Keywords
Braconidae, endoparasitoid, Heliothis virescens, cotton plant
Braconidae, endoparasitoid, Heliothis virescens, cotton plant
Infested plants emit volatile organic compounds (VOCs) as an indirect defense against herbivore damage1,2. Similarly, herbivores emit plant-associated VOCs that can guide parasitoids to their hosts3. However, such odor cues are usually released as a blend of various compounds in nature. Consequently, differentiating useful cues from ecologically irrelevant odors can be challenging for foraging parasitoids. Therefore, it is expected that antennal sensitivity of parasitoids will vary in response to different compounds. Antenna sensitivity in insects can be measured with electroantennogram (EAG) recording. EAG measures the summed activity of olfactory receptor neurons in the antenna and forms the basis for the level of biological activity elicited by various compounds4.
Microplitis croceipes (Hymenoptera: Braconidae) is an endoparasitoid of Heliothis virescens (Lepidoptera: Noctuidae), which is an important pest of cotton plant. In a previous related study5, 15 compounds in the volatile blend emitted by cotton-fed H. virescens larvae that attracted M. croceipes were identified and quantified using gas chromatography-mass spectrometry (GC/MS). The compounds in the attractive blend occurred in varying proportions (Table 1). However, the relative abundance of a blend component does not necessarily indicate its relevance to resource location in insects6. In the present study, olfactory response of M. croceipes to synthetic versions of 15 previously identified compounds was tested in EAG bioassays. Comparing EAG results in the present study and GC/MS analyses in a previous study5, we indicated the discrepancy between relative abundance of a volatile blend component and the level of antennal response in parasitoids.
This table was modified from Morawo and Fadamiro (doi: 10.1007/s10886-016-0779-7)5, with permission from the authors.
| ID1 | Compound | Relative abundance (%) | Chemical category |
|---|---|---|---|
| 1 | α-Pinene | 15.1 | Monoterpene |
| 2 | β-Pinene | 1.6 | Monoterpene |
| 3 | 1-Octen-3-ol | 1.4 | Alcohol |
| 4 | 3-Octanone | 0.8 | Ketone |
| 5 | Myrcene | 2.7 | Monoterpene |
| 6 | Unknown2 | 1.2 | - |
| 7 | Limonene | 9.1 | Monoterpene |
| 8 | 2-Ethylhexanol | 2.2 | Alcohol |
| 9 | Decanal | 1.0 | Aldehyde |
| 10 | Tridecane | 6.2 | Alkane |
| 11 | Tetradecane | 2.4 | Alkane |
| 12 | (E)-β-Caryophyllene | 29.2 | Sesquiterpene |
| 13 | α-Bergamotene2 | 0.7 | Sesquiterpene |
| 14 | α-Humulene | 6.5 | Sesquiterpene |
| 15 | α-Farnesene | 0.8 | Sesquiterpene |
| 16 | Bisabolene | 8.6 | Sesquiterpene |
| 17 | α-Bisabolol | 7.9 | Sesquiterpene |
Microplitis croceipes was reared on 2nd-3rd instar larvae of H. virescens and adult wasps were supplied with 10% sugar water upon emergence in our laboratory at Entomology & Plant Pathology Department, Auburn University. For more details about rearing protocol, see Lewis and Burton7. Female parasitoids used for EAG bioassays were 2–3 days-old, presumed mated (after at least 24 h of interaction with males), and inexperienced with oviposition or plant material. The general rearing conditions for all insects were 25±1 °C, 75±5 % relative humidity and 14:10 h (light:dark) photoperiod.
EAG responses of M. croceipes to 15 synthetic compounds (Table 1), previously identified in the headspace of cotton-fed H. virescens larvae5, were recorded according to the method described by Ngumbi et al.8 with modifications. Two compounds, α-bergamotene (not commercially available) and an unidentified compound reported in the previous study5 were not tested in the present study. α-Pinene, β-pinene, myrcene, limonene, 2-ethylhexanol, tridecane, (E)-β-caryophyllene, α-humulene, α-farnesene and α-bisabolol with purity 95–99% were purchased from Sigma-Aldrich® (St. Louis, MO, USA). 1-Octen-3-ol, 3-octanone, decanal, tetradecane and bisabolene with purity 96–99% were purchased from Alfa Aesar® (Ward Hill, MA, USA). Test compounds were formulated in hexane at 0.1 μg/μl and delivered onto Whatman®No.1 filter paper strips at an optimum dose of 1 µg. Impregnated filter papers were placed inside glass Pasteur pipettes and stimulus was introduced as 0.2 s odor puffs. A glass capillary reference electrode filled with 0.1 M KCl was attached to the back of the wasp head, and a similar recording electrode was connected to the excised tip of the wasp antenna. The analog signal was detected through a probe and processed with a data acquisition controller (IDAC-4, Syntech, The Netherlands). Data was assessed using EAG 2000 software (Syntech, The Netherlands). EAG responses to the 15 compounds and control (hexane) were sequentially recorded for each of 15 insect replicates. Each compound was assigned positions 1 through 15 across replicates to minimize positional bias.
Differences in absolute EAG values (EAG response to compound minus response to solvent control) of synthetic compounds was analyzed using the Kruskal-Wallis test, followed by Sidak’s multiple comparison test. The relationship between EAG response and relative abundance was analyzed with Proc Corr (correlation) procedure in SAS. All analyses were performed in SAS v9.2 (SAS Institute Inc., Cary, NC, USA) with P=0.05 level of significance.
Female M. croceipes showed varying EAG responses to test compounds (range: 0.05–0.82 mV; Figure 1). Decanal elicited the highest EAG response (0.82 mV; χ2 = 134.13; df = 14; P<0.0001), while β-pinene elicited the lowest response (0.05 mV) in parasitoids. Decanal, tridecane, 3-octanone, 2-ethylhexanol, 1-octen-3-ol, bisabolene, tetradecane and α-farnesene elicited EAG responses ≥0.22 mV (50th percentile rank). Four of the top bioactive compounds, decanal, 3-octanone, 1-octen-3-ol and 2-ethylhexanol were emitted in quantities ≤2.2% of the total blend (Table 1). On the other hand, (E)-β-caryophyllene, the most abundant (29.2% of total blend) component, elicited a relatively low EAG response (0.17 mV) in parasitoids (Figure 1). However, the negative correlation between EAG response and relative abundance of compounds was not statistically significant (r = -0.33; N = 15; P=0.23).
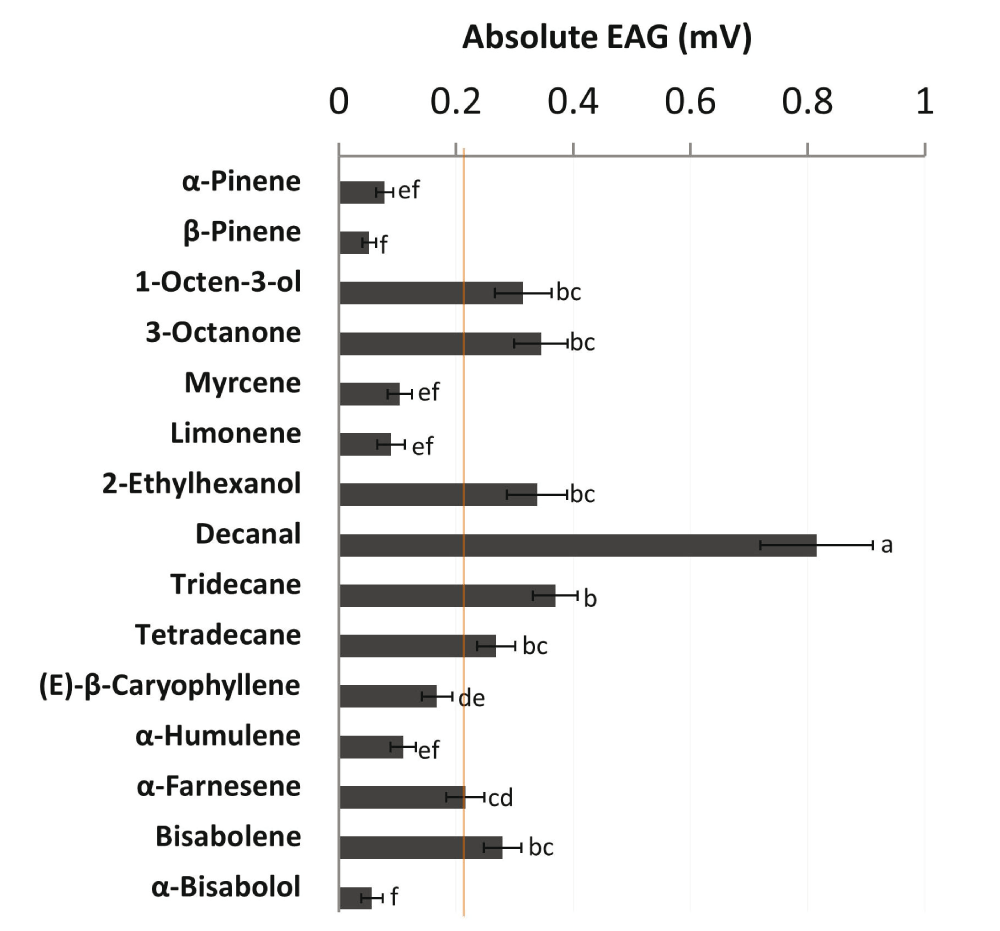
Mean absolute Electroantennogram (EAG) responses (mV ± SEM; N = 15) of female Microplitis croceipes to 15 volatile compounds identified in the headspace of cotton-fed Heliothis virescens larvae5. Synthetic compounds were formulated in hexane (solvent control) and tested at an optimum dose of 1 μg. Orange line indicates the arbitrary response threshold of 0.22 mV (50th percentile rank). Bars with no letters in common are significantly different (P<0.05; Kruskal-Wallis test followed by Sidak’s multiple comparison test).
EAG responses of Micropiltis croceipes in the present study indicated variation in biological activity elicited by test compounds at the peripheral level, and revealed a discrepancy between relative abundance and level of antennal responses in parasitoids. High EAG response elicited by decanal in M. croceipes agrees with previous reports on olfactory responses of the parasitoids, Microplitis mediator9 and Bracon hebetor10. Furthermore, decanal is a key attractant for host-seeking M. croceipes5. Although compounds are emitted in different quantities in natural blends, minor components can have a profound effect on resource location in parasitoids6,11. Interestingly, decanal constituted only 1% of the total blend emitted by cotton-fed H. virescens5, but elicited the highest EAG response in M. croceipes, supporting the “little peaks-big effects” concept6. On the other hand, (E)-β-caryophyllene, the most abundant blend component, elicited a relatively low EAG response in parasitoids.
Therefore, it is more likely that the ecological relevance of a compound, rather than its relative abundance determines the level of olfactory response in foraging insects. For instance, small amounts of isothiocyanates in the volatile blend of brassica plants serve as host location cues for parasitoids of brassica herbivores12,13. More importantly, blend components act in concert to provide parasitoids with complete information14. Consequently, certain compounds function as background odors to enhance detectability (olfactory contrast) of other attractive components in a blend12,15. It is possible that (E)-β-caryophyllene serves as a background odor in the blend emitted by cotton-fed H. virescens. Finally, it should be noted that while EAG measures the level of bioactivity, behavioral bioassays are usually needed to establish the functional role of various compounds.
Dataset 1. EAG responses of Microplitis croceipes to synthetic compounds and correlation with relative abundance of compounds. Electroantennogram (EAG) data shows actual EAG response readouts to different compounds for 15 insect replicates. Absolute EAG value for each compound in a replicate can be obtained by deducting the average of two controls (Control 1 and Control 2) from the actual EAG values. Correlation data shows relative abundance of 15 blend components and their corresponding mean absolute EAG values. Details of data analyses were indicated in the main text and Figure 1 legend. Raw data behind the representation shown in Figure 1 and analyses referred to in the Results section are included. DOI: 10.5256/f1000research.10104.d14344616.
TM and HF conceived the study. TM designed the experiment. TM and MB carried out the research. All authors contributed to writing and revision of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
References
1. Li Y, Dickens JC, Steiner WW: Antennal olfactory responsiveness ofMicroplitis croceipes (Hymenoptera: Braconidae) to cotton plant volatiles.J Chem Ecol. 1992; 18 (10): 1761-73 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
References
1. Morawo T, Fadamiro H: Identification of Key Plant-Associated Volatiles Emitted by Heliothis virescens Larvae that Attract the Parasitoid, Microplitis croceipes: Implications for Parasitoid Perception of Odor Blends.J Chem Ecol. 2016; 42 (11): 1112-1121 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
References
1. Tamiru A, Bruce T, Woodcock C, Birkett M, et al.: Chemical cues modulating electrophysiological and behavioural responses in the parasitic waspCotesia sesamiae. Canadian Journal of Zoology. 2015; 93 (4): 281-287 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 2 (revision) 09 Mar 17 |
read | |||
|
Version 1 21 Nov 16 |
read | read | read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)