Keywords
structural chromosomal aberrations, recurrent breakpoint genes, molecular characterization, cancer genome, copy number aberration profile, computational method
This article is included in the Bioconductor gateway.
This article is included in the RPackage gateway.
structural chromosomal aberrations, recurrent breakpoint genes, molecular characterization, cancer genome, copy number aberration profile, computational method
Tumor development is driven by irreversible somatic genomic aberrations such as small nucleotide variants (SNVs) and chromosomal aberrations including numerical as well as structural changes1,2. Genome-wide somatic DNA copy number aberrations (CNA) profiling is a widely established approach to characterize chromosomal aberrations in cancer genomes. At present, application of computational methods has mainly been focused on the analysis of numerical aberrations of chromosomal segments. Recently, evidence is emerging that genes affected by structural chromosomal aberrations, i.e. genes affected by chromosomal breaks, represent a biologically and clinically relevant class of mutations in many cancer types including solid tumors3–6. Importantly, the actual locations of chromosomal CNA-associated breakpoints, which are the points of copy number level shift in somatic CNA profiles, indicate underlying chromosomal breaks and thereby genomic locations affected by somatic structural aberrations5–12. Hence, the wide availability of large series of high-resolution DNA copy number data by for instance array-Comparative Genomic Hybridization (CGH) or by (low-pass) whole genome sequencing (WGS) approaches enables to systematically search for regions and genes that are affected by CNA-associated structural chromosomal changes. Computational methods determining numerical CNAs, consequently, also yield CNA-associated breakpoint locations. However, it is not trivial to identify genes that are recurrently affected by CNA-associated chromosomal breakpoints across (large) series of cancer samples since this methodology also requires dedicated computational methods including comprehensive statistical evaluation.
We here provide a computational method, ‘GeneBreak’, that identifies chromosomal breakpoint locations using DNA copy number profiles. A tailored annotation approach maps breakpoint locations to genes for each individual profile. Moreover, dedicated comprehensive cohort-based statistical analysis including correction for covariates that influence the probability to be a breakpoint gene and multiple testing pinpoints genes that are non-randomly and recurrently affected by chromosomal breaks across multiple tumor samples5. ‘GeneBreak’ is implemented in R (www.cran.r-project.org) and is available from Bioconductor (www.bioconductor.org/packages/release/bioc/html/GeneBreak.html). The Bioconductor vignette describes a detailed example workflow of CNA data obtained by analysis of 200 array-CGH samples. A schematic overview of computational methods is depicted in Figure 1.
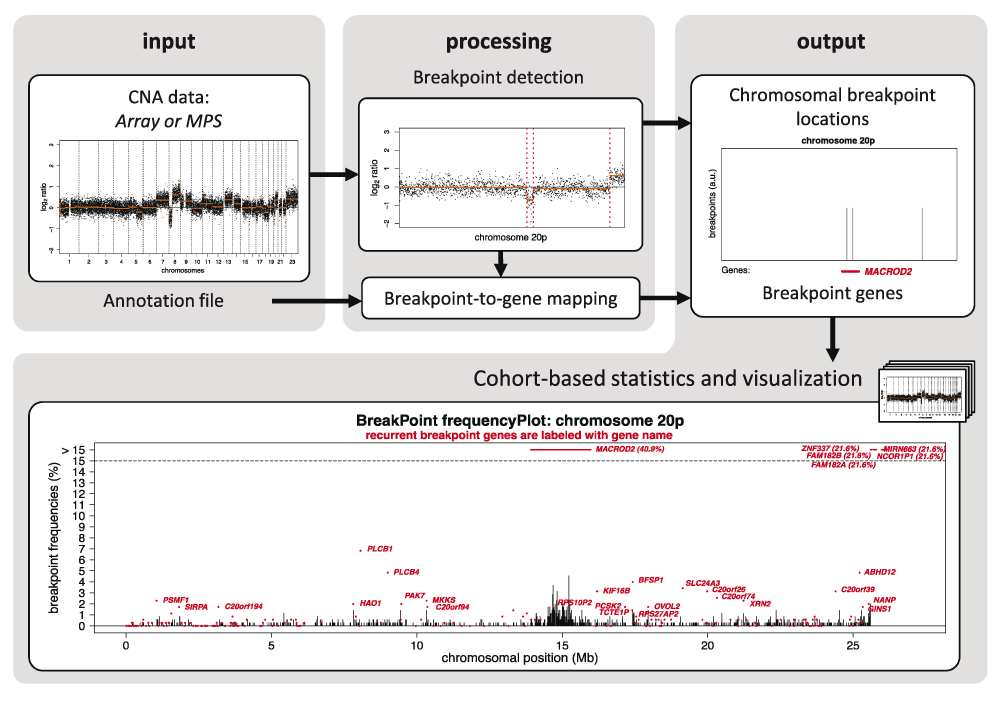
GeneBreak’ requires already segmented DNA copy number data from array-CGH or WGS approaches. The first step involves detection of breakpoint locations. Next, breakpoint locations will be mapped to gene annotations in order to identify genes affected by DNA breakpoints. The final step performs comprehensive cohort-based statistical analyses including correction for multiple testing to reveal both recurrent breakpoint locations and breakpoint genes. The breakpoint frequencies can be visualized with a built-in plot function. This example visualizes the breakpoint locations (vertical black bars) and breakpoint genes (horizontal red bars) on the p-arm of chromosome 20 identified in a cohort of 352 advanced colorectal cancers. The genes labeled with a name are statistically significant recurrent breakpoint genes (FDR<0.1).
The breakpoint detection method we provide is amenable for data from any DNA copy number discovery platform, e.g. array-CGH and (low-pass) WGS, and copy number detection algorithm. For optimal results, ‘GeneBreak’ takes DNA copy number data that are pre-processed by the R-package ‘CGHcall’13 or ‘QDNAseq’14, both based on the Circular Binary Segmentation algorithm15, as input. Alternatively, segmented values (log2-ratios) from a different copy number detection algorithm can be used. In addition, it is recommended to provide discrete DNA copy number states (e.g. loss, neutral, gain) that can be used for breakpoint selection. Bioconductor vignette and manual describe commands and workflows in detail (See Supplementary material).
Breakpoints are defined by the chromosomal locations that separate the contiguous DNA copy number segments pinpointed by a segmentation algorithm. ‘GeneBreak’ identifies chromosomal breakpoint locations for each individual DNA copy number profile. Instead of taking all detected breakpoints, users may want to define more precisely what breakpoints to take into account, based on the two flanking DNA copy number segment characteristics. One of the following three selection options can be applied. A) Copy number-deviation: this selects breakpoints where the shift in log2-ratio between two consecutive DNA copy number segments exceeds the user-defined threshold; B) CNA-associated breakpoints: this selects all breakpoints between consecutive DNA copy number segments, except for breakpoints flanked by two copy number neutral segments; C) CNA-breakpoints: this selects only those breakpoints flanked by segments with dissimilar discrete DNA copy number states.
Due to the typical granularity of the DNA copy number profile data localization (distance between microarray probes or bin size of WGS copy number data), the detected breakpoints that are defined by the genomic start position of the copy number segments, in fact represent a chromosomal interval.
For identification of genes affected by chromosomal breakpoints the built-in gene annotations can be used. Alternatively, a user-defined gene annotation file can be provided (see Bioconductor vignette and manual for further details). The implemented mapping approach identifies genes that are associated with one or multiple chromosomal breakpoint intervals.
Cohort-based identification of recurrent breakpoint events can be performed on both genome location- and gene-level. The default statistical analysis includes standard Benjamini-Hochberg false discovery rate (FDR) correction for multiple testing. This method assumes the same permutation null- distribution for all candidate breakpoint events for the analysis of breakpoints at the level of genomic location. For the gene level however, we recommend to apply the built-in regression-based correction for covariates that may influence the breakpoint probability including the number of breakpoints in a tumor profile, the number of gene-associated features and the gene length by gene-associated feature coverage. In addition, a more comprehensive and powerful dedicated Benjamini-Hochberg FDR correction that accounts for discreteness in the null-distribution is supplied16. Commands and example workflow can be found in Bioconductor vignette and manual.
We applied our method to 352 high-resolution array-CGH samples from a series of advanced colorectal cancers17 following CNA detection using ‘CGHcall’13. Array-CGH data are available in the Gene Expression Omnibus database under accession number GSE63216 (www.ncbi.nlm.nih.gov/projects/geo/). We selected for the CNA-associated breakpoints (setting: ‘CNA-associated’), used gene annotations from ensembl (human genome NCBI build36/hg18, release 54) and applied the dedicated Benjamini-Hochberg-type FDR correction (setting: ‘Gilbert’), for recurrent breakpoint gene identification. A total of 748 genes appeared to be recurrently affected by chromosomal breaks (FDR<0.1)5. Breakpoint frequencies of chromosome 20p are visualized with the built-in plot function (Figure 1; see Bioconductor vignette and manual for further details about this function). Interestingly, patient stratification based on recurrent gene breakpoints and well-known point mutations by propagation to the predefined STRING human protein interaction network revealed one CRC subtype with very poor prognosis, which supported clinical relevance of this class of somatic aberrations in advanced colorectal cancers5.
Genome instability including numerical and structural somatic chromosomal aberrations is a hallmark of cancer. Several tools are available that focus on detection of numerical aberrations of large chromosome segments. The R-package ‘GeneBreak’ extracts additional information from CNA data. ‘GeneBreak’ provides an easy-to-use algorithm, which handles identification of genomic breakpoint locations, mapping of breakpoints to genes and includes a comprehensive statistical approach to reveal recurrent breakpoint genes from series of tumor samples. Therefore, ‘GeneBreak’ can be applied to detect CNA-associated chromosomal breaks in individual tumor samples and facilitates detection of recurrent breakpoint genes across multiple tumor samples.
Publicly available copy number data used for the use case is deposited at Gene Expression Omnibus database under accession number GSE63216 (https://protect-eu.mimecast.com/s/6LQhBmNGvCG).
Software available from: C www.bioconductor.org/packages/release/bioc/html/GeneBreak.html and https://protect-eu.mimecast.com/s/aLGhBqmpgF2
Latest source code: https://github.com/F1000Research/GeneBreak/releases/tag/v1.0
Archived source code as at the time of publication: F1000Research/Genebreak, doi: 10.5281/zenodo.15393718
License: GPL 2
EvdB, GM, RF and SA conceived the study. EvdB, SvL, MvdW, GM, RF and SA designed the workflow and EvdB, SvL and MvdW developed and tested the code. MvdW provided expertise in biostatistics. CR and BY provided expertise in analysis of CNA data obtained by array-CGH and WGS. EvdB, RF and SA prepared the first draft of the manuscript. All authors were involved in the revision of the draft manuscript and have agreed to the final content.
This work was supported by the VUmc-Cancer Center Amsterdam [to E.vd.B.]; performed within the framework of the Center for Translational Molecular Medicine, DeCoDe project [03O-101]; and CTMM-TraIT [05T-401 to EvdB, SvL, BY, GM, RF and SA].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 06 Jul 17 |
read | |
|
Version 1 19 Sep 16 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)