2.
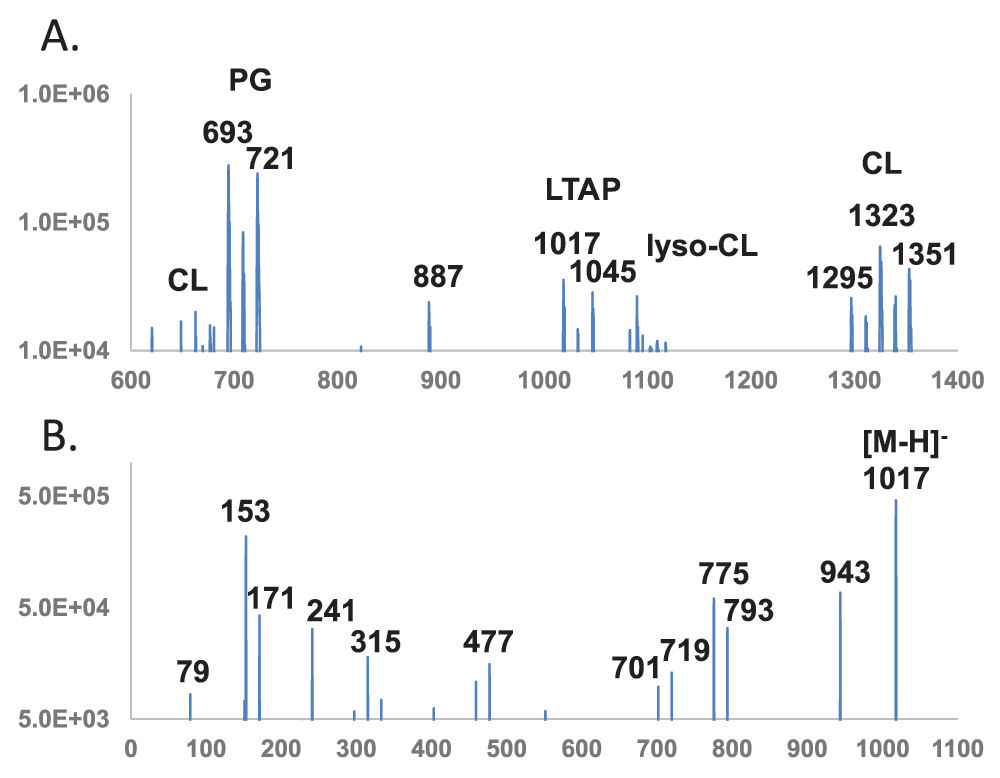
Horizontal axis denotes m/z values. Vertical axis denotes ion counts. DAG – diacylglycerol; PG – phosphatidylglycerol; P – phosphate; Gro – glycerol; Glc – glucose; LTAP – lipoteichoic acid primer; CL – cardiolipin. A. Precursor scan for 153 amu cyclo-glycerolphosphate anion. B. MS/MS spectrum of lipoteichoic acid primer (30:0) DAG-Glc-Glc-P-Gro (1017 amu).
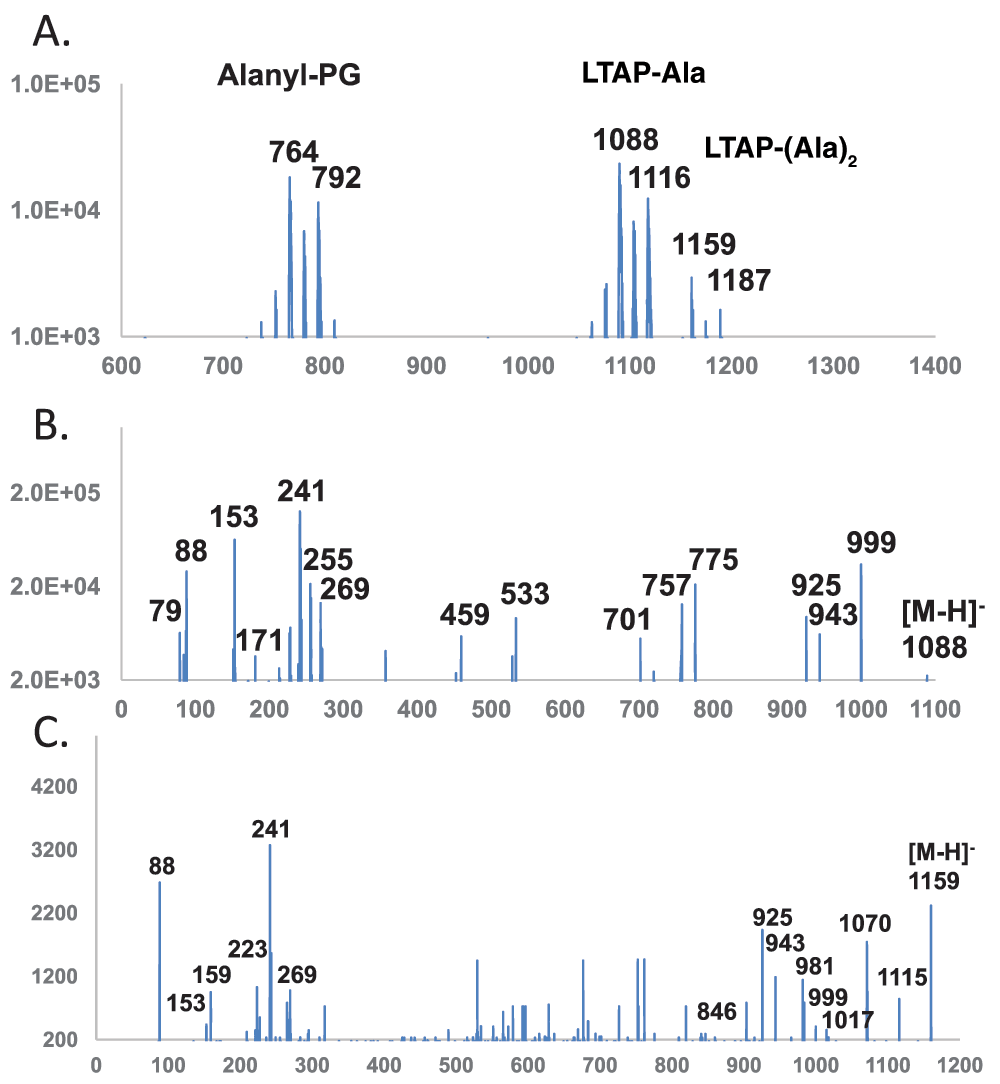
Horizontal axis denotes m/z values. Vertical axis denotes ion counts. DAG – diacylglycerol; PG – phosphatidylglycerol; P – phosphate; Gro – glycerol; Glc – glucose; Ala – alanine; LTAP – lipoteichoic acid primer. A. Precursor scan for 88 amu [Ala-H]-. B. MS/MS spectrum of mono-alanylated (30:0) lipoteichoic acid primer DAG-Glc-Glc-P-Gro-Ala (1088 amu). C. MS/MS spectrum of dialanylated (30:0) lipoteichoic acid primer DAG-Glc-Glc-P-Gro-(Ala)2 (1159 amu).
Note: The alphabetically labeled scissile bonds are shown in Figure 1D. FA – fatty acid; P – phosphate; Gro – glycerol; MAG – monoacylglycerol; DAG – diacylglycerol; Glc – glucose; LTAP – lipoteichoic acid primer DAG-Glc2-P-Gro. Cleavage at a1 and a2, as well as at b1 and b2 produces fragments of identical sizes.
| Observed mass | Calculated mass | Cleavage | Description |
|---|---|---|---|
| 88.0401 | 88.0399 | k or m | Ala – H |
| 152.9946 | 152.9953 | g & k & n | P-Gro – H3O |
| 159.0762 | 159.0770 | Figure 5A | Ala-Ala – H |
| 223.1704 | 223.1698 | (14:2) FA – H | |
| 241.2166 | 241.2169 | b1 or b2 | (15:0) FA – H |
| (846.4) | 846.3891 | b1 & l | (15:0) MAG-Glc2-P-Gro-Ala – H3O |
| (925.5) | 925.5293 | i | (30:0) DAG-Glc2-P – H3O |
| (943.5) | 943.5399 | j | (30:0) DAG-Glc2-P – H |
| (981.6) | 981.5550 | k & m | (30:0) LTAP – H5O2 |
| (999.5) | 999.5661 | k & n | (30:0) LTAP – H3O |
| 1017.5774 | 1017.5770 | l & n | (30:0) LTAP – H |
| 1070.6092 | 1070.6030 | k or m | (30:0) LTAP-Ala – H3O |
| (1115.7) | 1115.6610 | Figure 5B | (30:0) LTAP-Ala2 – CO2 – H |
| 1159.6527 | 1159.6510 | [M-H]- | (30:0) LTAP-Ala2 – H |
Note: The alphabetically labeled scissile bonds are shown in Figure 1. FA – fatty acid; P – phosphate; Gro – glycerol; MAG – monoacylglycerol; DAG – diacylglycerol; Glc – glucose; Ala – alanine; LTAP – LTA primer DAG-Glc2-P-Gro. Values in parentheses were observed only with the low-accuracy 4000 QTRAP system.
Profiling and tandem mass spectrometry of lipids with phosphoglycerol terminus – The phosphoglycerol head group has a molecular mass of 172 and produced a cyclic, equivalent to dehydrated, residual anion at 153 amu. The 153 amu fragment peak is most intense for phospholipids with a terminal phosphoglycerol, and weak for phospholipids - such as cardiolipin (CL) and aminoacylated PGs - with such an embedded group. This scan between 400 and 1700 amu at a collision energy of -95 electronvolts was most effective in hitting larger precursor ions (part of the mass range is shown in Figure 3A). The spectrum revealed a cluster of cardiolipin (CL) double anions in the 650–680 amu range and a more intense cluster centered around two major anions at 693 and 721 amu, corresponding to the dominant lipids of (30:0) and (32:0) PGs, respectively. There was an 887 amu unknown species as well as mostly dehydrated lyso-cardiolipins (lyso-CL) close to 1100 amu and cardiolipins close to 1300 amu. There were no noticeable hits below 600 amu or between 1400 and 1700 amu. Besides, the 1017 amu and 1045 amu anions matched expected masses of lipoteichoic acid primer (Figure 1B) with dominant fatty acyl compositions of (30:0) and (32:0), respectively.
The 1017 amu anion had two identical (15:0) fatty acyl chains and therefore made assignment of fragments less difficult. The MS/MS spectra of the 1017 ion acquired with the QTRAP system at a collision energy of -90 electronvolts is shown in Figure 3B, and m/z values of fragments are listed in Table 2. In addition to the 79 amu phosphate residue, the pair of 153 amu and 171 amu ions which corresponded to glycerolphosphate residue, the dominant fatty acid ion at 241 amu matched the expected (15:0) composition. Fragmentation at the two glycosyl bonds likely produced the 315 amu and 477 amu ions. At the other end of the spectrum, the 943 amu ion was likely due to the neutral loss of cycloglycerol (74 amu). A further loss of (15:0) fatty acid (242 amu) or ketene (224 amu) likely produced the pair of 701 and 719 amu ions, respectively. Another pair at 775 and 793 amu were produced similarly but from the molecular ion. The 1017 amu molecular ion matched structural characteristics of a lipoteichoic acid primer with a single glycerolphosphate unit attached to the lipid anchor of diglucosyldiacyglycerol.
Profiling of lipids with ester-linked alanine – In negative mode, ester-linked fatty acids are known to form intense fragment [FA-H]- ions. This is also true for ester-linked amino acids23. A precursor scan between 400 and 1700 amu at an optimized collision energy of -95 electronvolts for 88 amu [Alanine-H]- (part of the mass range is shown in Figure 4A) revealed as expected a cluster of alanyl-PGs with two dominant peaks at 764 and 792 amu corresponding to (30:0) and (32:0) alanyl-PG, respectively. The precursor scan also revealed two adjacent clusters of alanylated lipids separated by 71 amu which corresponded to the molecular mass of a dehydrated alanine. The first cluster with dominant 1088 and 1116 amu anions matched expected m/z values of mono-alanylated lipoteichoic acid primers (Figure 1C), while the second cluster centered around the 1159 and 1187 amu anions matched those of di-alanylated lipoteichoic acid primers (Figure 1D). There were no noticeable hits below 700 amu or between 1200 and 1700 amu.
The overall mass of the 1088 amu anion matched that of (30:0) alanyl-lipoteichoic acid primer. The MS/MS spectra of the 1088 ion acquired with the QTRAP system at a collision energy of -90 electronvolts is shown in Figure 4B, and m/z values of fragments are listed in Table 3. The 79, 153 and 171 amu ions corresponded to the putative glycerolphosphate backbone of this lipid. The dominant fatty acid ion at 241 amu matched the expected (15:0) composition and its putative ester linkage to the lipid. The 88 amu ion implied a terminal ester-linked alanine. The 224 amu ion corresponded to the dehydrated or cyclic form of the putative head group of alanylated glycerolphosphate. At the other end of the spectrum, the 999 amu ion was likely due to the neutral loss of alanine (89 amu) from the 1088 molecular anion. The pair of ions at 925 and 943 amu corresponded to the neutral loss of linear (163 amu) or cyclic (145 amu) alanyl-glycerol. In the mid-section of the spectrum, the 459 amu species corresponded to neutral losses of both DAG (540 amu) and alanine (89 amu), while the 533 amu ion corresponded to neutral losses of both fatty acids (2 × 242 = 484 amu) and a dehydrated alanine (71 amu). Fragments at 757 and 775 amu corresponded to the loss of one (15:0) fatty acid or ketene (242 or 224 amu) as well as alanine (89 amu). The smaller 701 and 719 amu fragments corresponded to loss of one (15:0) fatty acid or ketene as well as cyclo-alanyl-glycerol (145 amu). Except for the absence of 701 amu anion and the low-abundance 757 amu anion in the Q-TOF-acquired spectrum, all fragments matched expected m/z values within 0.004 amu. This 1088 amu is most likely mono-alanylated lipoteichoic acid primer.
The m/z value of the 1159 amu anion matched that of (30:0) di-alanyl-lipoteichoic acid primer. The MS/MS spectra of the 1159 ion acquired with the QTRAP system at a collision energy of -80 electronvolts is shown in Figure 4C, and m/z values of fragments are listed in Table 4. Due to the lower abundance of the 1159 anion, its MS/MS spectrum was noisier than those of the 1017 and 1088 anions. The spectrum shared 79, 88, 153, 171 and 241 amu ions with that of the 1088 amu anion. Unexpectedly, a 159 amu species corresponding precisely to deprotonated alanylalanine dipeptide was also observed. The mid-section of the spectrum did not reveal reoccurring ions in spectra collected at collision energies 10 electronvolts apart and therefore were likely noise due to the low abundance of this molecular ion. The whole head group of dialanylated glycerolphosphate was not observed. At the high end of the spectrum, an 846 amu fragment was probably generated by neutral losses of both (15:0) fatty acid (242 amu) and a cyclo-alanine (71 amu). The 925, 943 and 999 amu ions were in common with the fragments from the 1088 amu anion of mono-alanylated lipoteichoic acid primer. A further dehydrated 981 amu ion was observed, which corresponded to neutral losses of two alanine molecules (2 × 89 = 178 amu). A larger 1017 amu ion precisely matched that of (30:0) lipoteichoic acid primer. Another even larger 1070 amu ion corresponded to the 89 amu neutral loss of one alanine from the 1159 amu molecular anion. Surprisingly, a 1115 amu ion corresponding to a neutral loss of 44 amu was observed. As shown in Figure 5A, a two-step reaction may account for the formation of alanylalanyl-LTA primer and subsequent fragmentation into the 159 amu alanylalanine anion. An alternate reaction shown in Figure 5B may rearrange the putative bis-alanyl-LTA primer to expose a terminal carboxyl group in one of the two alanine residues, which could subsequently release the 44 amu CO2 and produce the 1115 amu fragment anion. Due to the lack of any fragment ion corresponding to linear (313 amu) or cyclic (295 amu) bis-alanyl-glycerolphosphate, the result was not definitive on the location of two ester-linked alanine residues. Based on its similar fragmentation pattern to that of mono-alanylated LTA primer, this 1159 amu species is tentatively assigned as bis-alanyl-LTA primer (Figure 1D).
Aminoacylated lipids play an apparent role in surface charge modulation of Gram-positive bacteria5. The least known part of charge modulation is arguably the D-alanylation pathway of lipoteichoic acids. The Bligh and Dyer method24 carried out at an icy temperature appeared to be essential for successful extraction of species that are almost certainly lipoteichoic acid primer and its mono- and di-alanylated derivatives. My lab has recently observed that lysate of B. subtilis lipids contained predominantly D-alanine. Lysate of lipids in this study showed the expected predominance of D-alanine over L-alanine. The lipoteichoic acid primers were possibly esterized with D-alanine. The possible existence of the putative bis-alanylated lipoteichoic acid primer indicates that these species are unlikely to be hydrolyzed fragments of lipoteichoic acids since hydrolysis could only produce mono-alanylated derivative. Hydrolysis of lipoteichoic acid should also produce detectable amount of residue with more than one phosphoglycerol units attached to the lipid anchor, which was apparently lacking in the lipid extract. It also implies that lipoteichoic acid is unlikely to be transferred as D-alanyl-glycerolphosphate unit directly from D-alanyl-PG to the growing lipoteichoic acid chain by the LtaS polymerase as that would only produce mono-alanylated derivative. The observable abundance of lipoteichoic acid primer also appear to suggest that one of the four LtaS paralogs in B. subtilis28 may indeed act like LtaP primase in Listeria monocytogenes for the biosynthesis of lipoteichoic acid primer29.
My lab has recently hypothesized that D-alanyl-PG may serve as the lipid intermediate for subsequent D-alanylation of teichoic acids. Taken together, a putative pathway is shown in Figure 6. It is known that DltA catalyzes the activation of D-alanine with the consumption of ATP and thioester formation with the D-alanyl carrier protein DltC. It is possible either DltD or DltB - with the former being more likely based on the best available evidences that DltD binds DltC and has thioesterase activity22 – catalyzes the transfer of thioester-bound D-alanyl group to PG in the bacterial membrane by a thermodynamically spontaneous esterification reaction. The other one of the pair of Dlt proteins, most likely DltB, then catalyzes the transfer of D-alanyl group from the PG carrier to lipoteichoic acid by a transesterification reaction that can only reach equilibrium. This thermodynamic nature of this final transesterification reaction would enable the accumulation of a significant amount of the D-alanyl-PG intermediate, which is consistent with my lab’s recent observation that alanyl-PG is somewhat abundant in lipids extracted from B. subtilis. Importantly, the diglucosyl-diacylglycerol anchor, lipoteichoic acid primer, D-alanylated lipoteichoic acid primer as well as D-alanylated phosphatidylglycerol can be monitored in lipids extracted from wild-type and mutant cells of B. subtilis and aid in the full elucidation of the D-alanylation pathway of lipoteichoic acids.
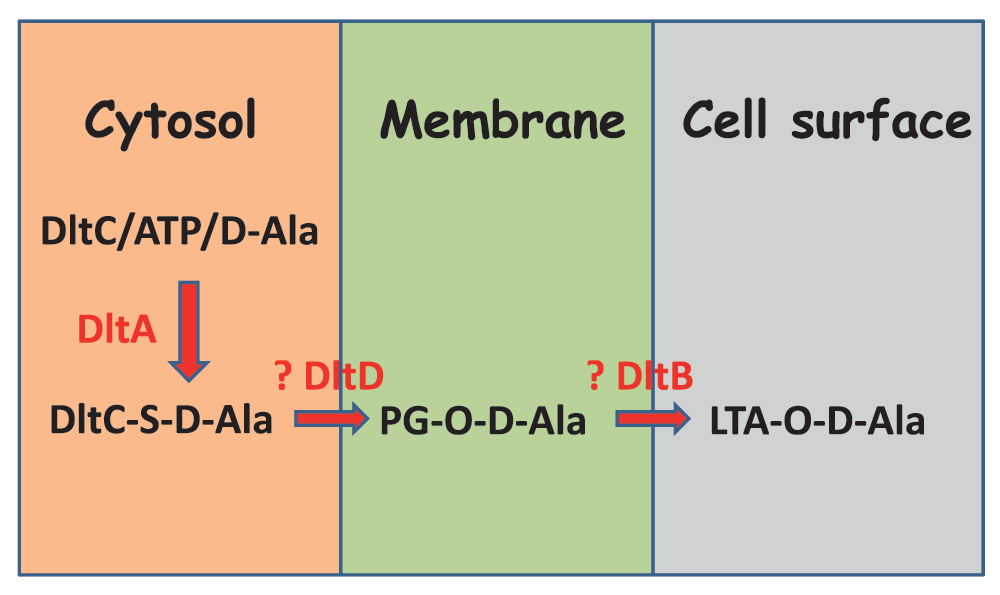
DltA catalyzes the loading of thioester-linked D-alanine to the carrier protein DltC. DltC-carried D-alanyl group is further transferred to PG in the membrane by forming an ester bond. The putative enzyme for this process is DltD. PG-attached D-alanyl group is further transferred to LTA by transesterification. The putative enzyme for this latter process is DltB.
F1000Research: Dataset 1. Raw data for ‘Alanylated lipoteichoic acid primer in Bacillus subtilis’, Luo 2016. README.txt contains a description of the files, 10.5256/f1000research.8007.d11343430
This work is supported by Saskatchewan Health Research Foundation Group Grant (2008–2010) and Phase 3 Team Grant (2010–2013) to the Molecular Design Research Group at University of Saskatchewan, a Natural Sciences and Engineering Research Council Discovery Grant (2010–2015) 261981-2010 to YL.
I confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
I thank Ms. Deborah Michel for training and maintenance of SCIEX 4000 QTRAP system at the Core Mass Spectrometry Facility at the University of Saskatchewan. I also thank Mr. Paulos Chumala and Dr. George Katselis for tuning and operating the Agilent Q-TOF 6550 system.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 11 Apr 16 |
read | read |
|
Version 1 10 Feb 16 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)