Keywords
Flavonoids, quercetin, antioxidants, Arabidopsis, tobacco, duckweed, paraquat
Flavonoids, quercetin, antioxidants, Arabidopsis, tobacco, duckweed, paraquat
Cellular redox homeostasis is maintained by a complex antioxidant defense system, which includes antioxidant enzymes and low-molecular-weight scavengers1–4. Concerted action of the enzymatic and non-enzymatic components of this defense system counteracts excessive levels of reactive oxygen species (ROS), which can damage cellular components, while preserving adequate levels of ROS required for signaling and cellular redox regulation5–10.
The rapid and excessive generation of ROS is a common response to abiotic stresses and thus can be viewed as a converging point for stress signaling and defense responses11–14. One of the common responses to stress-induced ROS generation is increased flavonoid biosynthesis15–19. Although there is a large body of evidence that supports a role for flavonoids as ROS scavengers, the actual in vivo function of flavonoids as antioxidants in plants was a matter of debate20–22. The main points of contention were (1) that flavonoids are mainly found in vacuoles and are thus compartmentalized from the main site of ROS production in plant cells (i.e., chloroplasts), (2) that flavonoids are enriched in epidermal cells and thus cannot play a significant role in protecting cells of the majority of plant tissues, and (3) that plant cells have an elaborate and efficient antioxidant defense system that successfully suppresses ROS accumulation and therefore the putative antioxidant role of flavonoids would be redundant22. However, recent studies both in Arabidopsis and other plant species have shown that the in vivo antioxidant function of flavonoids is important for the survival of plants under abiotic stress22,23.
Recent studies have also shown that those flavonoid species which, based on their chemical structure, are predicted to be the strongest antioxidants are indeed induced the most by stress22,23. These flavonoids, the dihydroxy B-ring-substituted flavonoids and their glycosides, are exemplified by quercetin and its derivatives22. Quercetin, one of the most abundant flavonoids in plants, also attracted significant attention in medical research because of its antioxidant, anti-inflammatory and anticancer effects with no human toxicity24,25. Here we have tested if quercetin feeding protects plants against the ROS-inducer paraquat (methyl viologen). Paraquat causes the formation of ROS in plants predominantly by impacting the chloroplastic electron transport systems1. Feeding Arabidopsis, tobacco and duckweed with quercetin indeed suppressed the toxic effects of paraquat, indicating that this flavonol can be used as an effective protectant against the harmful effects of ROS on plant growth.
All plants were grown and treated under sterile conditions. Arabidopsis wild type Lansberg erecta (Ler) and transparent testa (tt) mutant lines tt3-1, tt4-1 and tt5-1 (all in Ler background) were grown on solid half-strength Murashige and Skoog (MS/2, Phytotechology) media supplemented with 1% sucrose (pH 5.7). Nicotiana tabacum (Burley variety KT204LC) was grown on solid full-strength MS media with 3% sucrose (pH 5.7). Lemna gibba (Rutgers Duckweed Stock Cooperative ID 7749) was grown in liquid Schenk and Hilderbrandt Basal Salt media (SH, Phytotechology) without sucrose and vitamins. All tested compounds were added to the media after autoclaving. Paraquat and quercetin were obtained from Sigma. Plants were grown in continuous light with a light intensity of 80 µmol m−2 s−1 at 24°C. To measure fresh weight, at least seven pools of 10 plants per treatment were used. Chlorophyll levels were measured using CCM-300 chlorophyll content fluorometer (Opti-Sciences). Data were analyzed using Prism 5.0a software (GraphPad Software Inc.) and are presented as mean ± SD of at least two independent experiments. One-way ANOVA with the Bonferroni’s multiple compariston post-test was used to determine the significance of the difference between means.
For the protein carbonylation experiments, plants were grown on the denoted media for 2 weeks and then weighed. Tissue was disrupted with zirconium beads in a BeadBug bead beater (MidSci) in 2 volumes of extraction buffer (50 mM potassium phosphate buffer pH 7.0, 2 mM MgCl2, 5% glycerol and 5 mM 2-mercaptoethanol). Protein concentration was measured with a BioPhotometer (Eppendorf) using Bradford reagent (Bio-Rad) and bovine serum albumin (BSA, Bio-Rad) as the standard. Proteins were derivatized as described previously26. In brief, protein extracts containing the same amount of protein were mixed with one volume of 12% sodium dodecyl sulfate (SDS, Fisher Scientific) and 2 volumes of 20 mM dinitrophenylhydrazine (Sigma). Derivatization reactions were performed at room temperature in the dark for 60 minutes. Derivatization mixtures were then neutralized with 2M Tris Base and mixed with one volume of 2X SDS-PAGE loading buffer. Protein extracts used for the control gels were directly mixed with 1 volume of 2X SDS-PAGE loading buffer. After denaturation at 95°C for 5 minutes, protein samples were loaded onto SDS-PAGE gels (7.5% for derivatized proteins and 4–20% gradient for control proteins, both Mini-Protean TGX precast gels from Bio-Rad). Separated proteins were transferred to nitrocellulose membranes as previously described27. The commercial antibodies used were rabbit polyclonal anti-DNP antibody (D9656 Sigma; used at 1:1000), monoclonal anti-HSP70 1D9 (Enzo; used at 1:10,000) and polyclonal anti-BiP antibodies (Santa Cruz Biotechnology, sc-33757; used at 1:1000). Secondary antibodies (goat anti-rabbit IgG-HRP and goat anti-mouse IgG-HRP; Santa Cruz Biotechnology) were used at 1:1000. Immunoblots were developed using SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific). Signals were captured using ChemiDoc XRS (Bio-Rad).
To determine whether quercetin feeding protects plants from oxidative stress, we tested the response of Arabidopsis thaliana wild type and mutants with reduced flavonoid content to the ROS-generating compound paraquat in the presence or absence of quercetin. Paraquat is known to prevent germination at high concentrations and to retard growth and promote chlorosis at sub-lethal concentrations28–30. From the large collection of Arabidopsis flavonoid pathway mutants, we selected the three transparant testa (tt) mutants tt3-1, tt4-1 and tt5-1 in the Ler background31,32 and plated them on MS/2 media containing a range of paraquat doses (Figure 1A). The tt4-1 mutant, which carries a lesion in the first dedicated enzyme of the flavonoid biosynthesis pathway, has been previously tested for paraquat sensitivity and was shown to have a lower tolerance to paraquat than the wild type by monitoring loss of chlorophyll content as a measure of chloroplast damage23. Paraquat doses of 0.15 μM and 0.3 μM caused severe growth inhibition in both the wild type and tt mutants (Figure 1). Quercetin (at 100 μM) alone did not lead to any significant changes in fresh weight of any of the tested lines (Ler: 73.9±12.6 mg and 71.3±10.1 mg; tt4-1: 73.2±8.3 mg and 69.3±8.7 mg; tt5-1: 68.8±10.7 mg and 65.1±6.7 mg; tt3-1: 66.2±14.7 mg and 70.4±8.4 mg for 2-week-old plants grown on control and 100 µM quercetin supplemented media, respectively). When plants were grown on plates with 100 µM quercetin and 0.15 µM or 0.3 µM paraquat, they were partially protected from the toxic effect of the herbicide (Figure 1). As expected from their genetic backgrounds, the wild-type, tt3-1 and tt5-1 seedlings were rescued more efficiently by 100 µM quercetin then the tt4-1 mutant which has the strongest defect in flavonoid biosynthesis (Figure 1).
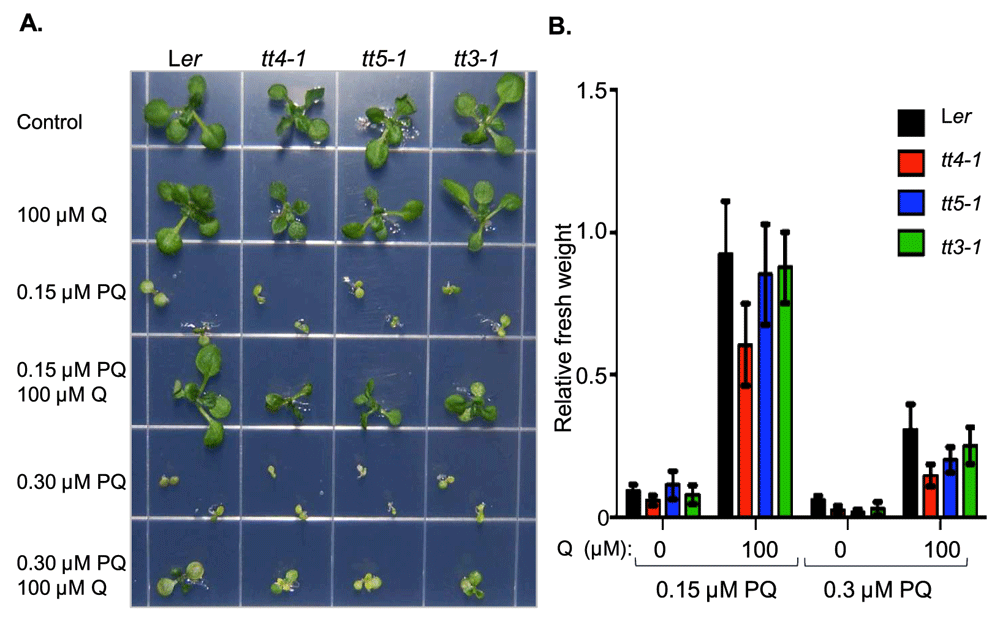
A. Seeds of the Arabidopsis wild type Landsberg erecta (Ler) and transparant testa (tt) mutants were sown and grown on half-strength Murashige and Skoog media containing the denoted compounds paraquat (PQ) and quercetin (Q). Representative plants were transferred to a new plate for photography 2 weeks after sowing. B. Relative fresh weight of plants grown on paraquat media with and without quercetin. Fresh weight of plants grown on control media was assigned the value of 1. Two-week-old plants were weighed in pools of 10 and the data are presented as mean ± SD (n≥7).
To test if quercetin-dependent protection from oxidative stress can be detected at the molecular level, we analyzed protein oxidation. Protein carbonylation is an irreversible type of protein oxidation that leads to loss of protein function and is often used as an indicator of oxidative stress9,33–35. We grew Arabidopsis wild-type plants on control plates and plates containing 100 µM quercetin for 10 days. Plants were then harvested and incubated in either water or 100 µM paraquat for 4 hours. Proteins were isolated, derivatized with dinitrophenylhydrazine, separated on SDS-PAGE gels, transferred to membranes and probed with the anti-diphenylhidrazone antibodies. The protective effect of quercetin was apparent from the reduced accumulation of derivatized proteins in paraquat-treated plants grown on media containing quercetin (Figure 2).
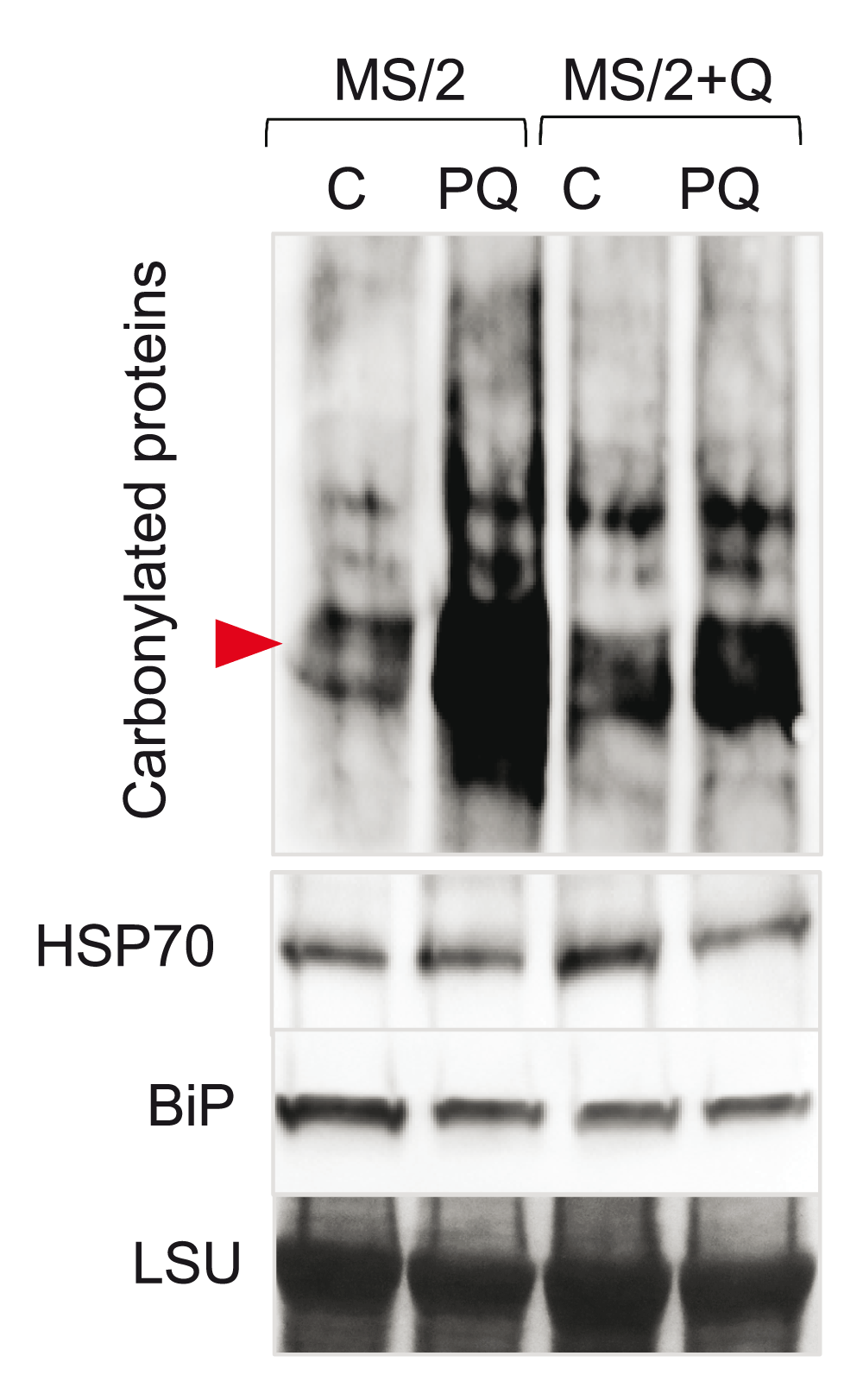
The wild-type plants (Ler) was grown for 10 days on control media or media supplemented with 100 µM Q. Plants were then removed from the plates, weighed and incubated for 4 hours with a mock (water) or 100 µM paraquat (PQ). Representative immunoblot of carbonylated proteins is shown. Arrowhead marks the position of the 50 kDa marker. HSP70 and BiP blots are shown to illustrate that the overall levels of proteostatic stress are not increased in the cytosol and endoplasmic reticulum, respectively. Region of the Ponceau S stained membrane encompassing the RuBiSCO large subunit (LSU) is shown as a loading control.
Next, we tested if quercetin protects other plant species from paraquat-induced oxidative stress. We chose to test tobacco as another dicot species that is distantly related to Arabidopsis and the aquatic monocot species Lemna gibba (duckweed) (Figure 3). Dose-response experiments showed that quercetin counteracts the toxic effects of paraquat in tobacco (Figure 3A and B). Whereas lower doses of quercetin (e.g. 10 µM) did not reverse seedling growth inhibition or chlorophyll loss, seedlings grown on paraquat and higher quercetin doses (e.g. 50 µM and 100 µM) showed no symptoms of toxicity. Seedlings grown on paraquat and the highest tested dose of quercetin (500 µM) remained green, but were stunted suggesting that quercetin concentrations higher than 100 µM are suboptimal for tobacco growth.
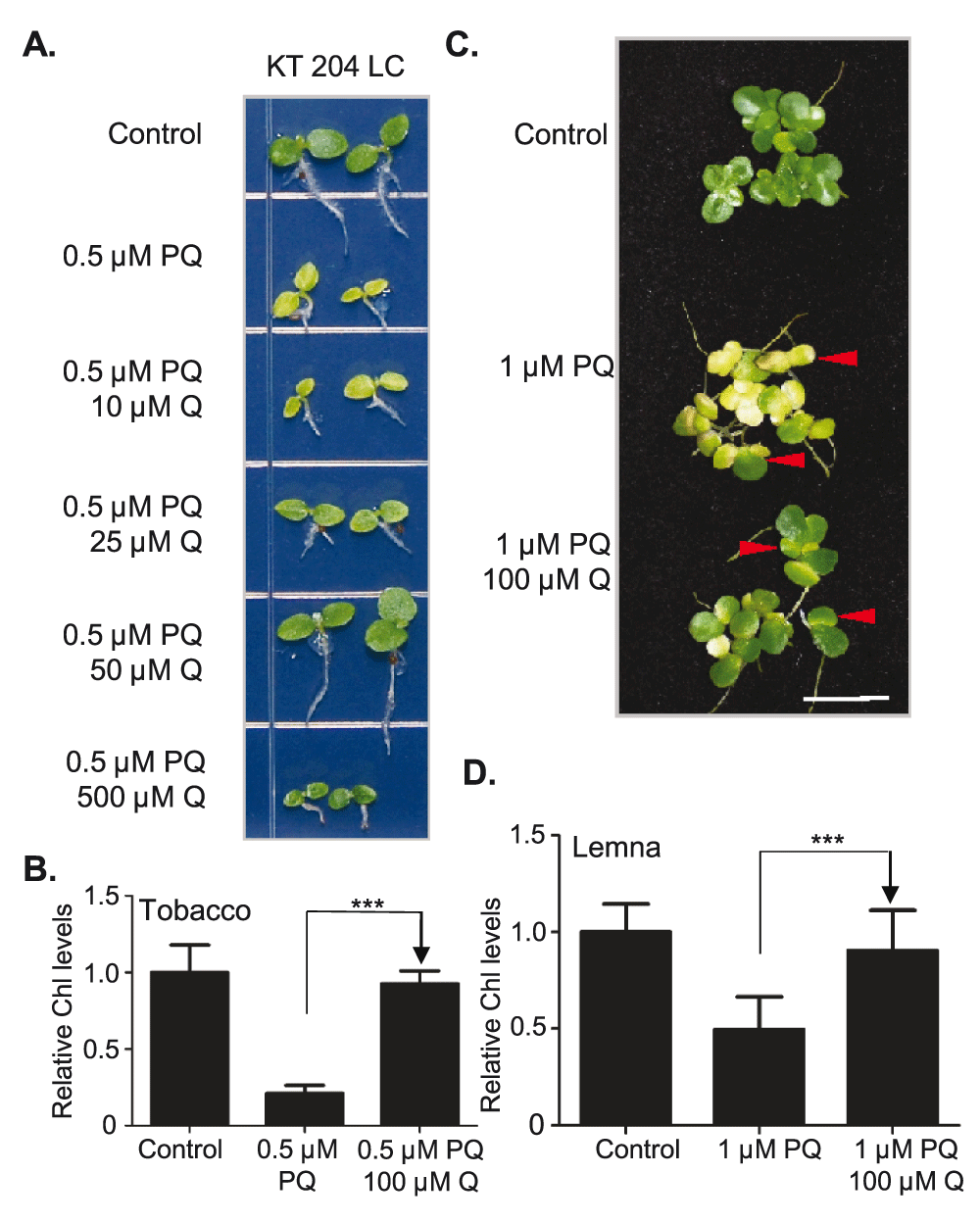
A. Tobacco KT204LC seeds were sown and grown on full-strength Murashige and Skoog media containing the denoted doses of PQ and/or Q. Plants were grown for 3 weeks prior to photography. Scale bar: 5 mm. B. Plants shown in A. were used to measure chlorophyll (Chl) content. One-way ANOVA was used to determine the significance of the difference between the PQ-treated sample and PQ and Q-treated sample. ***, p<0.001. C. Lemna gibba plantlets were transferred to Schenk and Hilderbrandt media with denoted doses of PQ and/or Q and incubated under continuous light with the denoted doses or PQ and/or Q for 5 days prior to photography. Arrowhead points to the newly grown fronds. Scale bar: 5 mm. D. Chl content was measured in all (young and mature) fronds from plantlets shown in C. One-way ANOVA was used to determine the significance of the difference between samples. ***, p<0.001.
We also observed a protective effect of quercetin against paraquat toxicity in the duckweed Lemna gibba. Duckweeds are the smallest, fastest growing and the most morphologically reduced flowering plants36,37. They have a frond (thalloid), no stem and one or more roots. When duckweed plantlets were grown for 36 hours in liquid media with 1 µM paraquat, new fronds emerged as chlorotic (Figure 3C). In contrast, new-grown fronds remained green when plantlets are grown in media containing 1 µM paraquat and 100 µM quercetin. Chlorophyll measurements showed that the overall chlorophyll level in paraquat-treated cultures decreased to ~50% of the control, whereas the chlorophyll level in cultures treated with paraquat and quercetin were the same as in the control plants (Figure 3D).
Here we have shown that feeding plants with quercetin suppressed paraquat toxicity, indicating that this particular flavonoid and its derivates have an important role in the protection of plant cells against increased ROS load. We also found that quercetin offers protection against ROS in a range of plant species, from the evolutionary distant dicots Arabidopsis and tobacco to the monocot and aquatic plant Lemna gibba. Thus, we can conclude that quercetin can be used as a general stress protectant. Considering the relatively low cost of quercetin and its low concentration (100 μM) required for protection against ROS, we propose that the inclusion of quercetin to growth media could be beneficial to promote stress tolerance of agricultural plants grown in tissue or aquaculture.
F1000Research: Dataset 1. Raw data of quercetin feeding protecting plants against oxidative stress, 10.5256/f1000research.9659.d13696438.
J.K., T.E.S. and J.A.S. designed the experiments. J.K. and T.E.S. performed the experiments. All authors analyzed the data, prepared figures and wrote the paper.
This work was funded by the Kentucky Tobacco Research and Development Center.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 03 Oct 16 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)